Comparison of Engine Performance between Nano- and Microemulsions of Solketal Droplets Dispersed in Diesel Assisted by Microwave Irradiation
Abstract
1. Introduction
2. Experimental Details
2.1. Experimental Materials
2.2. Preparation of Nano- and Microemulsions by Microwave Irradiation
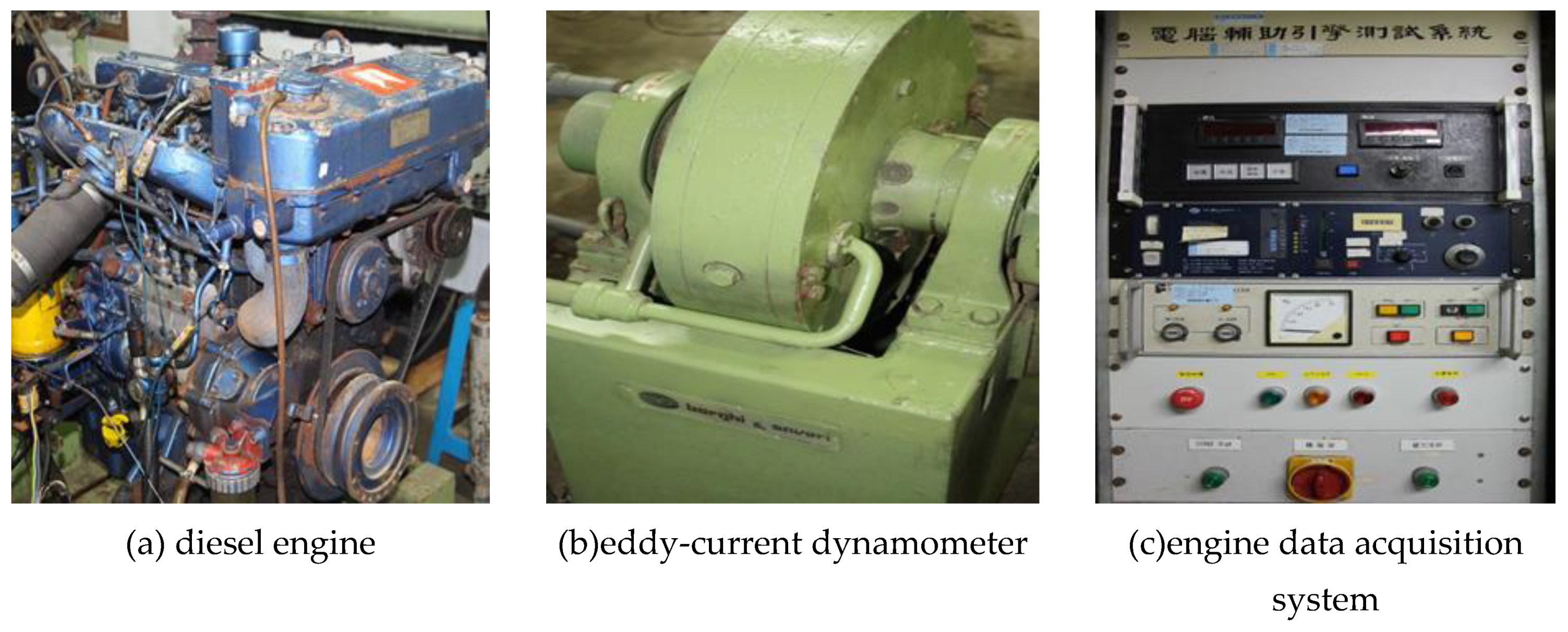
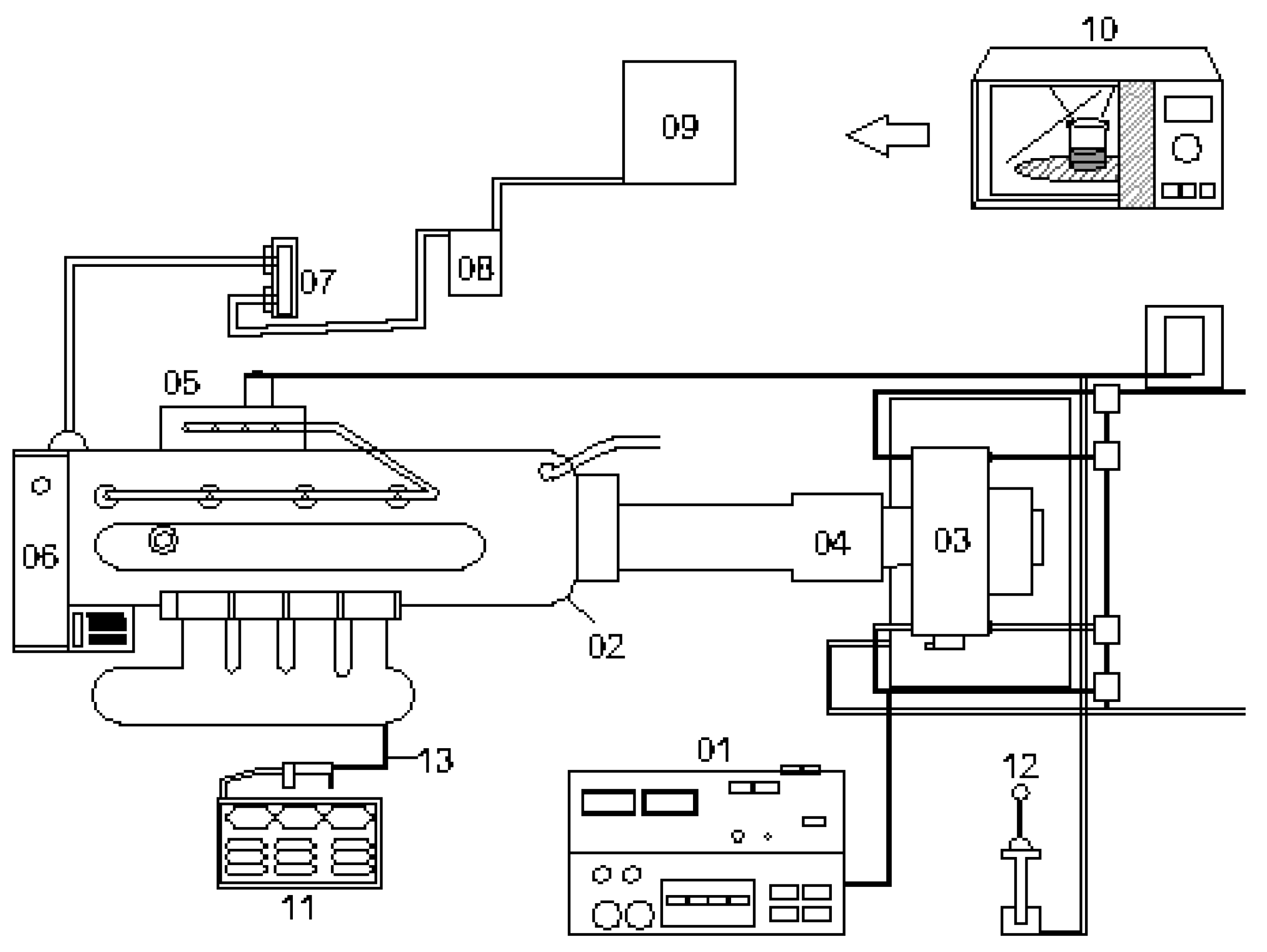
2.3. Measurement of Engine Performance
3. Results and Discussion
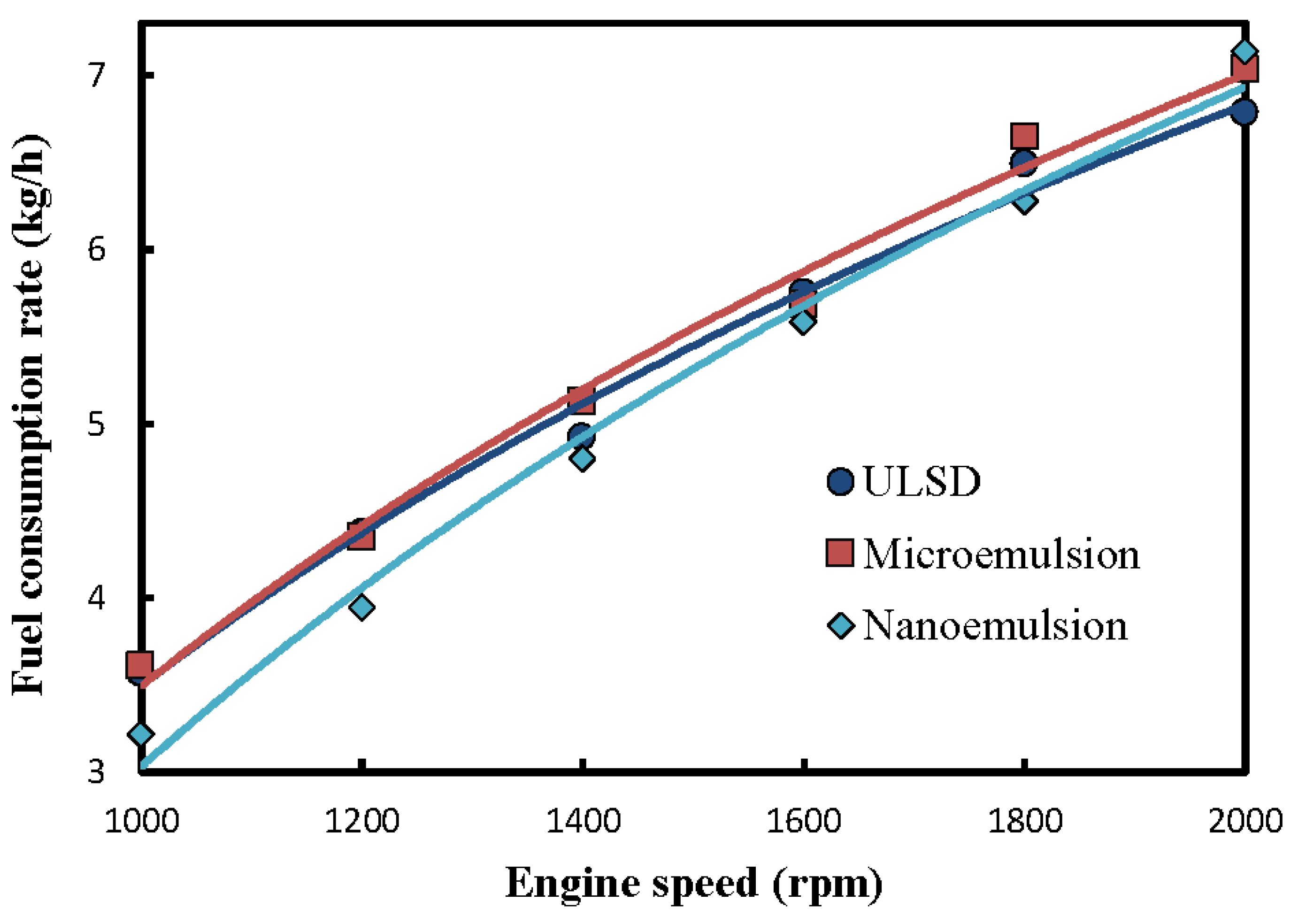
3.1. Fuel-Structure Effects on Fuel Consumption Rate
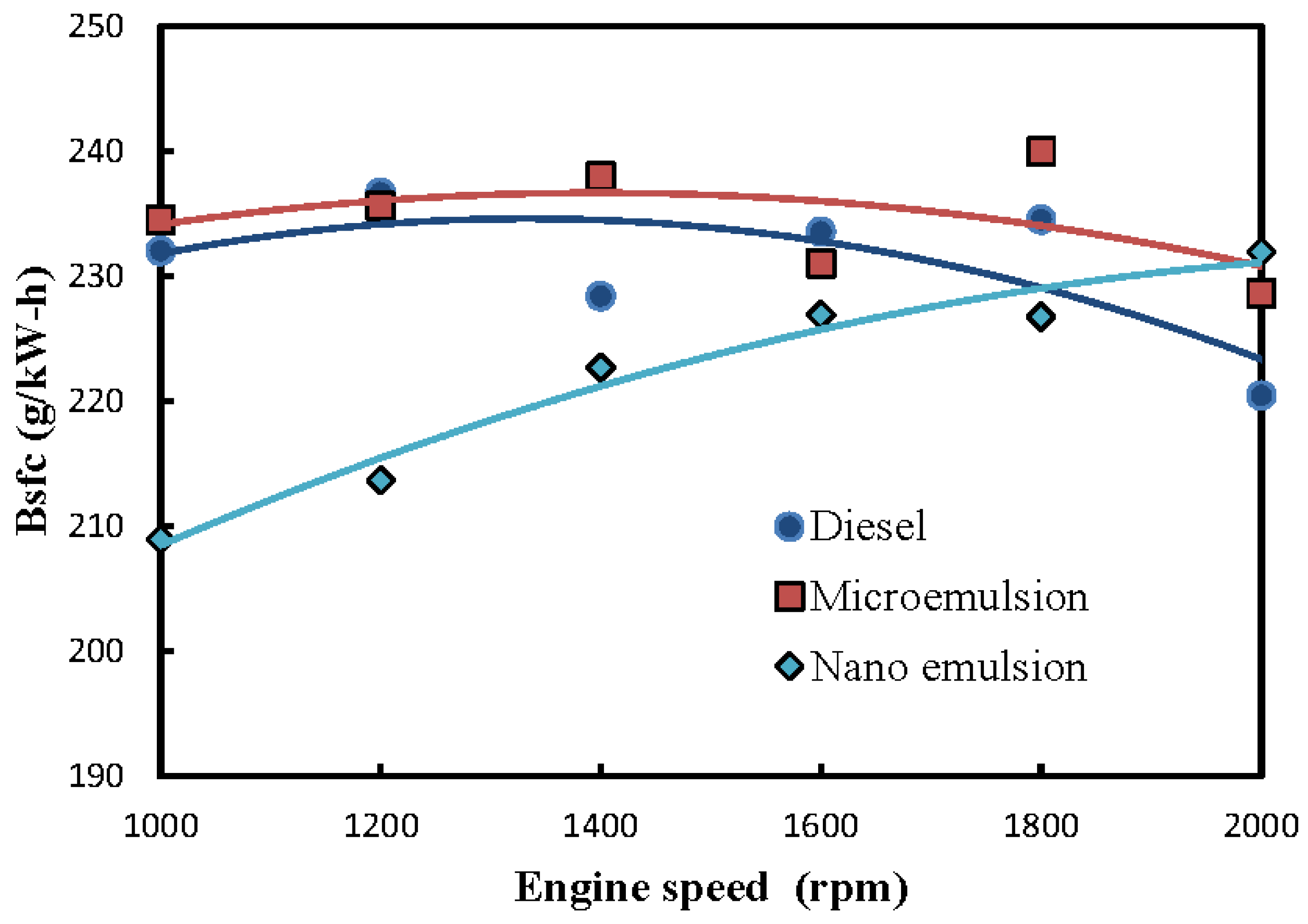
3.2. Fuel-Structure Effects on Brake Specific Fuel Consumption (Bsfc)
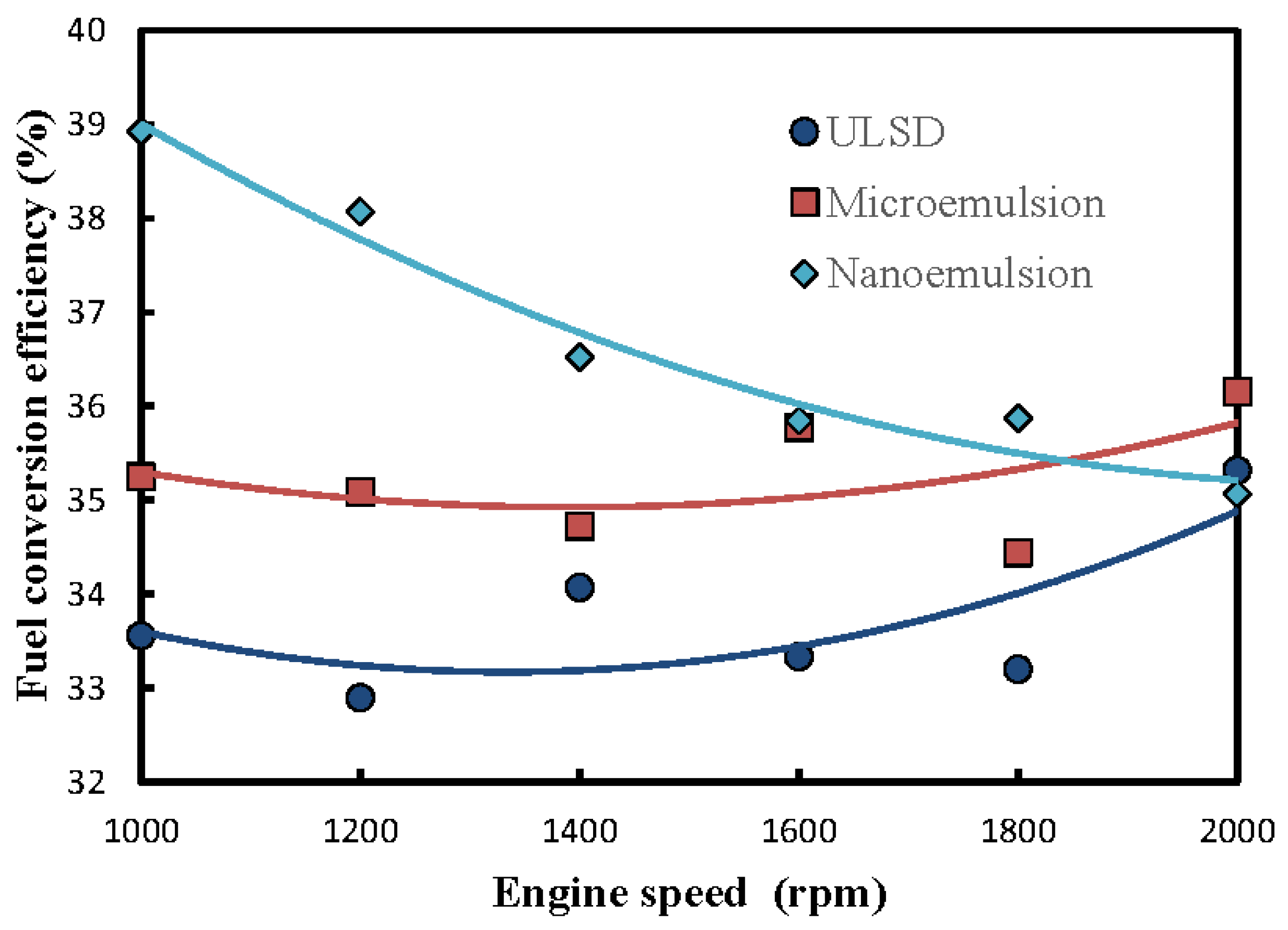
3.3. Fuel-Structure Effects on Fuel Conversion Efficiency
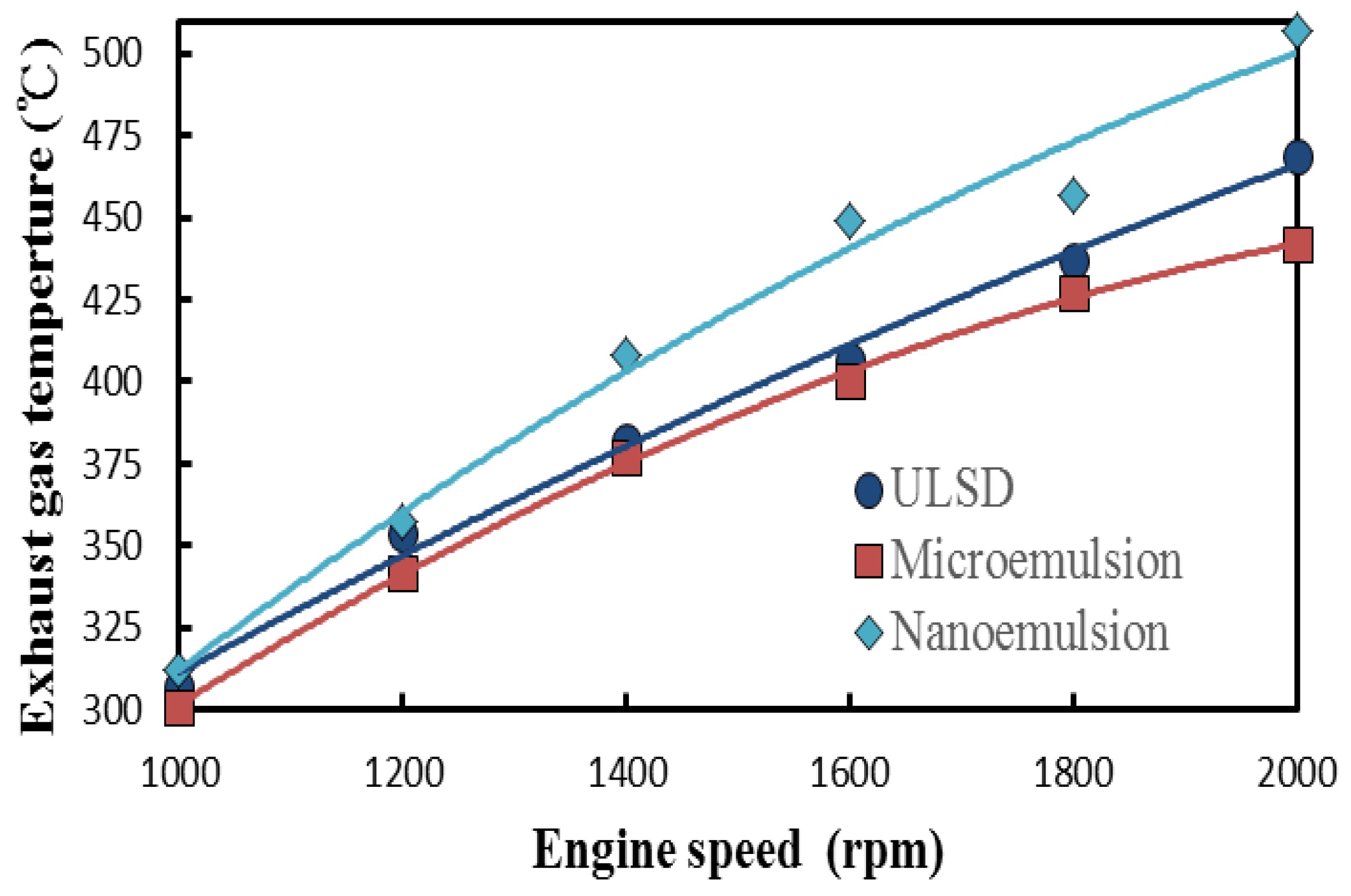
3.4. Fuel-Structure Effects on Exhasust Gas Temperature
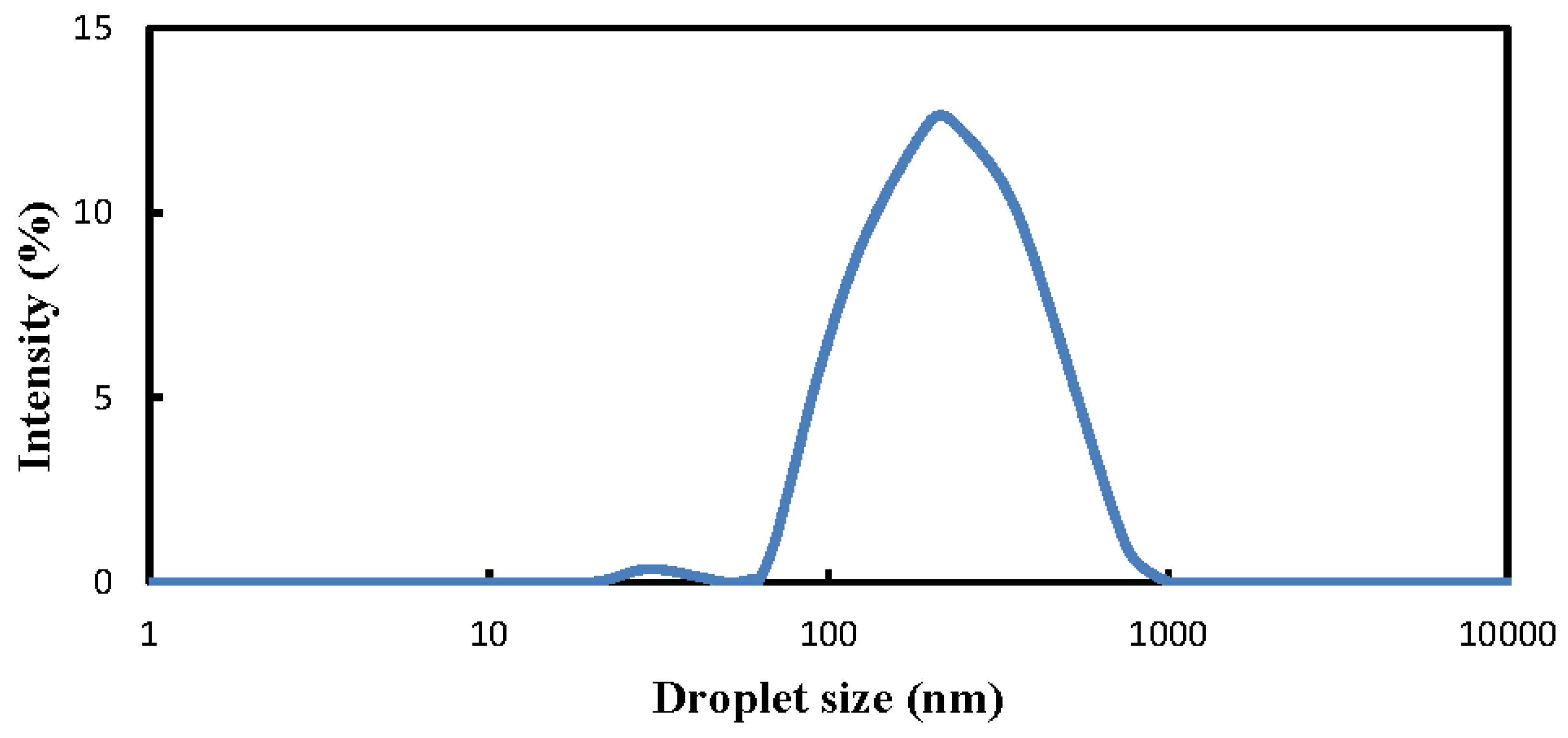
3.5. Fuel-Structure Effects on Distribution of Dispersed Droplet Size
4. Conclusions
- (1)
- The nanoemulsions of solketal-in-ULSD had the highest kinematic viscosity, lowest mean droplet size of the dispersed phase, the lowest carbon residue, and the lowest fuel consumption rate required for the same engine power output among the three test fuels. In addition, a larger fuel consumption rate was required at higher engine speed for all the test fuels;
- (2)
- In comparison with microemulsions of solketal droplets in ULSD and the neat ULSD, the nanoemulsions were found to have the lowest brake specific fuel consumption (Bsfc) and equvilently the highest fuel conversion efficiency. This implies that the nanoemulsions of solketal-in-ULSD had superior fuel economy and a lower fuel consumption rate and are thus preferrable to fuel diesel engines among those three fuels. In addition, the Bsfcs of the nanoemulsions increased while fuel conversion efficiency decreased under an increase of engine speed. The Bsfc and fuel conversion efficiency of the nanoemulsion gradually approached those bsfcs and fuel conversion efficiencies of the microemulsions and the neat ULSD particularly at a faster engine speed of over 1600 rpm;
- (3)
- The nanoemulsions of the dispersed solketal droplets within the ULSD were observed to have the highest amount of heat release and the highest exhaust gas temperature among those three test fuels;
- (4)
- Most of the dispersed solketal droplet sizes were concentrated around 127 nm, with peak intensity of 12.65%. In addition, the microwave-irradiating effects applied in this study were successful in preparing the nanoemulsions in which all the sizes of the dispersed solketal droplets distributed within their continuous ULSD phase were well below 1000 nm.
Author Contributions
Funding
Conflicts of Interest
References
- Renckens, S.; Skogstad, G.; Mondou, M. When normative and market power interact: The european union and global biofuels governance. J. Common Mark. Stud. 2017, 55, 1432–1448. [Google Scholar] [CrossRef]
- Khalife, E.; Tabatabaei, M.; Demirbas, A.; Aghbashlo, M. Impacts of additives on performance and emission characteristics of diesel engines during steady state operation. Progr. Energy Combust. Sci. 2017, 59, 32–78. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ibrahim, T.K.; Hammid, A.T.; Yusri, I.M.; Hamidi, M.A.; Yusop, A.F. Overview of the oxygenated fuels in spark ignition engine: Environmental and performance. Renew. Sust. Energy Rev. 2018, 91, 394–408. [Google Scholar] [CrossRef]
- Ozorio, L.P.; Pianzolli, R.; Mota, M.B.S.; Mota, C.J. Reactivity of glycerol/acetone ketal (solketal) and glycerol/formaldehyde acetals toward acid-catalyzed hydrolysis. J. Braz. Chem. Soc. 2012, 23, 931–937. [Google Scholar] [CrossRef]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Attia, A.M.; Kulchitskiy, A.R. Influence of the structure of water-in-fuel emulsion on diesel engine performance. Fuel 2014, 116, 703–708. [Google Scholar] [CrossRef]
- Melo-Espinosa, E.A.; Bellettre, J.; Tarlet, D.; Montillet, A.; Piloto-Rodríguez, R.; Verhelst, S. Experimental investigation of emulsified fuels produced with a micro-channel emulsifier: Puffing and micro-explosion analyses. Fuel 2018, 219, 320–330. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.T.; Chen, L.W. Influences of combustion improver content and motionless time on the stability of two-phase emulsions. Particul. Sci. Technol. 2018, 36, 91–95. [Google Scholar] [CrossRef]
- Hasannuddin, A.K.; Yahya, W.J.; Sarah, S.; Ithnin, A.M.; Syahrullail, S.; Sugeng, D.A.; Ramlan, N.A. Performance, emissions and carbon deposit characteristics of diesel engine operating on emulsion fuel. Energy. 2018, 142, 496–506. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 41–54. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Mehrnia, M.A.; Jafari, S.M.; Makhmal-Zadeh, B.S.; Maghsoudlou, Y. Crocin loaded nanoemulsions: Factors affecting emulsion properties in spontaneous emulsification. Int. J. Biol. Macromol. 2016, 84, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.N.; More, U.; Malek, N.; Chakraborty, M.; Parikh, P.A. Study of stability and thermodynamic properties of water-in-diesel nanoemulsion fuels with nano-Al additive. Appl. Nanosci. 2015, 5, 891–900. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, S.M. Emulsification characteristics of nano-emulsions of solketal in diesel prepared using microwave irradiation. Fuel 2018, 221, 165–170. [Google Scholar] [CrossRef]
- Debnath, B.K.; Saha, U.K.; Sahoo, N. A comprehensive review on the application of emulsions as an alternative fuel for diesel engines. Renew. Sust. Energy Rev. 2015, 42, 196–211. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Ong, M.Y. A review of microwave-assisted reactions for biodiesel production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef]
- Horikoshi, S.; Schiffmann, R.F.; Fukushima, J.; Serpone, N. Microwave heating. In Microwave Chemical and Materials Processing; Springer: Singapore, 2018; pp. 47–85. [Google Scholar]
- Abdurahman, N.H.; Yunus, R.M.; Azhari, N.H.; Said, N.; Hassan, Z. The potential of microwave heating in separating water-in-oil (w/o) emulsions. Energy Procedia 2017, 138, 1023–1028. [Google Scholar] [CrossRef]
- Yin, S.; Chen, K.; Srinivasakannan, C.; Guo, S.; Li, S.; Peng, J.; Zhang, L. Enhancing recovery of ammonia from rare earth wastewater by air stripping combination of microwave heating and high gravity technology. Chem. Eng. J. 2018, 337, 515–521. [Google Scholar] [CrossRef]
- Hyde, A.; Horiguchi, M.; Minamishima, N.; Asakuma, Y.; Phan, C. Effects of microwave irradiation on the decane-water interface in the presence of Triton X-100. Colloid Surf. A. 2017, 524, 178–184. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.T.; Chen, L.W. Comparison of the fuel properties of nitromethane emulsions in diesel and biodiesel assisted by microwave irradiation and magnetic stirring. J. Disper. Sci. Technol. 2016, 37, 1334–1340. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Buddin, M.S.; Ooi, B.S.; Kusumastuti, A. Utilization of environmentally benign emulsion liquid membrane (ELM) for cadmium extraction from aqueous solution. J. Water Proc. Eng. 2017, 15, 26–30. [Google Scholar] [CrossRef]
- Rhodia Company. Technical Data Sheet for Augeo SL-191 Product; Rhodia Company: Brussels, Belgium, 2016. [Google Scholar]
- Koneva, A.S.; Safonova, E.A.; Kondrakhina, P.S.; Vovk, M.A.; Lezov, A.A.; Chernyshev, Y.S.; Smirnova, N.A. Effect of water content on structural and phase behavior of water-in-oil (n-decane) microemulsion system stabilized by mixed nonionic surfactants Span 80/Tween 80. Colloid Surf. A. 2017, 518, 273–282. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.T. Emulsification characteristics of three-phase emulsion of biodiesel-in nitromethane-in-diesel prepared by microwave irradiation. Fuel 2015, 158, 50–56. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of flavor oil microemulsions, nanoemulsions and emulsions: Influence of composition and preparation method. J. Agr. Food. Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Tsai, S.M. Comparison of fuel properties of nanoemulsions of diesel fuel dispersed with solketal by microwave irradiation and mechanical homogenization methods. Energy Fuels 2018, 32, 11814–11820. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ebrahimzadeh, M. Preparation of polyamide nanocapsules of Aloe vera L. delivery with in vivo studies. AAPS PharmSciTech. 2015, 16, 242–249. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Hamze, S.; Agresti, F.; Estellé, P.; Barison, S.; Fedele, L.; Bobbo, S. Dynamic viscosity, surface tension and wetting behavior studies of paraffin-in-water nano-emulsions. Energies 2019, 12, 3334. [Google Scholar] [CrossRef]
- Saravanan, S. Effect of exhaust gas recirculation (EGR) on performance and emissions of a constant speed DI diesel engine fueled with pentanol/diesel blends. Fuel 2015, 160, 217–226. [Google Scholar]
- Fayad, M.A.; Tsolakis, A.; Fernández-Rodríguez, D.; Herreros, J.M.; Martos, F.J.; Lapuerta, M. Manipulating modern diesel engine particulate emission characteristics through butanol fuel blending and fuel injection strategies for efficient diesel oxidation catalysts. Appl. Energy 2017, 190, 490–500. [Google Scholar] [CrossRef]
- Venkatesh, A.P.; Muniyappan, M.; Joel, C.; Padmanabhan, S. Investigation on the effect of nanofluid on performance behaviour of a waste cooking oil on a small diesel engine. Int. J. Ambient Energy 2018, 1–6. [Google Scholar] [CrossRef]
- Yang, W.M.; An, H.; Chou, S.K.; Chua, K.J.; Mohan, B.; Sivasankaralingam, V.; Raman, V.; Maghbouli, A.; Li, J. Impact of emulsion fuel with nano-organic additives on the performance of diesel engine. Appl. Energy 2013, 112, 1206–1212. [Google Scholar] [CrossRef]
- Bidita, B.S.; Suraya, A.R.; Shazed, M.A.; Salleh, M.M.; Idris, A. Preparation, characterization and engine performance of water in diesel nanoemulsions. J. Energy Inst. 2016, 89, 354–365. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion micro emulsion and nano emulsion: A review. Syst. Rev. Pharm. 2017, 8, 39. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds solketal and surfactants are available from the authors. |
| Property | Component | ||
|---|---|---|---|
| Solketal | Tween 80 | Span 80 | |
| Chemical formula | C6H12O3 | C64H124O26 | C24H44O6 |
| Molecular weight (g/mol) | 132 | 1308 | 428 |
| Flash point (°C) | 91 | 149 | 186 |
| Amount of heat release (MJ/kg) | 14.99 | 29.77 | 35.23 |
| Carbon residue (wt. %) | 1.81 | 2.48 | 2.87 |
| Property | Fuel | ||
|---|---|---|---|
| Neat ULSD | Microemulsion of Solketal-in- ULSD | Nanoemulsion of Solketal-in- ULSD | |
| Amount of heat release (MJ/kg) | 46.26 | 43.58 | 44.26 |
| Carbon residue (wt. %) | 1.05 | 0.29 | 0.26 |
| Kinematic viscosity (mm2/s) | 3.4 | 3.6 | 7.2 |
| Flash point (°C) | 79 | 84 | 87 |
| Mean droplet size (µm) | - | 26.51 | 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Tsai, S.-M. Comparison of Engine Performance between Nano- and Microemulsions of Solketal Droplets Dispersed in Diesel Assisted by Microwave Irradiation. Molecules 2019, 24, 3497. https://doi.org/10.3390/molecules24193497
Lin C-Y, Tsai S-M. Comparison of Engine Performance between Nano- and Microemulsions of Solketal Droplets Dispersed in Diesel Assisted by Microwave Irradiation. Molecules. 2019; 24(19):3497. https://doi.org/10.3390/molecules24193497
Chicago/Turabian StyleLin, Cherng-Yuan, and Shih-Ming Tsai. 2019. "Comparison of Engine Performance between Nano- and Microemulsions of Solketal Droplets Dispersed in Diesel Assisted by Microwave Irradiation" Molecules 24, no. 19: 3497. https://doi.org/10.3390/molecules24193497
APA StyleLin, C.-Y., & Tsai, S.-M. (2019). Comparison of Engine Performance between Nano- and Microemulsions of Solketal Droplets Dispersed in Diesel Assisted by Microwave Irradiation. Molecules, 24(19), 3497. https://doi.org/10.3390/molecules24193497





