Facile Synthesis of a Series of Non-Symmetric Thioethers Including a Benzothiazole Moiety and Their Use as Efficient In Vitro anti-Trypanosoma cruzi Agents
Abstract
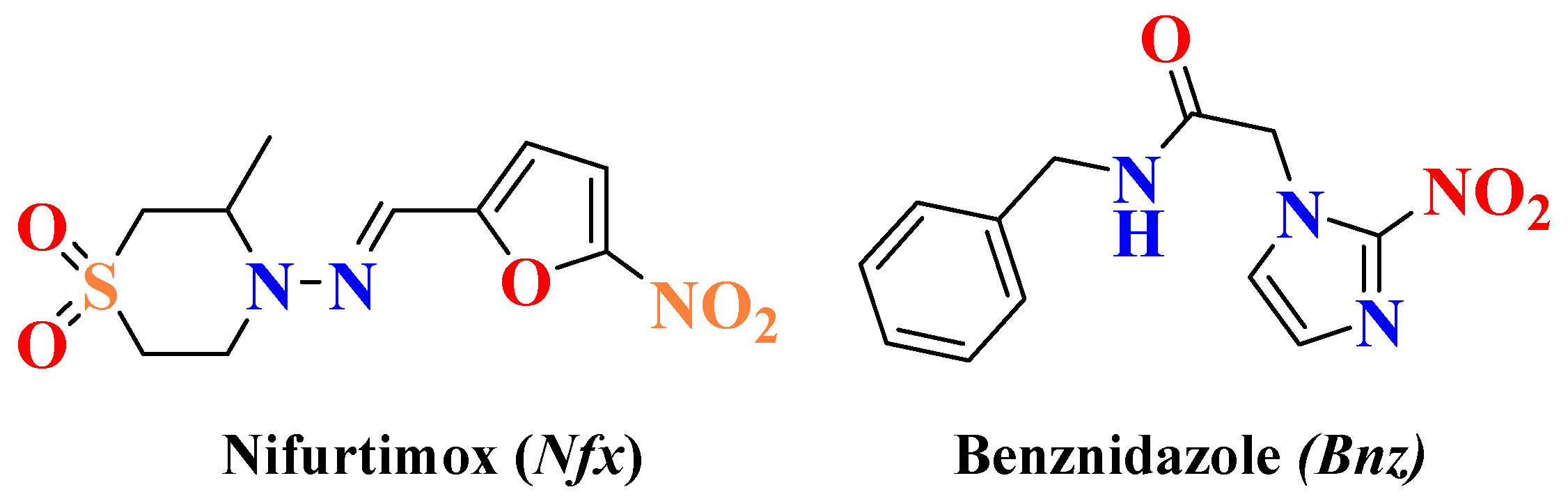
:1. Introduction
2. Results and Discussion
2.1. Synthesis
Spectroscopic Characterization
2.2. Biological Evaluation
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Synthesis of 2-benzylsulfanyl BTAs Derivatives (1–7)
3.3. Biological Activity
3.3.1. Trypanocidal Activity Evaluation
3.3.2. Cytotoxic Activity Evaluation and SI
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mariappan, G.; Prabhat, P.; Sutharson, L.; Banerjee, J.; Patangia, U.; Nath, S. Synthesis and antidiabetic evaluation of benzothiazole derivatives. J. Korean Chem. Soc. 2012, 56, 251–256. [Google Scholar] [CrossRef]
- Malik, S.; Bahare, R.S.; Khan, S.A. Design, synthesis and anticonvulsant evaluation of N-(benzo[d]thiazol-2-ylcarbamoyl)-2-methyl-4-oxoquinazoline-3(4H)-carbothioamide derivatives: A hybrid pharmacophore approach. Eur. J. Med. Chem. 2013, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, N.M.; Patel, H.M.; Kaur, M. Benzothiazoles: Search for anticancer agents. Eur. J. Med. Chem. 2012, 54, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Kamboj, P.; Alam, O.; Amir, M. 1,2,4-Triazole-based benzothiazole/benzoxazole derivatives: Design, synthesis, p38α MAP kinase inhibition, anti-inflammatory activity and molecular docking studies. Bioorg. Chem. 2018, 81, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.; Sawhey, S.N. Antimalarials. III. Benzothiazole amino alcohols. J. Med. Chem. 1968, 11, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Shadab, M.; Khan, S.; Ali, N.; Khan, A.T. One-pot synthesis and evaluation of antileishmanial activities of functionalized S-Alkyl/Aryl benzothiazole-2-carbothioate scaffold. J. Org. Chem. 2016, 81, 3149–3160. [Google Scholar] [CrossRef] [PubMed]
- Gabriel Navarrete-Vázquez, G.; Chávez-Silva, F.; Colín-Lozano, B.; Estrada-Soto, S.; Hidalgo-Figueroa, S.; Guerrero-Álvarez, J.; Méndez, S.T.; Reyes-Vivas, H.; Oria-Hernández, J.; Canul-Canché, J.; et al. Synthesis of nitro(benzo)thiazole acetamides and in vitro antiprotozoal effect against amitochondriate parasites Giardia intestinalis and Trichomonas vaginalis. Bioorg. Med. Chem. 2015, 23, 2204–2210. [Google Scholar] [CrossRef]
- Ge, J.F.; Zhang, Q.Q.; Lu, J.M.; Kaiser, M.; Wittlin, S.; Brunb, R.; Ihara, M. Synthesis of cyanine dyes and investigation of their in vitro antiprotozoal activities. Med. Chem. Commun. 2012, 3, 1435–1442. [Google Scholar] [CrossRef]
- Pudhom, K.; Kasai, K.; Terauchi, H.; Inoue, H.; Kaiser, M.; Brun, R.; Iharaa, M.; Takasu, K. Synthesis of three classes of rhodacyanine dyes and evaluation of their in vitro and in vivo antimalarial activity. Bioorg. Med. Chem. 2006, 14, 8550–8563. [Google Scholar] [CrossRef]
- Hout, S.; Azas, N.; Darque, A.; Robin, M.; Di Giorgio, C.; Gasquet, M.; Galy, J.; Timon-David, P. Activity of benzothiazoles and chemical derivatives on Plasmodium falciparum. Parasitology 2004, 129, 525–542. [Google Scholar] [CrossRef]
- Ferraza, L.R.M.; Alvesa, A.É.G.; Nascimentoa, D.D.S.S.; Amarizb, I.A.E.; Ferreira, A.S.; Costa, S.P.M.; Rolimb, L.A.A.; Limac, Á.A.N.; Rolim Neto, P.J. In vitro activity of steroidal dendrimers on Trypanosoma cruzi epimastigote form with PAMAM dendrons modified by “click” chemistry. Acta Trop. 2018, 185, 127–132. [Google Scholar] [CrossRef]
- World and Health Organization. Neglected Tropical Diseases. Available online: https://www.who.int/en/news-room/fact-sheets/detail/chagas-disease-(american-rypanosomiasis) (accessed on 1 February 2019).
- Papadopoulou, M.V.; Bloomer, W.D.; Rosenzweig, H.S.; Kaiser, M.; Eric Chatelain, E.; Ioset, J.-R. Novel 3-nitro-1H-1,2,4-triazole-based piperazines and 2-amino-1,3-benzothiazoles as antichagasic agents. Bioorg. Med. Chem. 2013, 21, 6600–6607. [Google Scholar] [CrossRef]
- Schmunis, G.A.; Yadon, Z.E. Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop. 2010, 115, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Chiguer, D.L.; Márquez-Navarroa, A.; Nogueda-Torres, B.; León-Ávila, G.L.; Pérez-Villanueva, J.; Hernández-Campos, A.; Castillo, R.; Ambrosio, J.R.; Nieto-Menesesa, R.; Yépez-Mulia, L.; et al. In vitro and in vivo trypanocidal activity of some benzimidazole derivatives against two strains of Trypanosoma cruzi. Acta Trop. 2012, 122, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Bern, C.N. Chagas’ Disease. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Ballari, M.S.; Cano, N.H.; Lopez, A.G.; Wunderlin, D.A.; Feresín, G.E.; Santiago, A.N. Green synthesis of potential antifungal agents: 2-benzyl substituted thiobenzoazoles. J. Agric. Food Chem. 2017, 65, 10325–10331. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.; Zhang, H.; Jiang, Y.; Ma, D. Synthesis of 2-thio-substituted benzothiazoles via a domino condensation/S-arylation/heterocyclization process. J. Org. Chem. 2011, 76, 4200–4204. [Google Scholar] [CrossRef]
- Chu, X.-Q.; Jiang, R.; Fang, Y.; Gu, Z.-Y.; Meng, H.; Wang, S.-Y.; Ji, S.-J. Acidic-functionalized ionic liquid as an efficient, green, and metal-free catalyst for benzylation of sulfur, nitrogen, and carbon nucleophiles to benzylic alcohols. Tetrahedron 2013, 69, 1166–1174. [Google Scholar] [CrossRef]
- Klimesova, V.; Koci, J.; Palat, K.; Stolarikova, J.; Dahse, H.-M.; Mollmann, U. Structure-activity relationships of 2-benzylsulfanylbenzothiazoles: Synthesis and selective antimycobacterial properties. Med. Chem. 2012, 8, 281–291. [Google Scholar] [CrossRef]
- Brenière, S.F.; Waleckx, E.; Barnabé, C. Over Six thousand Trypanosoma cruzi strains classified into Discrete Typing Units (DTUs): Attempt at an inventory. PLoS Negl. Trop. Dis. 2016, 10, e0004792. [Google Scholar]
- Dos-Santos, V.A.F.F.; Leite, K.M.; da Costa-Siqueira, M.; Regasini, L.O.; Martinez, I.; Nogueira, C.T.; Kolos Galuppo, M.; Stolf, B.S.; Soares-Pereira, A.M.; Cicarelli, R.M.B.; et al. Antiprotozoal activity of quinonemethide triterpenes from Maytenus ilicifolia (Celastraceae). Molecules 2013, 18, 1053–1062. [Google Scholar] [CrossRef]
- Chacón-Vargas, K.F.; Nogueda-Torres, B.; Sánchez-Torres, L.E.; Suarez-Contreras, E.; Villalobos-Rocha, J.C.; Torres-Martinez, Y.; Lara-Ramirez, E.E.; Fiorani, G.; Krauth-Siegel, R.L.; Bolognesi, M.L.; et al. Trypanocidal activity of quinoxaline 1,4 di-N-oxide derivatives as trypanothione reductase inhibitors. Molecules 2017, 22, 220–238. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
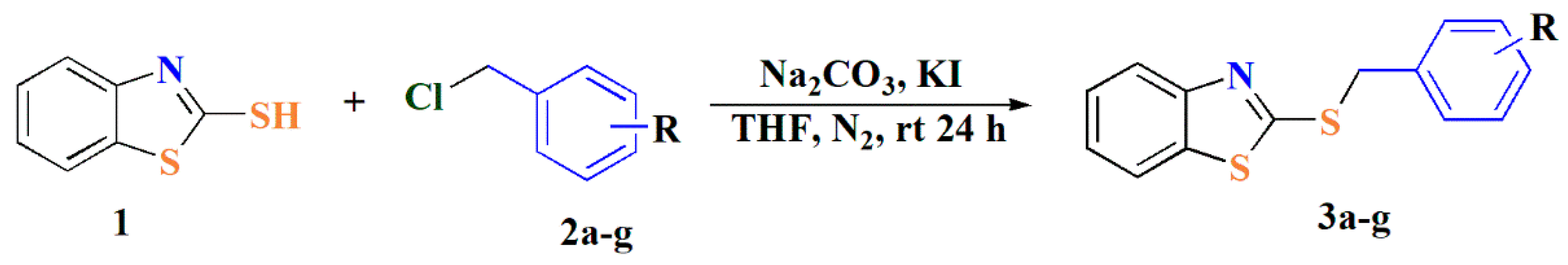
| Entry | Benzyl chloride | Compound | Yield a (%) |
|---|---|---|---|
| 1 | 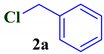 | 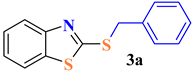 | 75 |
| 2 | 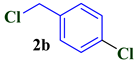 | 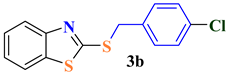 | 80 |
| 3 | 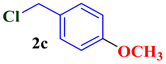 | 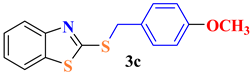 | 80 |
| 4 | 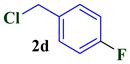 | 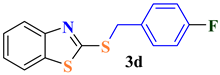 | 73 |
| 5 | 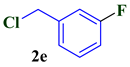 | 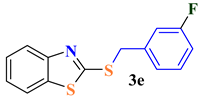 | 83 |
| 6 | 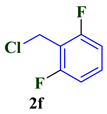 | 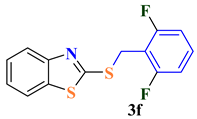 | 77 |
| 7 | 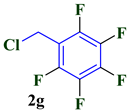 | 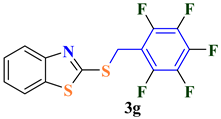 | 87 |
| Compound | Bloodstream Trypomastigotes LC50 (µM) | Macrophages J774A.1 CC50 (µM) | SI (CC50 /LC50) | ||
|---|---|---|---|---|---|
| T. cruzi NINOA | T. cruzi INC-5 | T. cruzi NINOA | T. cruzi INC-5 | ||
| 3a | 123.44 ± 21.86 | 335.59 ±35.7 | >900 | >7.29 | >2.68 |
| 3b | >350 | 262.62 ± 12.33 | >900 | Nd | >3.42 |
| 3c | 307.82 ± 28.88 | 275.67 ± 22.96 | >900 | >2.92 | >3.26 |
| 3d | >350 | >350 | 869.91 ± 26.8 | Nd | Nd |
| 3e | >350 | >350 | 784.82 ± 25.45 | Nd | Nd |
| 3f | 109.76 ± 23.18 | 259.81 ± 25.56 | 491.88 ± 10.09 | 4.48 | 1.91 |
| 3g | 146.95 ± 21.01 | 185.35 ± 12.95 | 249.56 ± 9.44 | 1.69 | 1.34 |
| Nfx | 96.26 ± 11.48 | 127.63 ±14.97 | 347.32 ± 18.26 | 3.61 | 2.72 |
| Bnz | 173.46 ± 15.89 | 216.57 ± 23.08 | 223.43 ± 11.23 | 1.28 | 1.05 |
| Nd: Not determined. | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Sorrosa, A.; Tapia-Alvarado, J.D.; Nogueda-Torres, B.; Chacón-Vargas, K.F.; Díaz-Cedillo, F.; Vargas-Díaz, M.E.; Morales-Morales, D. Facile Synthesis of a Series of Non-Symmetric Thioethers Including a Benzothiazole Moiety and Their Use as Efficient In Vitro anti-Trypanosoma cruzi Agents. Molecules 2019, 24, 3077. https://doi.org/10.3390/molecules24173077
Avila-Sorrosa A, Tapia-Alvarado JD, Nogueda-Torres B, Chacón-Vargas KF, Díaz-Cedillo F, Vargas-Díaz ME, Morales-Morales D. Facile Synthesis of a Series of Non-Symmetric Thioethers Including a Benzothiazole Moiety and Their Use as Efficient In Vitro anti-Trypanosoma cruzi Agents. Molecules. 2019; 24(17):3077. https://doi.org/10.3390/molecules24173077
Chicago/Turabian StyleAvila-Sorrosa, Alcives, Jazz D. Tapia-Alvarado, Benjamín Nogueda-Torres, Karla Fabiola Chacón-Vargas, Francisco Díaz-Cedillo, María Elena Vargas-Díaz, and David Morales-Morales. 2019. "Facile Synthesis of a Series of Non-Symmetric Thioethers Including a Benzothiazole Moiety and Their Use as Efficient In Vitro anti-Trypanosoma cruzi Agents" Molecules 24, no. 17: 3077. https://doi.org/10.3390/molecules24173077
APA StyleAvila-Sorrosa, A., Tapia-Alvarado, J. D., Nogueda-Torres, B., Chacón-Vargas, K. F., Díaz-Cedillo, F., Vargas-Díaz, M. E., & Morales-Morales, D. (2019). Facile Synthesis of a Series of Non-Symmetric Thioethers Including a Benzothiazole Moiety and Their Use as Efficient In Vitro anti-Trypanosoma cruzi Agents. Molecules, 24(17), 3077. https://doi.org/10.3390/molecules24173077