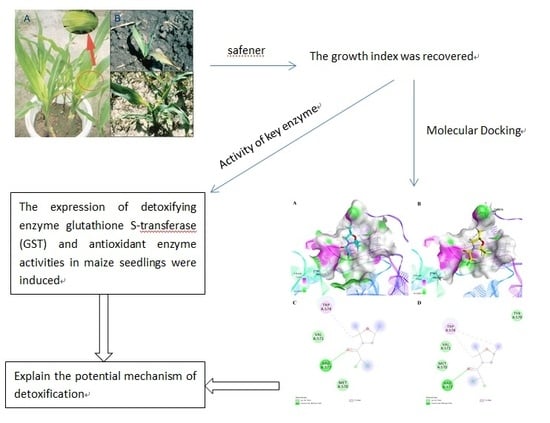
Protective Responses Induced by Chiral 3-Dichloroacetyl Oxazolidine Safeners in Maize (Zea mays L.) and the Detoxification Mechanism
Abstract
1. Introduction
2. Results
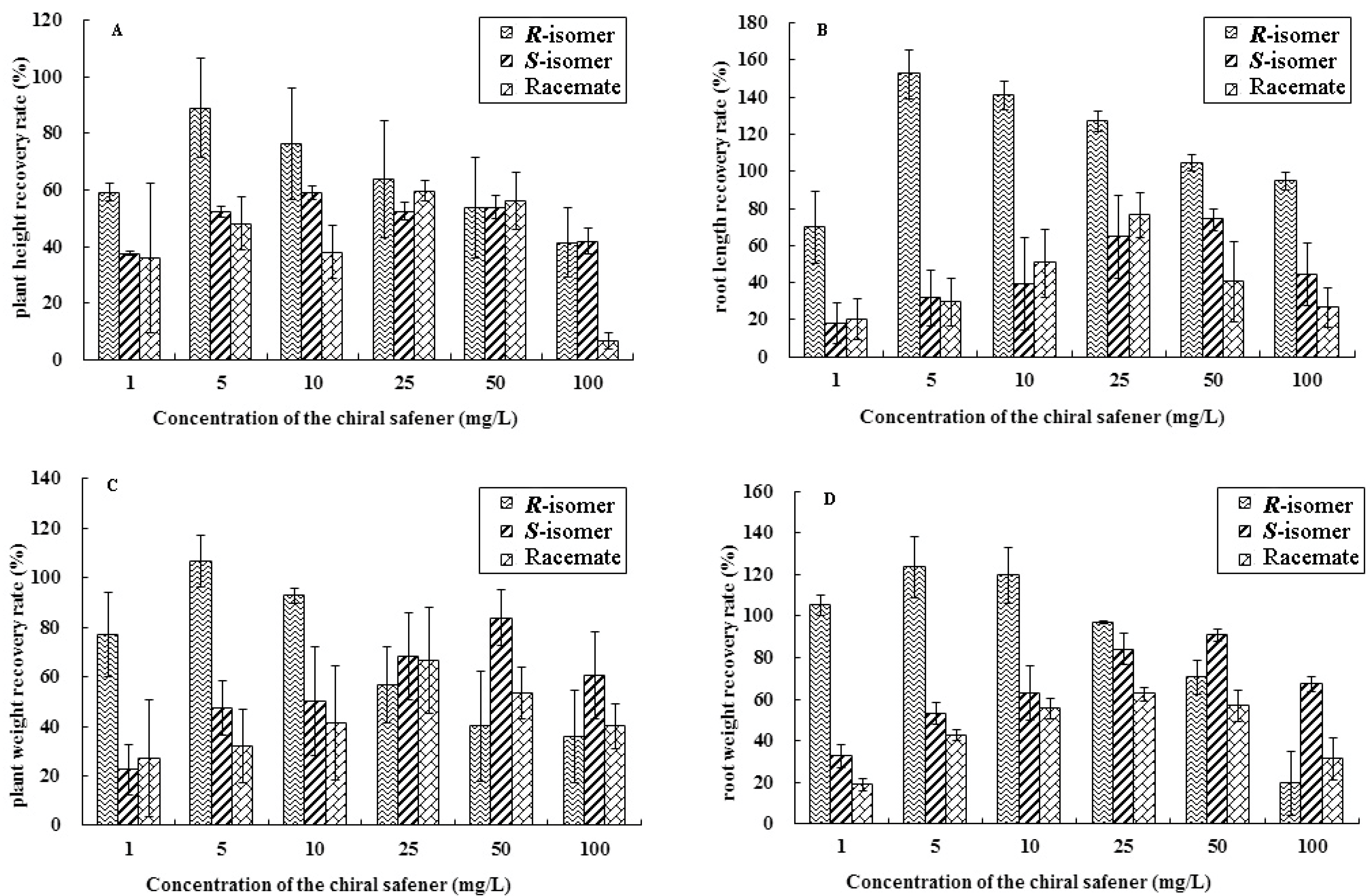
2.1. Results of Determination of the Growth Index
2.2. GSH and GST Results
2.2.1. GSH Content
2.2.2. GST Activity
2.2.3. Kinetic Parameters of GST
2.3. POD and CAT Activity
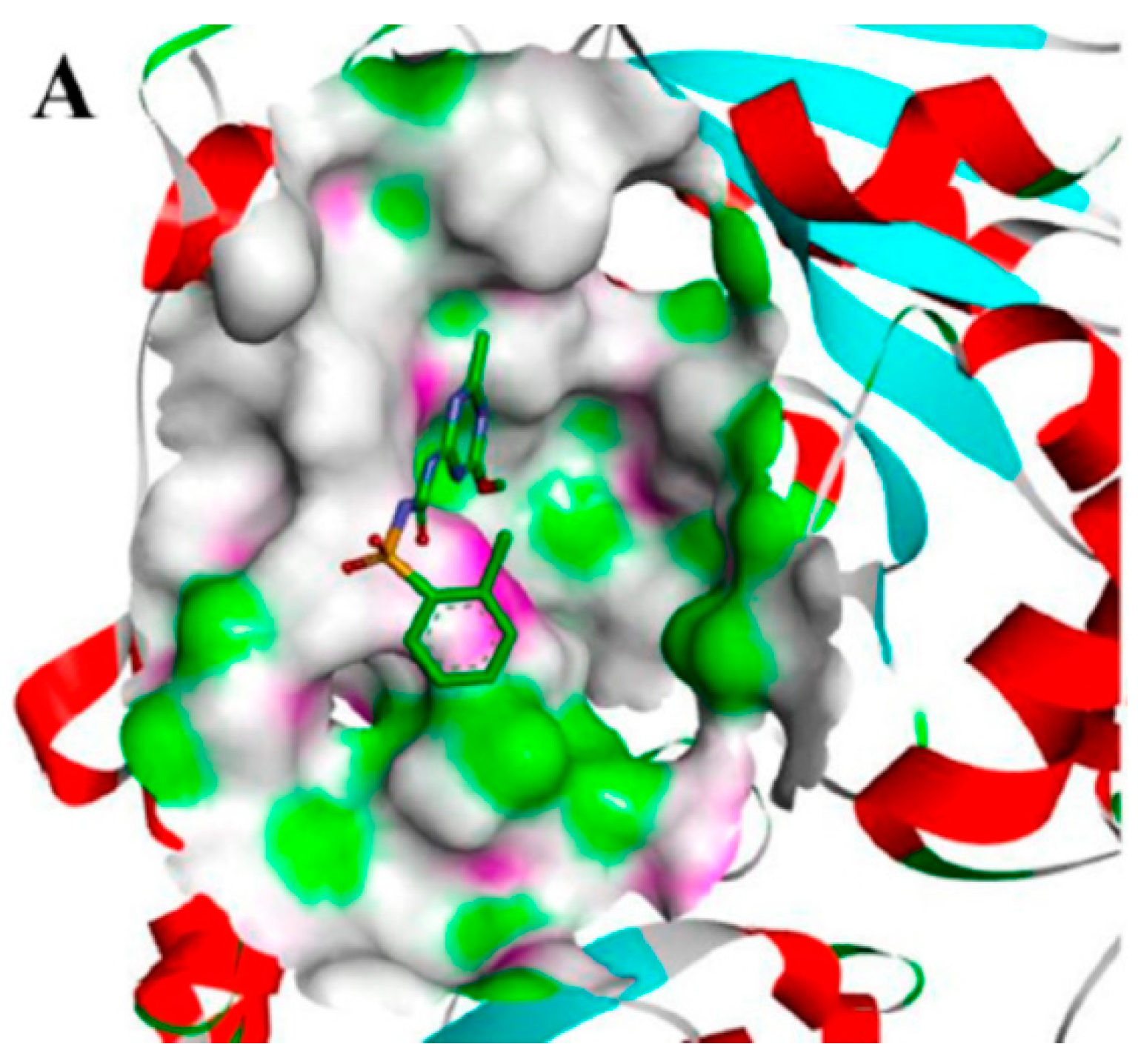
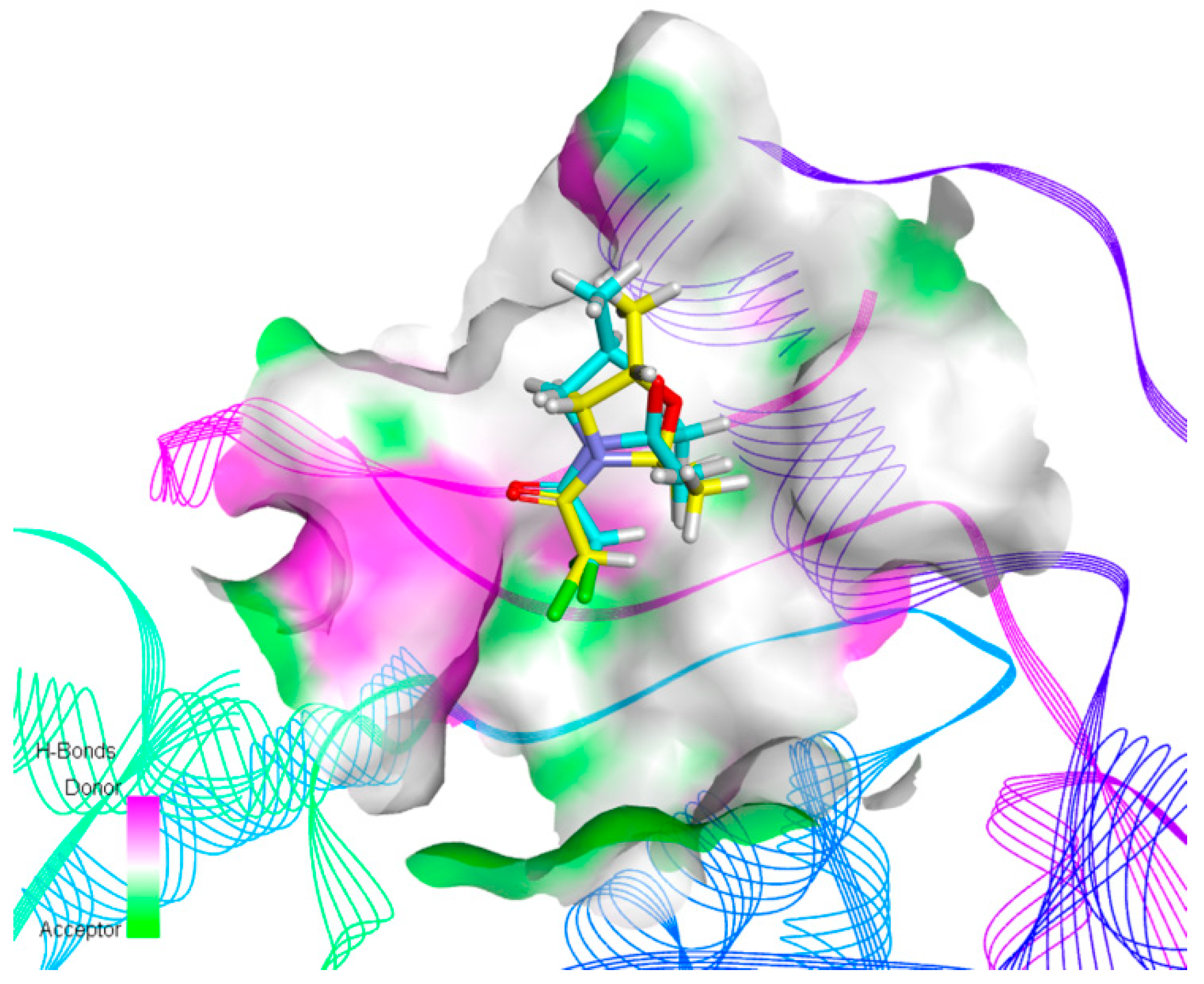
2.4. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Materials and Chemical Reagents
4.2. Plant Material and Growth Conditions
4.3. GSH Content Assay
4.4. Extraction and Activity Determination of GST
4.5. GST Kinetic Parameters Assay (CDNB)
4.6. CAT Activity Assay
4.7. POD Enzyme Assay
4.8. Statistical Analysis
4.9. Molecular Docking Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- VanGessel, M.J.; Johnson, Q.R.; Scott, B.A. Evaluating postemergence herbicides, safener, and tolerant hybrids for corn response. Weed Technol. 2016, 30, 869–877. [Google Scholar] [CrossRef]
- Damalas, C.A.; Gitsopoulos, T.K.; Koutroubas, S.D.; Alexoudis, C.; Georgoulas, I. Weed control and selectivity in maize (Zea mays L.) with tembotrione mixtures. Int. J. Pest Manag. 2018, 64, 11–18. [Google Scholar] [CrossRef]
- Hollaway, K.L.; Kookana, R.S.; Noy, D.M.; Smith, J.G.; Wilhelm, N. Crop damage caused by residual acetolactate synthase herbicides in the soils of south-eastern Australia. Aust. J. Exp. Agric. 2006, 46, 1323–1331. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Ji, Q.; Fu, Y.; Zhao, L.; Li, C.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Fu, Y.; Kang, J.X.; Wang, Y.K.; Liu, J.; Zhao, L.X.; Gao, S.; Ye, F. Design, synthesis and biological activity of novel sulfonylurea oxazolidines. Heterocycles 2016, 92, 740–750. [Google Scholar]
- Elmore, M.T.; Brosnan, J.T.; Armel, G.R.; Vargas, J.J.; Breeden, G.K. Herbicide safeners increase creeping bentgrass (Agrostis stolonifera) tolerance to pinoxaden and affect weed control. Weed Technol. 2016, 30, 919–928. [Google Scholar] [CrossRef]
- Scarponi, L.; Quagliarini, E.; Del Buono, D. Induction of wheat and maize glutathione S-transferase by some herbicide safeners and their effect on enzyme activity against butachlor and terbuthylazine. Pest Manag. Sci. 2006, 62, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Behringer, C.; Bartsch, K.; Schaller, A. Safeners recruit multiple signalling pathways for the orchestrated induction of the cellular xenobiotic detoxification machinery in Arabidopsis. Plant Cell Environ. 2011, 34, 1970–1985. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Bie, C.; Chen, X.S.; Zhao, L.X.; Fu, Y.; Li, C.Y.; Ye, F. Inducing Glutathione S-Transferase Expression and Activity using an Herbicide Safener against Metolachlor in Maize. Int. J. Agric. Biol. 2017, 19, 1357–1362. [Google Scholar]
- Ye, F.; Cao, H.F.; Chen, X.S.; Zhang, M.; Fu, Y.; Li, C.Y.; Gao, S. Effects of chiral 3-Dichloroacetyl Oxazolidine on glutathione S-Transferase and antioxidant enzymes activity in maize treated with Acetochlor. J. Agric. Sci. 2018, 24, 422–429. [Google Scholar] [CrossRef]
- Ye, F.; Zhai, Y.; Kang, T.; Wu, S.L.; Li, J.J.; Gao, S.; Zhao, L.X.; Fu, Y. Rational design, synthesis and structure-activity relationship of novel substituted oxazole isoxazole carboxamides as herbicide safener. Pest. Biochem. Physiol. 2019, 157, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Lu, Y.C.; Zhang, J.J.; Tan, L.R.; Yang, H. Accumulation and toxicological response of atrazine in rice crops. Ecotox. Environ. Saf. 2014, 102, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Cao, H.F.; Wei, L.N.; Wang, C.; Fu, Y.; Li, C.Y.; Gao, S. Chiral 3-dichloroacetyl Oxazolidines induced the activity of enzymes in maize for the detoxified herbicide. Int. J. Agric. Biol. 2018, 20, 1027–1032. [Google Scholar]
- Basantani, M.; Srivastava, A.; Sen, S. Elevated antioxidant response and induction of tau-class glutathione S-transferase after glyphosate treatment in Vigna radiata (L.) Wilczek. Pest. Biochem. Physiol. 2011, 99, 111–117. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Gar’kova, A.N.; Bochkarjova, A.S.; Nushtaeva, O.V.; da Silva, J.A.T. Treatment with the herbicide TOPIK induces oxidative stress in cereal leaves. Pest. Biochem. Physiol. 2013, 105, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Ashraf, M.; Al-Qurainy, F. Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus L.) plants under saline regimes. Sci. Hortic. 2012, 142, 143–148. [Google Scholar] [CrossRef]
- Li, L.H.; Yi, H.L. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Raiesi, F. Mycorrhizal fungi and earthworms reduce antioxidant enzyme activities in maize and sunflower plants grown in Cd-polluted soils. Soil Biol. Biochem. 2015, 86, 87–97. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, K.; Wang, P.; Kang, J.X.; Gao, S.; Zhao, L.X.; Ye, F. Design, synthesis, and herbicidal activity evaluation of novel aryl-naphthyl methanone derivatives. Front. Chem. 2019, 7, 2. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, S.-Q.; Liu, Y.-X.; Wang, J.-Y.; Gao, S.; Zhao, L.-X.; Ye, F. Design, synthesis, SAR and molecular docking of novel green niacin-triketone HPPD inhibitor. Ind. Crop. Prod. 2019, 137, 566–575. [Google Scholar] [CrossRef]
- Sartori, S.K.; Alvarenga, E.S.; Franco, C.A.; Ramos, D.S.; Oliveira, D.F. One-pot synthesis of anilides, herbicidal activity and molecular docking study. Pest Manag. Sci. 2018, 74, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Z.; Yang, Z.H.; Deng, X.L.; Lin, D.; Wu, M.F.; Li, J.M.; Chen, A.Y.; Ye, J.; Hu, A.X.; Liao, H.D. Novel aryloxyphenoxypropionate derivates containing benzofuran moiety: Design, synthesis, herbicidal activity, docking study and theoretical calculation. Pest. Biochem. Physiol. 2019, 154, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Ma, P.; Zhang, Y.Y.; Li, P.; Yang, F.; Fu, Y. Herbicidal activity and molecular docking study of novel ACCase inhibitors. Front. Plant Sci. 2018, 9, 1850. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Caseley, J.C. Herbicide safeners: A review. Pestic. Sci. 1999, 55, 1043–1058. [Google Scholar] [CrossRef]
- Kang, Y.F.; Liu, L.; Wang, R.; Zhou, Y.F.; Yan, W.J. Enantioselective alkynylation of aromatic ketones catalyzed by new chiral oxazolidine ligands. Adv. Synth. Catal. 2005, 347, 243–247. [Google Scholar] [CrossRef]
- Sriharsha, S.N.; Shashikanth, S. Synthesis and antimicrobial activity of novel 1,3-oxazolidine nucleoside analogues. Heterocycl. Commun. 2006, 12, 213–218. [Google Scholar] [CrossRef]
- Gao, S.; Fu, Y.; Zhao, L.-X.; Xing, Z.-Y.; Ye, F. Synthesis and crystal structure of novel chiral N-Dichloroacetyl-2-substituted-5-methyl-1,3-oxazolidines. Heterocycles 2012, 85, 903. [Google Scholar]
- Taylor, V.L.; Cummins, I.; Brazier-Hicks, M.; Edwards, R. Protective responses induced by herbicide safeners in wheat. Environ. Exp. Bot. 2013, 88, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jablonkai, I. Herbicide safeners: Effective tools to improve herbicide selectivity. In Herbicides-Current Research and Case Studies in Use; Kelton, J., Price, A., Eds.; Agricultural Research Service: Beltsville, MD, USA, 2013. [Google Scholar]
- Obermeier, M.; Schroder, C.A.; Helmreich, B.; Schroder, P. The enzymatic and antioxidative stress response of Lemna minor to copper and a chloroacetamide herbicide. Environ. Sci. Pollut. Res. 2015, 22, 18495–18507. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, D.; Ioli, G. Glutathione S-Transferases of Italian Ryegrass (Lolium multiflorum): Activity toward some chemicals, safener modulation and persistence of atrazine and fluorodifen in the shoots. J. Agric. Food Chem. 2011, 59, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Pascal, S.; Scalla, R. Purification and characterization of a safener-induced glutathione S-transferase from wheat (Triticum aestivum). Physiol. Plant. 1999, 106, 17–27. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xu, J.Y.; Lu, F.F.; Jin, S.F.; Yang, H. Detoxification of atrazine by low molecular weight thiols in alfalfa (Medicago sativa). Chem. Res. Toxicol. 2017, 30, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Xu, W. Advances in the studies on physiological mechanism of safener. Acta Phytophylacica Sinica 2008, 35, 367–372. [Google Scholar]
- Ye, F.; Cao, H.F.; Fu, Y.; Zhao, L.X.; Gao, S. The safener effect of chiral derivatives of 3-dichloroacetyl oxazolidine against haloxyfop-P-methyl-induced toxicity in maize. Zemdirbyste 2016, 103, 29–34. [Google Scholar] [CrossRef][Green Version]
- Habib, K.; Kumar, S.; Manikar, N.; Zutshi, S.; Fatma, T. Biochemical effect of Carbaryl on oxidative stress, antioxidant enzymes and osmolytes of cyanobacterium calothrix brevissima. Bull. Environ. Contam. Toxicol. 2011, 87, 615–620. [Google Scholar] [CrossRef] [PubMed]
- He, H.Z.; Li, Y.J.; Chen, T.F.; Huang, X.L.; Guo, Q.; Li, S.F.; Yu, T.H.; Li, H.S. Butachlor induces some physiological and biochemical changes in a rice field biofertilizer cyanobacterium. Pest. Biochem. Physiol. 2013, 105, 224–230. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wang, Y.K.; Zhou, J.H.; Xie, F.; Guo, Q.N.; Lu, F.F.; Jin, S.F.; Zhu, H.M.; Yang, H. Reduced phytotoxicity of propazine on wheat, maize and rapeseed by salicylic acid. Ecotox. Environ. Saf. 2018, 162, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, Y.; Jia, L.X.; Lin, J.L.; Liu, Y.; Pan, B.; Lin, Y. Biological responses of wheat (Triticum aestivum) plants to the herbicide simetryne in soils. Ecotox. Environ. Saf. 2016, 127, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Sytykiewicz, H. Transcriptional responses of catalase genes in maize seedlings exposed to cereal aphids’ herbivory. Biochem. Syst. Ecol. 2015, 60, 131–142. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, D.; Kang, T.; Guo, Y.Y.; Chen, W.G.; Gao, S.; Ye, F. Fragment splicing-based design, synthesis and safener activity of novel substituted phenyl oxazole derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 570–576. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Papenbrock, J. The effects of patulin from Penicillium vulpinum on seedling growth, root tip ultrastructure and glutathione content of maize. Eur. J. Plant Pathol. 2014, 139, 497–509. [Google Scholar] [CrossRef]
- Del Buono, D.; Michell, M.; Scarponi, L.; Standardi, A. Activity of glutathione S-transferase toward some herbicides and its regulation by benoxacor in non-embryogenic callus and in vitro regenerated tissues of Zea mays. Pest. Biochem. Physiol. 2006, 85, 61–67. [Google Scholar] [CrossRef]
- Hemanth Kumar, N.K.; Meena, S.K.; Meena, V.S.; Shobha, J. Oxidative stress and antioxidant metabolic enzymes response of maize (Zea Mays L.) seedlings to a biotic stress (Alachlor) condition. Int. J. Agric. Sci. 2016, 8, 2227–2231. [Google Scholar]
- Fu, Y.; Liu, Y.X.; Yi, K.H.; Li, M.Q.; Li, J.Z.; Ye, F. Quantitative Structure Activity Relationship Studies and Molecular Dynamics Simulations of 2-(Aryloxyacetyl)cyclohexane-1,3-Dones Derivatives as 4-Hydroxyphenylpyruvate Dioxygenase Inhibitors. Front. Chem. 2019, 7, 556. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3-dichloroacetyl substituted oxazolidines are available from the authors. |

| Treatment | GSH Content in Roots (μg·g−1) | GSH Content in Shoots (μg·g−1) |
|---|---|---|
| Control | 3.775 ± 0.036 d | 7.841 ± 0.024 d |
| Chlorsulfuron | 4.862 ± 0.028 c | 15.164 ± 0.045 c |
| R-isomer + Chlorsulfuron | 7.068± 0.025 a | 26.662 ± 0.072 a |
| S-isomer + Chlorsulfuron | 6.339 ± 0.044 b | 21.767 ± 0.058 b |
| R-29148 + Chlorsulfuron | 6.155 ± 0.053 b | 20.053 ± 0.032 b |
| Treatment | GST Activity In Vivo (μmol·min−1·mg−1 Protein) | Treatment | GST Activity In Vitro (nmol·min−1·mg−1 Protein) |
|---|---|---|---|
| Control | 8.97 ± 0.30 d | Control | 123.73 ± 2.95 d |
| Chlorsulfuron | 10.43 ± 0.16 c | Chlorsulfuron | - |
| R-isomer + Chlorsulfuron | 19.44 ± 0.28 a | R-isomer | 195.67 ± 3.15 a |
| S-isomer + Chlorsulfuron | 12.19 ± 0.67 b | S-isomer | 162.43 ± 1.05 b |
| R-29148 + Chlorsulfuron | 11.84 ± 0.35 b | R-29148 | 139.39 ± 1.88 c |
| Treatment | Vmax (nmol·min−1·mg−1 Protein) | Km (mmol·L−1) |
|---|---|---|
| Control | 14.87 ± 0.30 c | 0.52 ± 0.04 a,b |
| Chlorsulfuron | 5.42 ± 0.44 d | 0.55 ± 0.15 a |
| R-isomer | 23.82 ± 0.23 a | 0.39 ± 0.05 c |
| S-isomer | 12.73 ± 0.67 d | 0.50 ± 0.12 a,b |
| R-29148 | 17.54 ± 0.35 b | 0.48 ± 0.06 b |
| Treatment | CAT Activity (μmol·min−1·g−1 FW) | POD Activity (mmol·min−1·g−1 FW) |
|---|---|---|
| Control | 2.09 ± 0.12 a | 1135 ± 2.05 b |
| Chlorsulfuron | 1.60 ± 0.06 b | 1387 ± 1.06 a |
| R-isomer + Chlorsulfuron | 1.04 ± 0.03 d | 863 ± 3.63 d |
| S-isomer + Chlorsulfuron | 1.20 ± 0.08 c | 1381 ± 2.35 a |
| R-29148 + Chlorsulfuron | 1.32 ± 0.10 c | 1047 ± 3.24 c |
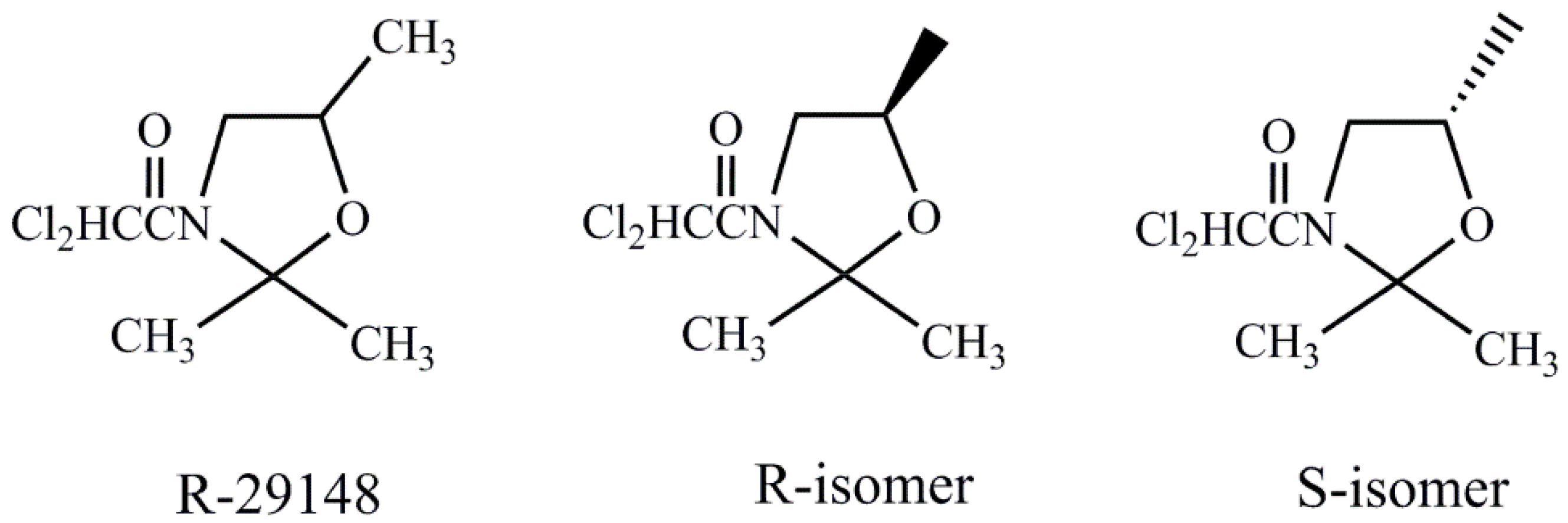
| Safener | Chemical Name |
|---|---|
| R-29148 | (R,S)-3-dichloroacetyl-2,2,5-trimethyl-1,3-oxazolidine |
| R-isomer | (R)- 3-dichloroacetyl-2,2,5-trimethyl-1,3-oxazolidine |
| S-isomer | (S)- 3-dichloroacetyl-2,2,5-trimethyl-1,3-oxazolidine |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Liu, Y.-Y.; Jiang, J.-Y.; Fu, Y.; Zhao, L.-X.; Li, C.-Y.; Ye, F. Protective Responses Induced by Chiral 3-Dichloroacetyl Oxazolidine Safeners in Maize (Zea mays L.) and the Detoxification Mechanism. Molecules 2019, 24, 3060. https://doi.org/10.3390/molecules24173060
Gao S, Liu Y-Y, Jiang J-Y, Fu Y, Zhao L-X, Li C-Y, Ye F. Protective Responses Induced by Chiral 3-Dichloroacetyl Oxazolidine Safeners in Maize (Zea mays L.) and the Detoxification Mechanism. Molecules. 2019; 24(17):3060. https://doi.org/10.3390/molecules24173060
Chicago/Turabian StyleGao, Shuang, Yan-Yan Liu, Jing-Yu Jiang, Ying Fu, Li-Xia Zhao, Chun-Yan Li, and Fei Ye. 2019. "Protective Responses Induced by Chiral 3-Dichloroacetyl Oxazolidine Safeners in Maize (Zea mays L.) and the Detoxification Mechanism" Molecules 24, no. 17: 3060. https://doi.org/10.3390/molecules24173060
APA StyleGao, S., Liu, Y.-Y., Jiang, J.-Y., Fu, Y., Zhao, L.-X., Li, C.-Y., & Ye, F. (2019). Protective Responses Induced by Chiral 3-Dichloroacetyl Oxazolidine Safeners in Maize (Zea mays L.) and the Detoxification Mechanism. Molecules, 24(17), 3060. https://doi.org/10.3390/molecules24173060