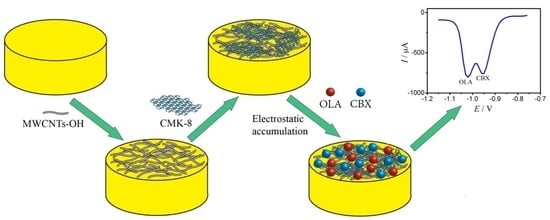
Novel Electrochemical Sensor Fabricated for Individual and Simultaneous Ultrasensitive Determination of Olaquindox and Carbadox Based on MWCNT-OH/CMK-8 Hybrid Nanocomposite Film
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of Materials
2.2. Electrochemical Characteristics
2.3. Electrochemical Impedance Spectroscopy
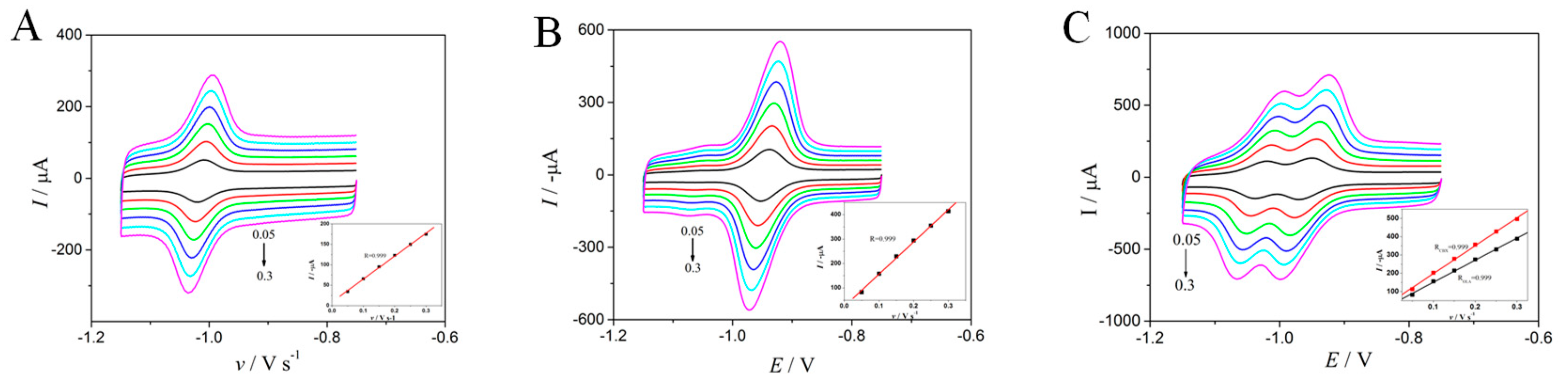
2.4. Effect of Scan Rate
2.5. Optimization of Experimental Factors
2.5.1. Influence of Modified Coating Amount
2.5.2. Influence of Electrolyte Concentration
2.5.3. Influence of Enrichment Potential
2.5.4. Influence of Enrichment Time
2.5.5. Influence of Stirring Speed
2.5.6. Influence of pH Measurement
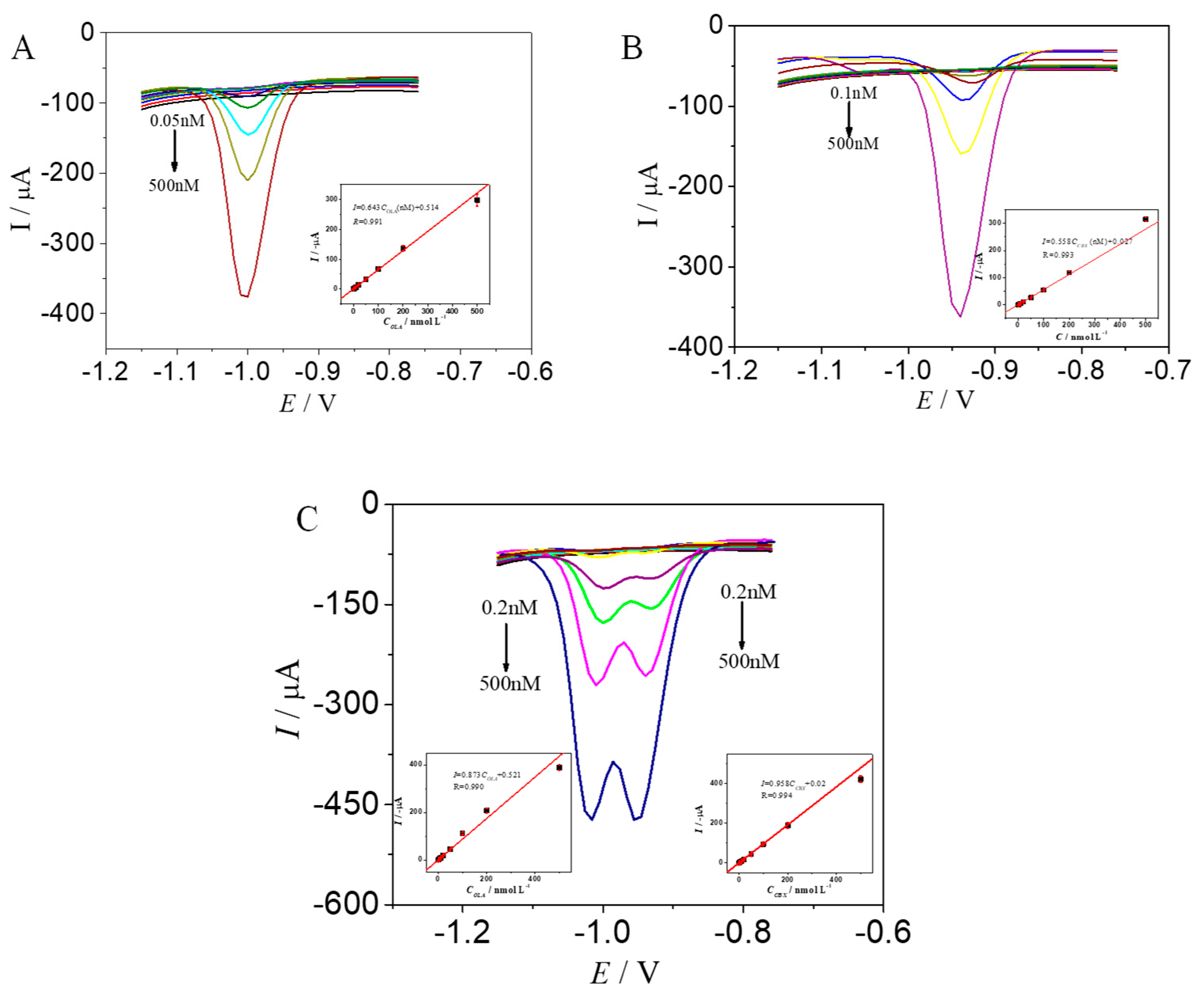
2.6. Determination of OLA and CBX
2.7. Repeatability, Reproducibility, and Stability of MWCNTs−OH/CMK−8/GE
2.8. Interference Study
2.9. Analytical Application
3. Materials and Methods
3.1. Chemicals
3.2. Instrumentation
3.3. Synthesis of CMK−8
3.4. Preparation of the Modified GE
3.5. Electrochemical Test Method
3.6. Sample Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, G.; Li, B.; Wang, C.; Zhang, H.; Liang, G.; Weng, Z.; Hao, H.; Wang, X.; Liu, Z.; Dai, M.; et al. Systematic and molecular basis of the antibacterial action of quinoxaline 1,4-di-N-oxides against Escherichia coli. PLoS ONE 2015, 10, e0136450. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.; Luo, X.; Ihsan, A.; Liu, X.; Dai, M.; Cheng, G.; Hao, H.; Wang, X.; Yuan, Z. Further investigations into the genotoxicity of quinoxaline-di-N-oxides and their primary metabolites. Food Chem. Toxicol. 2016, 93, 145–157. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Wang, Y.; Ihsan, A.; Dai, M.; Huang, L.; Chen, D.; Tao, Y.; Peng, D.; Liu, Z.; et al. Two generation reproduction and teratogenicity studies of feeding quinocetone fed to Wistar rats. Food Chem. Toxicol. 2012, 50, 1600–1609. [Google Scholar] [CrossRef]
- Ihsan, A.; Wang, X.; Zhang, W.; Tu, H.; Wang, Y.; Huang, L.; Iqbal, Z.; Cheng, G.; Pan, Y.; Liu, Z.; et al. Genotoxicity of quinocetone, cyadox and olaquindox in vitro and in vivo. Food Chem. Toxicol. 2013, 59, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Boison, J.O.; Lee, S.C.; Gedir, R.G. A determinative and confirmatory method for residues of the metabolites of carbadox and olaquindox in porcine tissues. Anal. Chim. Acta 2009, 637, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tang, S.; Jin, X.; Zou, J.; Chen, K.; Zhang, T.; Xiao, X. Investigation of the genotoxicity of quinocetone, carbadox and olaquindox in vitro using Vero cells. Food Chem. Toxicol. 2009, 47, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Huang, L.; Tao, Y.; Yuan, Z.; Chen, D. Simultaneous determination of five quinoxaline-1,4-dioxides in animal feeds using ultrasonic solvent extraction and high-performance liquid chromatography. Anal. Chim. Acta 2006, 569, 97–102. [Google Scholar] [CrossRef]
- Hutchinson, M.J.; Young, P.B.; Kennedy, D.G. Confirmation of carbadox and olaquindox metabolites in porcine liver using liquid chromatography–electrospray, tandem mass spectrometry. J. Chromatogr. B 2005, 816, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Zhu, L.; Yu, H. Dual-label quantum dot-based immunoassay for simultaneous determination of Carbadox and Olaquindox metabolites in animal tissues. Food Chem. 2016, 199, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Q.; Zhi, A.; Yang, J.; Zhi, Y.; Li, Q.; Hu, X.; Deng, R.; Casas, J.; Tang, L.; et al. Development of a lateral flow colloidal gold immunoassay strip for the rapid detection of olaquindox residues. J. Agri. Food Chem. 2011, 59, 9319–9326. [Google Scholar] [CrossRef]
- European Communities. Commission Regulation 2788/98, Official Journal L347. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31998R2788 (accessed on 22 December 1998).
- Sin, D.W.M.; Chung, L.P.K.; Lai, M.M.C.; Siu, S.M.P.; Tang, H.P.O. Determination of quinoxaline-2-carboxylic acid, the major metabolite of carbadox, in porcine liver by isotope dilution gas chromatography–electron capture negative ionization mass spectrometry. Anal. Chim. Acta 2004, 508, 147–158. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, H.; Wang, Y.; Huang, L.; Tao, Y.; Chen, D.; Peng, D.; Liu, Z.; Yuan, Z. Development of a high-performance liquid chromatography method for the simultaneous quantification of quinoxaline-2-carboxylic acid and methyl-3-quinoxaline-2-carboxylic acid in animal tissues. J. Chromatogr. A 2007, 1146, 1–7. [Google Scholar] [CrossRef]
- Kesiunaite, G.; Naujalis, E.; Padarauskas, A. Matrix solid-phase dispersion extraction of carbadox and olaquindox in feed followed by hydrophilic interaction ultra-high-pressure liquid chromatographic analysis. J. Chromatogr. A 2008, 1209, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, X.; Wang, Y.; Pan, Y.; Liu, Z.; Chen, D.; Sheng, F.; Yuan, Z. An immunoaffinity column for the selective purification of 3-methyl-quinoxaline-2-carboxylic acid from swine tissues and its determination by high-performance liquid chromatography with ultraviolet detection and a colloidal gold-based immunochromatographic assay. Food Chem. 2017, 237, 290–296. [Google Scholar] [PubMed]
- Zhao, Y.; Yue, T.; Tao, T.; Wang, X.; Huang, L.; Xie, S.; Pan, Y.; Peng, D.; Chen, D.; Yuan, Z. Simultaneous Determination of Quinoxalines in Animal Feeds by a Modified QuEChERS Method with MWCNTs as the Sorbent Followed by High-Performance Liquid Chromatography. Food Anal. Methods 2016, 10, 2085–2091. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Beier, R.C.; Cao, X.; Shen, J.; Zhang, S. Simultaneous determination of mequindox, quinocetone, and their major metabolites in chicken and pork by UPLC-MS/MS. Food Chem. 2014, 160, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Zhang, J.; Yan, Z.; Zhang, S.; Chen, S.; Fang, Y. A sensitive and selective immunoaffinity column clean up coupled to UPLC-MS/MS for determination of trace methyl-3-quinoxaline-2-carboxylic acid in animal tissues. J. Chromatogr. B 2018, 1074–1075, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Souza Dibai, W.L.; de Alkimin Filho, J.F.; da Silva Oliveira, F.A.; Sampaio de Assis, D.C.; Camargos Lara, L.J.; de Figueiredo, T.C.; de Vasconcelos Cancado, S. HPLC-MS/MS method validation for the detection of carbadox and olaquindox in poultry and swine feedingstuffs. Talanta 2015, 144, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, B.; Zhang, H.; Chen, X.; Mei, G. Determination of marker residue of Olaquindox in fish tissue by ultra performance liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2011, 34, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shen, J.; Wang, Z.; Jiang, W.; Zhang, S. A sensitive and specific ELISA for determining a residue marker of three quinoxaline antibiotics in swine liver. Anal. Bioanal. Chem. 2013, 405, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Beier, R.C.; Wang, Z.; Wu, Y.; Shen, J. Simultaneous Screening Analysis of 3-Methyl-quinoxaline-2-carboxylic Acid and Quinoxaline-2-carboxylic Acid Residues in Edible Animal Tissues by a Competitive Indirect Immunoassay. J. Agric. Food Chem. 2013, 61, 10018–10025. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Yao, S.; Hu, G. Fabrication of super pure single−walled carbon nanotube electrochemical sensor and its application for picomole detection of olaquindox. Anal. Chim. Acta 2019, 1049, 82–90. [Google Scholar] [CrossRef]
- Wang, H.; Yao, S.; Liu, Y.; Wei, S.; Su, J.; Hu, G. Molecularly imprinted electrochemical sensor based on Au nanoparticles in carboxylated multi-walled carbon nanotubes for sensitive determination of olaquindox in food and feedstuffs. Biosens. Bioelectron. 2017, 87, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Song, J.; Li, L.; Qiao, X.; Chen, H. Development of an on-line molecularly imprinted chemiluminescence sensor for determination of trace olaquindox in chick feeds. J. Sci. Food Agric. 2012, 92, 2696–2702. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, L.; Yang, J.; Li, N.; Yang, L.; Jiang, X. Development of electrochemical method for the determination of olaquindox using multi-walled carbon nanotubes modified glassy carbon electrode. Talanta 2013, 109, 185–190. [Google Scholar] [CrossRef]
- Ma, T.; Liu, L.; Yuan, Z. Direct synthesis of ordered mesoporous carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template mediated structural transformation. J. Mater. Chem. B 1999, 103, 7743–7746. [Google Scholar]
- Ndamanisha, J.C.; Guo, L. Ordered mesoporous carbon for electrochemical sensing: A review. Anal. Chim. Acta 2012, 747, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Walcarius, A. Recent Trends on Electrochemical Sensors Based on Ordered Mesoporous Carbon. Sensors 2017, 17, 1863. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Fan, Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Mater. Chem. Front. 2017, 1, 1001–1027. [Google Scholar] [CrossRef]
- Dong, X.; Guo, Z.; Song, Y.; Hou, M.; Wang, J.; Wang, Y.; Xia, Y. Flexible and Wire-Shaped Micro-Supercapacitor Based on Ni(OH)2-Nanowire and Ordered Mesoporous Carbon Electrodes. Adv. Funct. Mater. 2014, 24, 3405–3412. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef]
- Kim, T.W.; Kleitz, F.; Paul, B.; Ryoo, R. MCM-48-like large mesoporous silicas with tailored pore structure: Facile synthesis domain in a ternary triblock copolymer-butanol-water system. J. Amer. Chem. Soc. 2005, 127, 7601–7610. [Google Scholar] [CrossRef] [PubMed]
- Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F.; Mahjani, M.G. Impedance spectroscopy analysis of glucose electro-oxidation on Ni-modified glassy carbon electrode. Electrochim. Acta 2008, 53, 6602–6609. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (CMK−8, OLA and CBX) are available from the authors. |


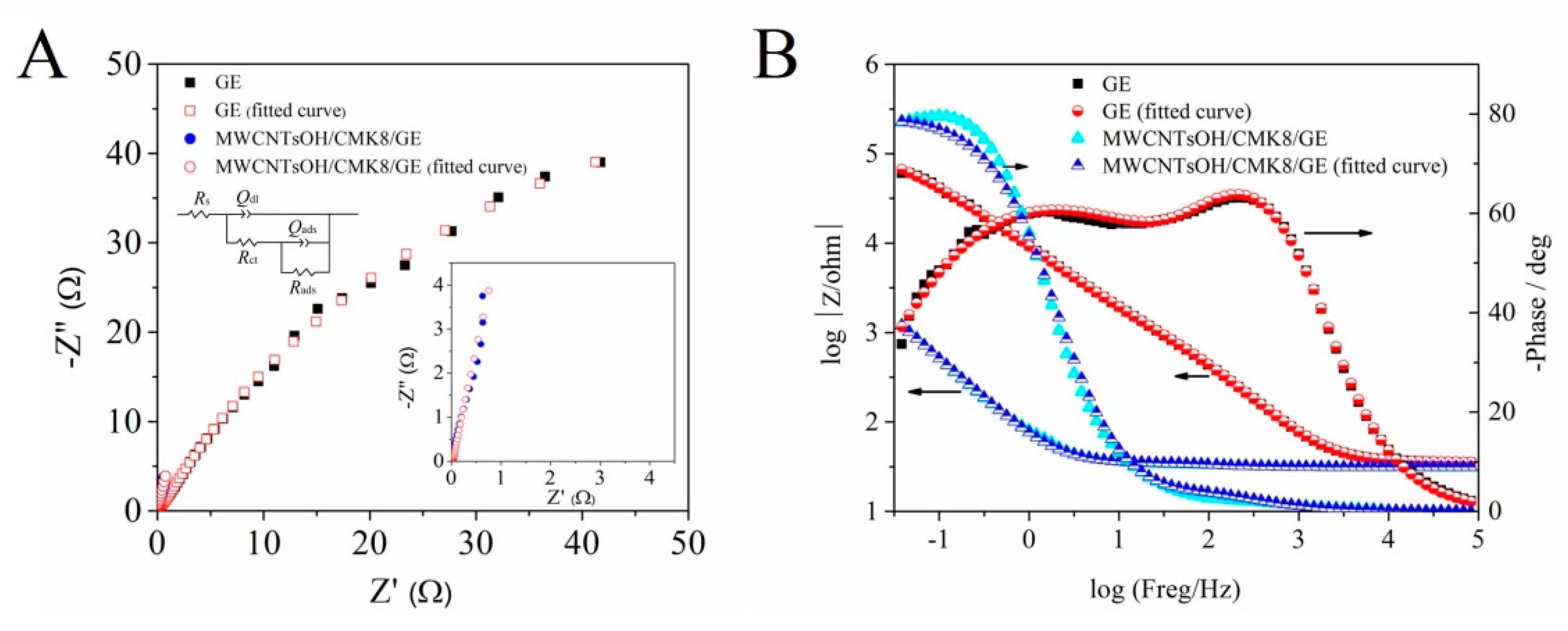


| Electrodes | Rs (Ω) | Rct (Ω) | Qdl (F) | Rads (Ω) | Qasd (F) | n1 | n2 |
|---|---|---|---|---|---|---|---|
| GE | 3.538 | 1.176 × 103 | 6.209 × 10−6 | 1.456 × 105 | 2.313 × 10−5 | 0.89 | 0.67 |
| Error (%) | 0.461 | 5.793 | 4.117 | 6.122 | 1.847 | 0.52 | 0.761 |
| MWCNTs−OH/CMK−8/GE | 3.132 | 5.884 | 1.175 × 10−3 | — | 1.816 × 10−3 | 0.81 | 0.95 |
| Error (%) | 0.740 | 8.89 | 10.31 | — | 9.73 | 2.178 | 2.223 |
| Title 1 | Added (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|
| OLA | 1 | 96.10 | 8.18 |
| 10 | 96.59 | 1.11 | |
| 100 | 107.78 | 6.71 | |
| CBX | 1 | 96.10 | 8.18 |
| 10 | 96.59 | 1.11 | |
| 100 | 107.78 | 6.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hu, G.; Wang, H.; Yao, S.; Ye, Y. Novel Electrochemical Sensor Fabricated for Individual and Simultaneous Ultrasensitive Determination of Olaquindox and Carbadox Based on MWCNT-OH/CMK-8 Hybrid Nanocomposite Film. Molecules 2019, 24, 3041. https://doi.org/10.3390/molecules24173041
Liu Y, Hu G, Wang H, Yao S, Ye Y. Novel Electrochemical Sensor Fabricated for Individual and Simultaneous Ultrasensitive Determination of Olaquindox and Carbadox Based on MWCNT-OH/CMK-8 Hybrid Nanocomposite Film. Molecules. 2019; 24(17):3041. https://doi.org/10.3390/molecules24173041
Chicago/Turabian StyleLiu, Yanqing, Gengxin Hu, Hongwu Wang, Su Yao, and Yinjian Ye. 2019. "Novel Electrochemical Sensor Fabricated for Individual and Simultaneous Ultrasensitive Determination of Olaquindox and Carbadox Based on MWCNT-OH/CMK-8 Hybrid Nanocomposite Film" Molecules 24, no. 17: 3041. https://doi.org/10.3390/molecules24173041
APA StyleLiu, Y., Hu, G., Wang, H., Yao, S., & Ye, Y. (2019). Novel Electrochemical Sensor Fabricated for Individual and Simultaneous Ultrasensitive Determination of Olaquindox and Carbadox Based on MWCNT-OH/CMK-8 Hybrid Nanocomposite Film. Molecules, 24(17), 3041. https://doi.org/10.3390/molecules24173041