Alnus Sibirica Extracts Suppress the Expression of Inflammatory Cytokines Induced by Lipopolysaccharides, Tumor Necrosis Factor-α, and Interferon-γ in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Results
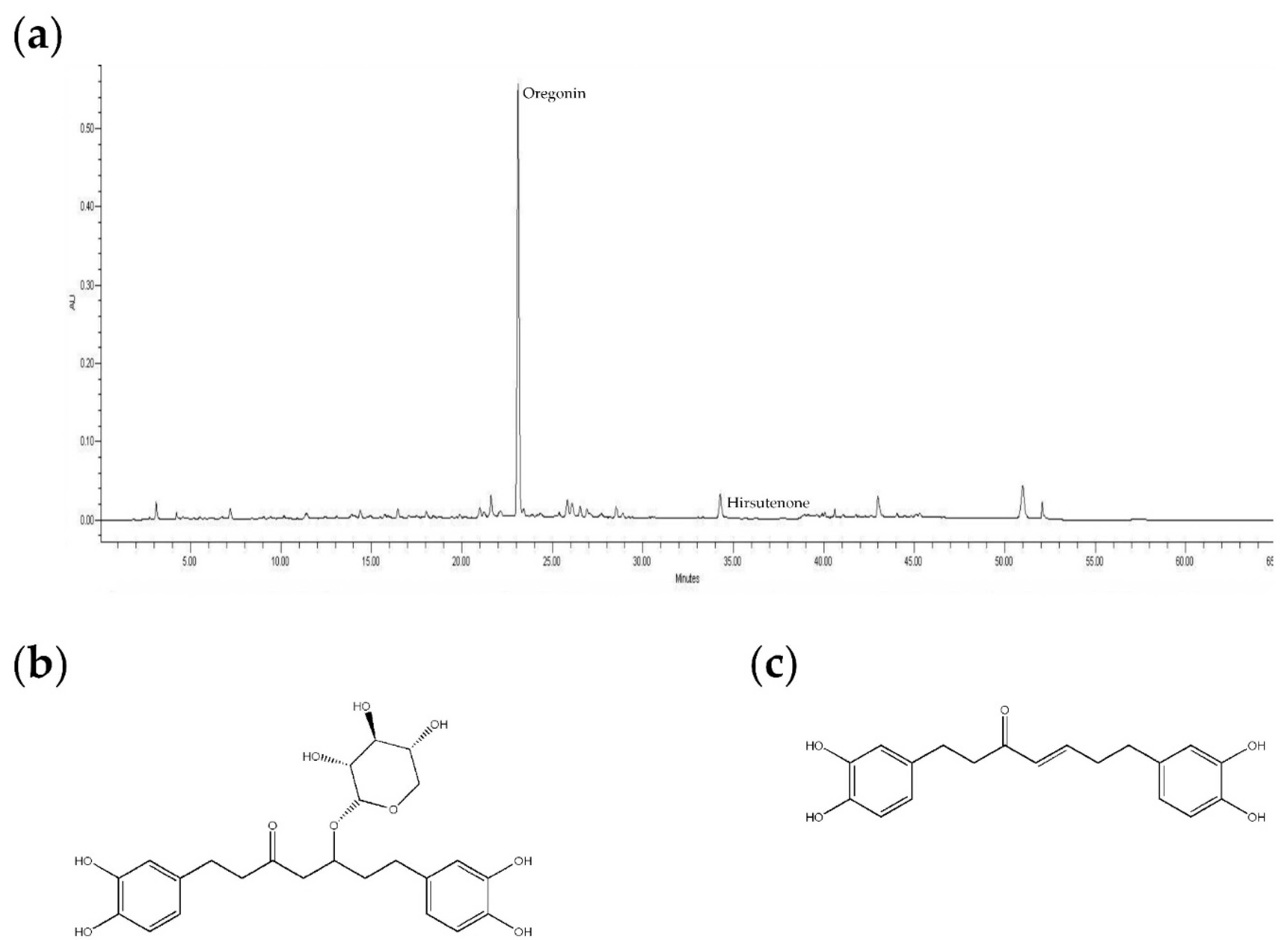
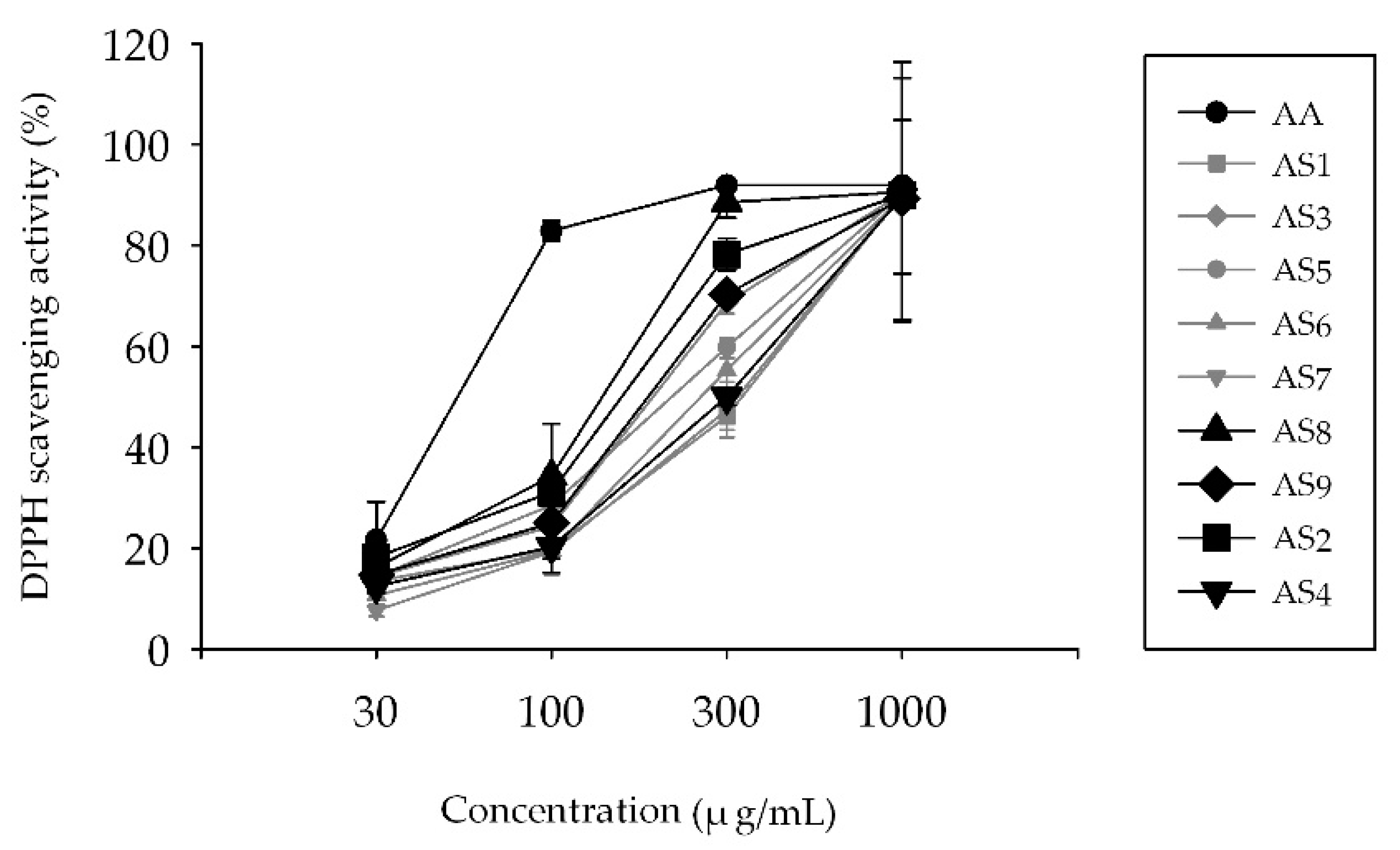
2.1. Measurement of 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
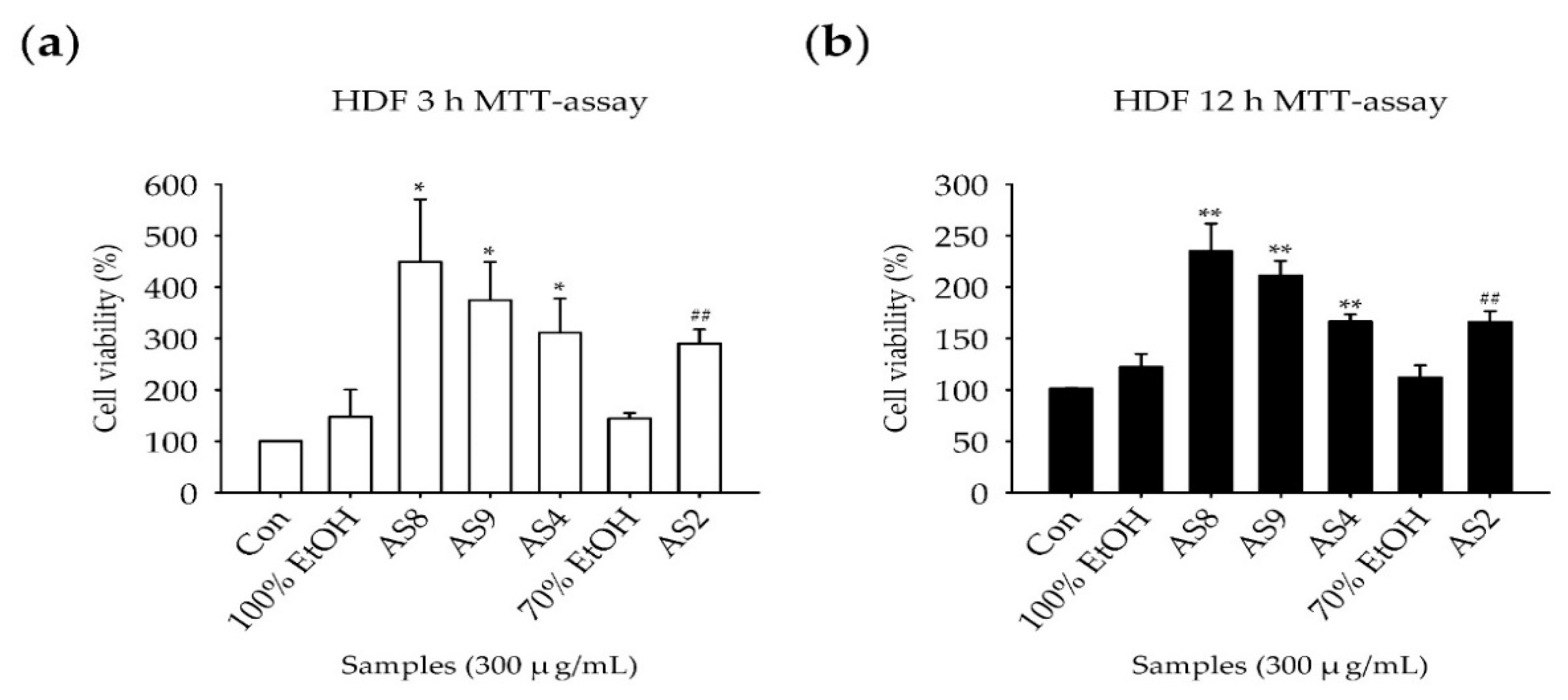
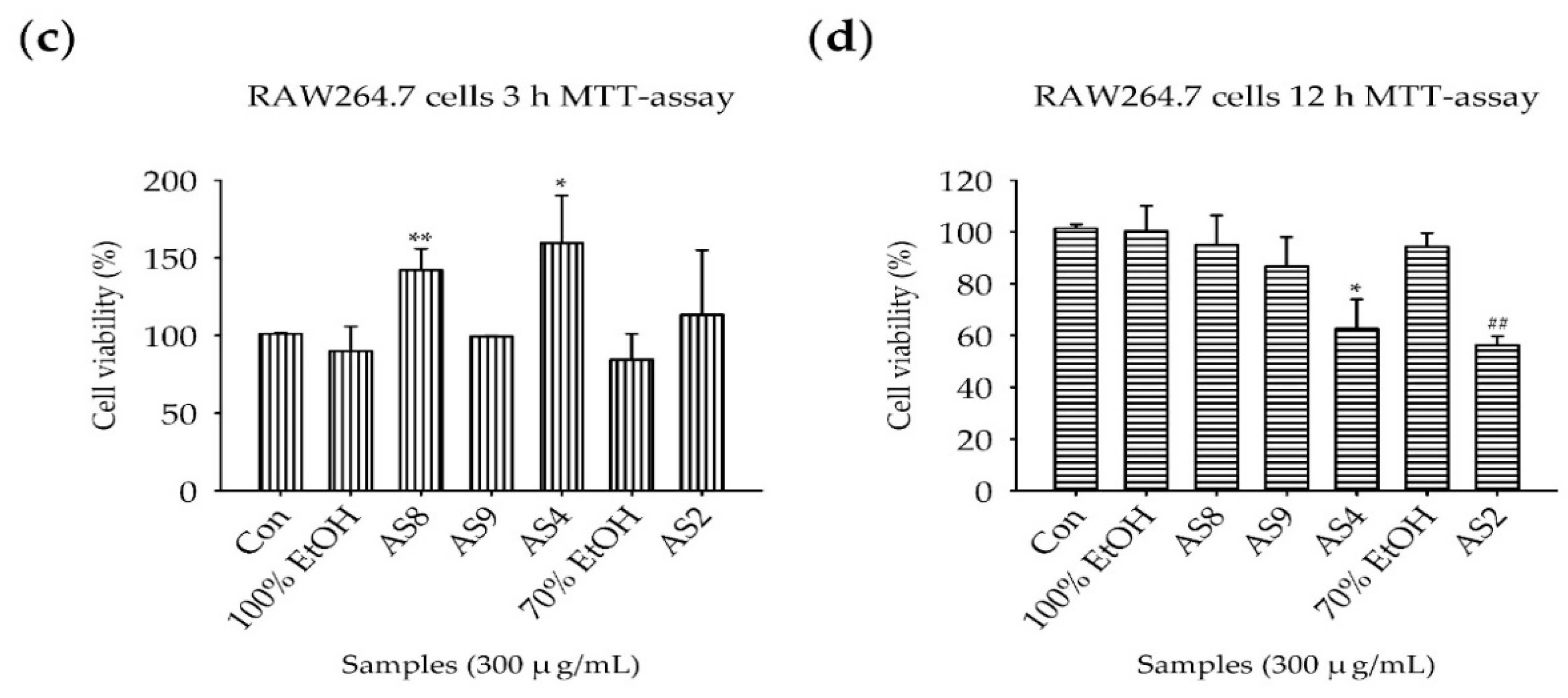
2.2. Measurement of Cell Viability
2.3. Reverse-Transcription Polymerase Chain Reaction
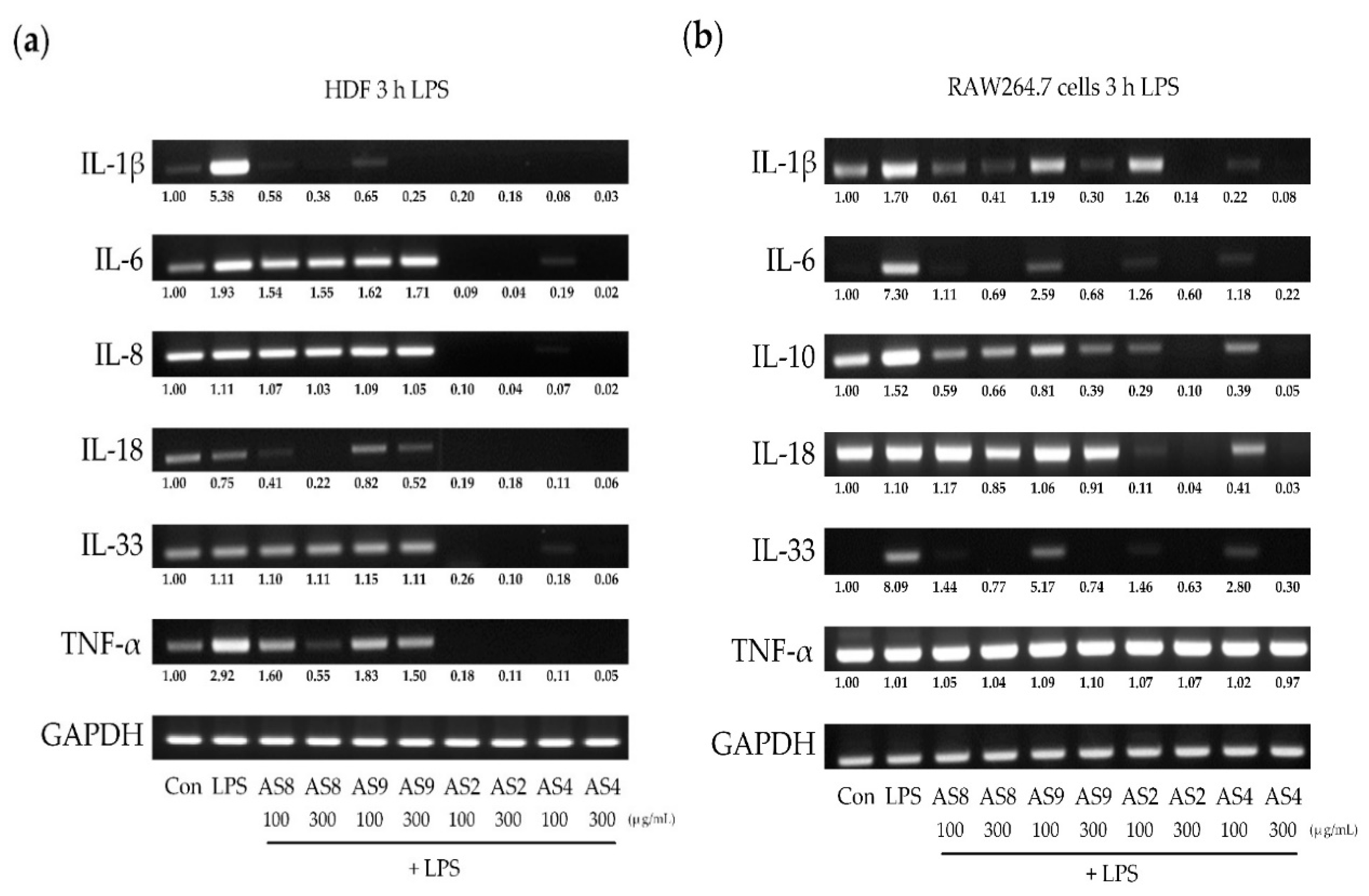
2.4. Effects of LPS
2.5. Effects of TNF-α
2.6. Effects of IFN-γ
3. Discussion
4. Materials and Methods
4.1. Plant Material, Extraction, and Isolation
4.2. HPLC Analysis
4.3. Measurement of DPPH Radical Scavenging Activity
4.4. Cell Culture
4.5. Measurement of Cell Viability
4.6. Inflammatory Stimulants and AS Extract Treatments
4.7. RNA Isolation and cDNA Synthesis
4.8. Reverse-Transcriptase Polymerase Chain Reaction
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kessler-Becker, D.; Krieg, T.; Eckes, B. Expression of pro-inflammatory markers bu human dermal fibroblasts in a three-dimensional culture model is mediated by an autocrine interleukin-1. Biochem. J. 2004, 379, 351–358. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic target—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef] [PubMed]
- Prefontaine, D.; Lajoie-Kadoch, S.; Foley, S.; Audusseau, S.; Olivenstein, R.; Halayko, A.J.; Lemiere, C.; Martin, J.G.; Hamid, Q. Increased expression of IL-33 in severe asthma: Evidence of expression by Airway smooth muscle cells. J. Immunol. 2009, 183, 5094–5103. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Packard, R.R.S.; Libby, P. Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin. Chem. 2008, 54, 24–38. [Google Scholar] [CrossRef]
- Wittmann, M.; McGonagle, D.; Werfel, T. Cytokines as therapeutic targets in skin inflammation. Cytokine Growth Factor Rev. 2014, 25, 443–451. [Google Scholar] [CrossRef]
- Nedorost, S.T. Generalized Dermatitis in Clinical Practice; Springer Science & Business Media: Berlin, Germany, 2012; Volume 1. [Google Scholar]
- Roeleveld, D.M.; Koenders, M.I. The role of the Th17 cytokines IL-17 and IL-22 in Rheumatoid Arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine 2015, 74, 101–107. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Zheng, X.-Y.; Zhu, J. Th1/Th2/Th17/Treg cytokines in Guillain–Barré syndrome and experimental autoimmune neuritis. Cytokine Growth Factor Rev. 2013, 24, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, R.; Abdullah, N.; Radhakrishnan, A.K.; Raj, V.L.; Liam, C. Relationship between various cytokines implicated in asthma. Hum. Immunol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Muchamuel, T.; Andrade, S.; Menon, S. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 1993, 177, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Mancuso, G.; Cusumano, V.; Marco, R.D.; Zaccone, P.; Bendtzen, K.; Teti, G. Prevention of endotoxin-induced lethality in neonatal mice by interleukin-13. Eur. J. Immunol. 1997, 27, 1580–1583. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.; Correa, P.A.; Gómez, L.M.; Cadena, J.; Molina, J.F.; Anaya, J.-M. Th1/Th2 cytokines in patients with systemic lupus erythematosus: Is tumor necrosis factor α protective? Semin. Arthritis Rheum. 2004, 6, 404–413. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kubatzky, K.F.; Mitra, D.K. An Update on Interleukin-9: From Its Cellular Source and Signal Transduction to Its Role in Immunopathogenesis. Int. J. Mol. Sci. 2019, 20, 2113. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Chang, C.; Lu, L.; Lau, C.S.; Lu, Q. Th9 cells and IL-9 in autoimmune disorders: Pathogenesis and therapeutic potentials. Human Immunol. 2017, 78, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Di Marco, R.; Patti, F.; Reggio, E.; Nicoletti, A.; Zaccone, P.; Stivala, F.; Meroni, P.; Reggio, A. Blood levels of transforming growth factor-beta 1 (TGF-β1) are elevated in both relapsing remitting and chronic progressive multiple sclerosis (MS) patients and are further augmented by treatment with interferon-beta 1b (IFN-β1b). Clin. Exp. Immunol. 1998, 113, 96–99. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Leung, D.Y.M.; Bieber, T. Atopic dermatitis. Lancet 2003, 361, 151–160. [Google Scholar] [CrossRef]
- Wollenberg, A.; Bieber, T. Atopic dermatitis from the genes to skin lesions. Allergy 2000, 55, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dokmeci, E.; Herrick, C.A. The immune system and atopic dermatitis. Semin. Cutan. Med. Surg. 2008, 27, 138. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Arthritis and Musculoskeetal and Skin Diseases. Handout on Health; Atopic Dermatitis: Bethesda, MD, USA, 1999; pp. 1–16. [Google Scholar]
- Lee, A.Y. Immunologic barrier in skin of atopic dermatitis. Atopic Dermat. Symp. 2010, S143–S148. [Google Scholar]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Van Linthout, S.; Miteva, K.; Tschope, C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc. Res. 2014, 102, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Yamamoto, S.; Hitomi, E.; Inada, Y.; Suyama, Y.; Sugioka, T.; Hamasaki, Y. Interleukin 33 is induced by tumor necrosis factor A and interferon G in keratinocytes and contributes to allergic contact dermatitis. J. Investig. Allergol. Clin. Immunol. 2013, 23, 428–434. [Google Scholar] [PubMed]
- Sims, J.E.; Smith, D.E. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Moritz, D.R.; Gheyselinck, J.; Klemenz, R.; Rodewald, H.R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J. Immunol. 1998, 161, 4866. [Google Scholar]
- Xie, Q.; Shen, W.-W.; Zhong, J.; Huang, C.; Zhang, L.; Li, J. Lipopolysaccharide/adenosine triphosphate induces IL-1 beta and IL-18 secretion through the NLRP3 inflammasome in RAW264.7 murine macrophage cells. Int. J. Mol. Med. 2014, 34, 341–349. [Google Scholar] [CrossRef]
- Santarlasci, V.; Cosmi, L.; Maggi, L.; Liotta, F.; Annunziato, F. IL-1 and T helper immune responses. Front. Immunol. 2013, 4, 182. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Ann. Rev. Immunol. 2009, 27, 519. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.V.; McManus, A.T.; Chambers, J.P. Production of tumor necrosis factor-alpha, interleukin 1-beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to Substance P. Neuropeptides 2003, 37, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, K.; Morishita, K.; Yoshimura, T.; Lavu, S.; Kobayashi, Y.; Lew, W.; Appella, E.; Kung, H.F.; Leonard, E.J.; Oppenheim, J.J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J. Exp. Med. 1988, 167, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, M.; Walz, A.; Kunkel, S.L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Investig. 1989, 84, 1045. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Koch, A.E.; Antony, V.B.; Fick, R.B., Jr.; Standiford, T.J.; Kunkel, S.L. The immunopathology of chemotactic cytokines: The role of interleukin-8 and monocyte chemoattractant protein-1. J. Lab. Clin. Med. 1994, 123, 183. [Google Scholar]
- Dembic, Z. The Cytokines of the Immune System: The Role of Cytokines in Disease Related to Immune Response; Academic Press: London, UK, 2015. [Google Scholar]
- Barbulescu, K.; Becker, C.; Schlaak, J.F.; Schmitt, E.; Meyer zum Büschenfelde, K.H.; Neurath, M.F. IL-12 and IL-18 differentially regulate the transcriptional activity of the human IFN-gamma promoter in primary CD4+ T lymphocytes. J. Immunol. 1998, 160, 3642. [Google Scholar]
- Coondoo, A. Cytokines in dermatology—A basic overview. Indian J. Dermatol. 2011, 56, 368–374. [Google Scholar] [CrossRef]
- Löhning, M.; Stroehmann, A.; Grogan, J.L.; Radbruch, A.; Kamradt, T.; Coyle, A.J.; Lin, S.; Gutierrez-Ramos, J.C.; Levinson, D. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl. Acad. Sci. USA 1998, 95, 6930. [Google Scholar] [CrossRef]
- Stolarski, B.; Kurowska-Stolarska, M.; Kewin, P.; Xu, D.; Liew, F.Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 2010, 185, 3472–3480. [Google Scholar] [CrossRef]
- Schneider, E.; Petit-Bertron, A.-F.; Bricard, R.; Levasseur, M.; Ramadan, A.; Girard, J.-P.; Herbelin, A.; Dy, M. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J. Immunol. 2009, 183, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, E.; Van, L.P.; Samson, M.; Diem, S.; Barra, A.; Roga, S.; Gombert, J.-M.; Schneider, E.; Dy, M.; Gourdy, P.; et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur. J. Immunol. 2009, 39, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Rank, M.A.; Kobayashi, T.; Kozaki, H.; Bartemes, K.R.; Squillace, D.L.; Kita, H. IL-33–activated dendritic cells induce an atypical T H2-type response. J. Allerg. Clin. Immunol. 2009, 123, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Saluja, R.; Khan, M.; Church, M.K.; Maurer, M. The role of IL-33 and mast cells in allergy and inflammation. Clin. Transl. Allerg. 2015, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Prim. 2018, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.S.; Long, H.A.; Wu, K.C.; Reynolds, N.J.; Newman, C.F.; Wilson, P.A.; West, A.; McGill, P.J.; Donaldson, M.J. Proteomic analysis of filaggrin deficiency identifies molecular signatures characteristic of atopic eczema. J. Allerg. Clin. Immunol. 2017, 140, 1299. [Google Scholar] [CrossRef] [PubMed]
- Tracey, M.D.K.J.; Cerami, P.D.A. Tumor necrosis factor: A pleiotropic cytokine and therapuetic target. Ann. Rev. Med. 1994, 45, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Lee, S.-J. Korean folk medicine. Korean J. Pharmacogn. 1966, 7, 1–33. [Google Scholar]
- Pui-hay, B.P.; Sung, C.K. International Collation of Traditional and Folk Medicine; World Scientific: Singapore, 2001; Volume 4. [Google Scholar]
- Kim, M.H.; Park, K.H.; Kim, S.R.; Park, K.J.; Oh, M.H.; Heo, J.H.; Yoon, K.H.; Yin, J.; Yoon, K.H.; Lee, M.W. Two new phenolic compounds from the leaves of Alnus sibirica Fisch. ex Turcz. Nat. Prod. Res. 2016, 30, 206–213. [Google Scholar] [CrossRef]
- Suga, T.; Iwata, N.; Asakawa, Y. Chemical constituents of the male flower of Alnus pendula (BETULACEAE). Bull. Chem. Soc. Jpn. 1972, 45, 2058–2060. [Google Scholar] [CrossRef]
- Choi, S.E.; Kim, K.H.; Kwon, J.H.; Kim, S.B.; Kim, H.W.; Lee, M.W. Cytotoxic activities of diarylheptanoids from Alnus japonica. Arch. Pharm. Res. 2008, 31, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ko, H.H.; Lee, M.W.; Bang, H.; Lee, C.S. Inhibition of activated responses in dendritic cells exposed to lipopolysaccharide and lipoteichoic acid by diarylheptanoid oregonin. Int. Immunopharmacol. 2008, 8, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, N.; Park, M.; Ahn, K.; Toh, S.; Hahn, D.; Kim, Y.; Chung, H. Diarylheptanoids with in vitro inducible nitric oxide synthesis inhibitory activity from Alnus hirsuta. Planta Med. 2000, 66, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Park, K.H.; Jeong, M.S.; Kim, H.H.; Lee, D.I.; Joo, S.S.; Lee, C.S.; Bang, H.; Choi, Y.W.; Lee, M.K.; et al. Effect of Alnus japonica extract on a model of atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2011, 136, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Ohta, S.; Ohta, E.; Aoki, T. A C31-secodammarane-type triterpenic acid, 12-deoxy alnustic acid, from the female flowers of alnus pendula. Phytochemistry 1981, 25, 1243–1244. [Google Scholar] [CrossRef]
- Nomura, M.; Tokoroyama, T.; Kubota, T. Biarylheptanoids and other constituents from wood of Alnus japonica. Phytochemistry 1981, 20, 1097–1104. [Google Scholar] [CrossRef]
- Lee, M.; Pak, M.; Jeong, D.; Kim, K.; Kim, H.; Toh, S. Diarylheptanoids from the leaves of Alnus hirsuta Turcz. Arch. Pharm. Res. 2000, 23, 50–53. [Google Scholar] [CrossRef]
- Joo, S.S.; Kim, S.G.; Choi, S.E.; Kim, Y.B.; Park, H.Y.; Seo, S.J.; Choi, Y.W.; Lee, M.W.; Lee, D.I. Suppression of T cell activation by hirsutenone, isolated from the bark of Alnus japonica, and its therapeutic advantages for atopic dermatitis. Eur. J. Pharmacol. 2009, 614, 98–105. [Google Scholar] [CrossRef]
- Joo, S.-S.; Kim, M.-S.; Oh, W.-S.; Lee, D.-I. Enhancement of NK cytotoxicity, antimetastasis and elongation effect of survival time in B16-F10 melanoma cells by oregonin. Arch. Pharm. Res. 2002, 25, 493–499. [Google Scholar] [CrossRef]
- Yin, J.; Yoon, S.H.; Ahn, H.S.; Lee, M.W. Inhibitory activity of allergic contact dermatitis and atopic dermatitis-like skin in BALB/c mouse through oral administration of fermented barks of Alnus sibirica. Molecules 2018, 23, 450. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J. Allerg. Clin. Immunol. 2000, 105, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Narazaki, M.; Kishimoto, T. The two-faced cytokine IL-6 in host defense and diseases. Int. J. Mol. Sci. 2018, 19, 3528. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.H.; Moore, K.W.; Spits, H. Differential effects of IL-4 and IL-10 on IL-2-induced IFN-gamma synthesis and lymphokine-activated killer activity. Int. Immunol. 1992, 4, 563. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.Y.; Liu, J.; Homma, Y.; Zhang, Y.; Brendolan, A.; Saggese, M.; Han, J.; Silverstein, R.; Selleri, L.; Ma, X. Interleukin-10 expression in macrophages during Phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity 2007, 27, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse t helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Thl clones. J. Exp. Med. 1989, 170, 2081. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; Vieira, P.; Fiorentino, D.F.; Trounstine, M.L.; Khan, T.A.; Mosmann, T.R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 1990, 248, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Fuss, I.J.; Boirivant, M.; Lacy, B.; Strober, W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J. Immunol. 2002, 168, 900–908. [Google Scholar] [CrossRef]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263. [Google Scholar] [CrossRef]
- Berg, D.J.; Leach, M.W.; Kühn, R.; Rajewsky, K.; Müller, W.; Davidson, N.J.; Rennick, D. Interleukin 10 but not interleukin 4 is a natural suppressant of cutaneous inflammatory responses. J. Exp. Med. 1995, 182, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.J.; Davidson, N.; Grünig, G.; Rennick, D.; Kühn, R.; Rajewsky, K.; Müller, W.; Menon, S. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Investig. 1995, 96, 2339. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Das, M.P.; Howard, E.D.; Weiner, H.L.; Kuchroo, V.K.; Sobel, R.A. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol. 1998, 161, 3299. [Google Scholar] [PubMed]
- Wittmann, M.; Macdonald, A.; Renne, J. IL-18 and skin inflammation. Autoimmun. Rev. 2009, 9, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Gracie, J.A.; Robertson, S.E.; McInnes, I.B. Interleukin-18. J. Leukoc. Biol. 2003, 73, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Sokołowska-Wojdyło, M.; Roszkiewicz, J.; Nowicki, R.J.; Ruckemann-Dziurdzińska, K. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: Atopic dermatitis, psoriasis and skin mastocytosis. Postep. Dermatol. Alergol. 2014, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- Zelova, H.; Hosek, J. TNF-alpha signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, K.; Morizane, S.; Takiguchi, T.; Iwatsuki, K. Dexamethasone but not tacrolimus suppresses TNF-alpha-induced thymic stromal lymphopoietin expression in lesional keratinocytes of atopic dermatitis model. J. Dermatol. Sci. 2015, 80, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Junghans, V.; Gutgesell, C.; Jung, T.; Neumann, C. Epidermal cytokines IL-1β, TNF-α, and IL-12 in patients with atopic dermatitis: Response to application of house dust mite antigens. J. Investig. Dermatol. 1998, 111, 1184. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. IL-33: An alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr. Opin. Immunol. 2014, 31, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M. Role of IL-33 in inflammation and disease. J. Inflamm. 2011, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Nabe, T. Interleukin (IL)-33: New therapeutic target for atopic diseases. J. Pharmacol. Sci. 2014, 126, 85. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-L.; Fang, S.-H.; Wang, S.-C.; Cheng, W.-C.; Liu, P.-L.; Su, C.-C.; Chen, C.-S.; Huang, M.-Y.; Hua, K.-F.; Shen, K.-H. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Yanagawa, Y.; Hiraide, S.; Iizuka, K. Cyclic AMP signaling enhances lipopolysaccharide sensitivity and interleukin-33 production in RAW264.7 macrophages. Microbiol. Immunol. 2016, 60, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Meephansan, J.; Tsuda, H.; Komine, M.; Tominaga, S.; Ohtsuki, M. Regulation of IL-33 expression by IFN-gamma and tumor necrosis factor-alpha in normal human epidermal keratinocytes. J. Investig. Dermatol. 2012, 132, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Meephansan, J.; Komine, M.; Tsuda, H.; Karakawa, M.; Tominaga, S.-I.; Ohtsuki, M. Expression of IL-33 in the epidermis: The mechanism of induction by IL-17. J. Dermatol. Sci. 2013, 71, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Seltmann, J.; Werfel, T.; Wittmann, M. Evidence for a regulatory loop between IFN-γ and IL-33 in skin inflammation. Exp. Dermatol. 2013, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Shaik-Dasthagirisaheb, Y.B.; Conti, P. Chemokine network involved in inflammatory skin diseases. Ann. Clin. Lab. Sci. 2015, 45, 452. [Google Scholar]
- Borish, L.C.; Steinke, J.W. Cytokines and chemokines. J. Allerg. Clin. Immunol. 2003, 111, S460. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the AS1–AS9 are available from the authors. |


| No. | Name | Substrate |
|---|---|---|
| AS1 | SB | Barks, Songrim, Korea |
| AS2 | HL | Leaves, Hamyang, Korea |
| AS3 | JL | Leaves, Jacheon, Korea |
| AS4 | HB | Barks, Hamyang, Korea |
| AS5 | JB | Barks, Jacheon, Korea |
| AS6 | JBR | Barks, reflux extraction, Jacheon, Korea |
| AS7 | JLR | Leaves reflux extraction, Jacheon, Korea |
| AS8 | JBRE | Barks, reflux extraction, and EA fraction, Jacheon, Korea |
| AS9 | JLRE | Leaves, reflux extraction, and EA fraction, Jacheon, Korea |
| Column | Hector C18 HPLC Column (5 µm, 250 × 4.6 mm) | ||||||
|---|---|---|---|---|---|---|---|
| Detector | Waters 112 UV/VIS (280 nm) | ||||||
| 0 min | 20 min | 35 min | 38 min | 45 min | 47 min | 55 min | |
| H2O | 95 | 75 | 60 | 0 | 0 | 95 | 95 |
| ACN | 5 | 25 | 40 | 100 | 100 | 5 | 5 |
| Primer | bp | °C | Sense | Antisense |
|---|---|---|---|---|
| hGAPDH | 343 | 58 | 5′-CAGTGAGCTTCCCGTTCAG-3′ | 5′-GCCAAAAGGGTCATCATCTC-3′ |
| hIL-1β | 391 | 62 | 5′-AAACAGATGAAGTGCTCCTTCCAGG-3 | 5′-TGGAGAACACCACTTGTTGCTCCA-3′ |
| hIL-6 | 264 | 62 | 5′-ATGAACTCCTTCTCCACAAGC-3′ | 5′-GTTTTCTGCCAGTGCCTCTTTG-3′ |
| hIL-8 | 293 | 58 | 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ | 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ |
| hIL-18 | 342 | 53 | 5′-GCTTGAATCTAAATTATCAGTC-3′ | 5′-GAAGATTCAAATTGCATCTTAT-3′ |
| hIL-33 | 180 | 58 | 5′-CAAAGAAGTTTGCCCCATGT-3′ | 5′-AAGGCAAAGCACTCCACAGT-3′ |
| hTNF-α | 237 | 62 | 5′-GAGCTGAGAGATAACCAGCTGGTG-3′ | 5′-CAGATAGATGGGCTCATACCAGGG-3′ |
| mGAPDH | 343 | 58 | 5′-CAGTGAGCTTCCCGTTCAG-3′ | 5′-GCCAAAAGGGTCATCATCTC-3′ |
| mIL-1β | 213 | 53 | 5′-AAGGAGAACCAAGCAACGACAAAA-3′ | 5′-TGGGGAACTCTGCAGACTCAAACT-3′ |
| mIL-6 | 141 | 62 | 5′-AGGATACCACTCCCAACAGACCT-3′ | 5′-CAAGTGCATCATCGTTGTTCATAC-3′ |
| mIL-10 | 252 | 55 | 5′-TACCTGGTAGAAGTGATGCC-3′ | 5′-CATCATGTATGCTTCTATGC-3′ |
| mIL-18 | 434 | 53 | 5′-ACTGTACAACCGCAGTAATACGC-3′ | 5′-AGTGAACATTACAGATTTATCCC-3′ |
| mIL-33 | 155 | 61 | 5′-GGTGTGGATGGGAAGAAGCTG-3′ | 5′-GAGGACTTTTTGTGAAGGACG-3′ |
| mTNF-α | 384 | 61 | 5′-GCGACGTGGAACTGGCAGAAG-3′ | 5′-GGTACAACCCATCGGCTGGCA-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Moon, S.; Bae, H.; Kim, Y.-W.; Lee, D.; Kim, S.; Seo, Y.; Wang, H.S.; Choi, Y.W.; Lee, M.W.; et al. Alnus Sibirica Extracts Suppress the Expression of Inflammatory Cytokines Induced by Lipopolysaccharides, Tumor Necrosis Factor-α, and Interferon-γ in Human Dermal Fibroblasts. Molecules 2019, 24, 2883. https://doi.org/10.3390/molecules24162883
Choi J, Moon S, Bae H, Kim Y-W, Lee D, Kim S, Seo Y, Wang HS, Choi YW, Lee MW, et al. Alnus Sibirica Extracts Suppress the Expression of Inflammatory Cytokines Induced by Lipopolysaccharides, Tumor Necrosis Factor-α, and Interferon-γ in Human Dermal Fibroblasts. Molecules. 2019; 24(16):2883. https://doi.org/10.3390/molecules24162883
Chicago/Turabian StyleChoi, Jeongyoon, Sunghee Moon, Hyemi Bae, Young-Won Kim, Donghee Lee, Seongtae Kim, Yelim Seo, Hye Soo Wang, Young Wook Choi, Min Won Lee, and et al. 2019. "Alnus Sibirica Extracts Suppress the Expression of Inflammatory Cytokines Induced by Lipopolysaccharides, Tumor Necrosis Factor-α, and Interferon-γ in Human Dermal Fibroblasts" Molecules 24, no. 16: 2883. https://doi.org/10.3390/molecules24162883
APA StyleChoi, J., Moon, S., Bae, H., Kim, Y.-W., Lee, D., Kim, S., Seo, Y., Wang, H. S., Choi, Y. W., Lee, M. W., Ko, J.-H., Lim, I., & Bang, H. (2019). Alnus Sibirica Extracts Suppress the Expression of Inflammatory Cytokines Induced by Lipopolysaccharides, Tumor Necrosis Factor-α, and Interferon-γ in Human Dermal Fibroblasts. Molecules, 24(16), 2883. https://doi.org/10.3390/molecules24162883





