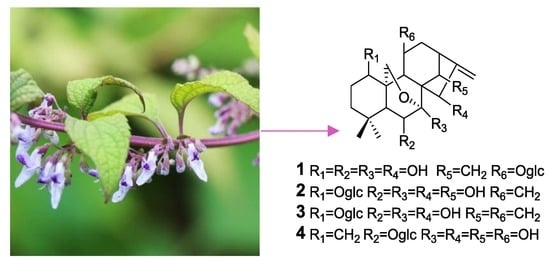
Four New ent-Kaurane Diterpene Glycosides from Isodon henryi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of New Compounds
2.2. Cytotoxicity Assay
2.3. Analysis of Structure–Activity Relationships
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; PU, J.X.; Li, J. Diterpenoids chemodiversity of the genus Isodon spach from Lamiaceae. Plant Divers. Resour. 2013, 35, 81–88. [Google Scholar]
- Sun, H.D.; Jiang, B. Study on the Diterpenoids of Isodon sculponeata (Vaniot) Hara. Zhongcaoyao 2001, 5, 15–17. [Google Scholar]
- Li, J.C.; Liu, C.J.; An, X.Z. The structure of henryin. Acta Bot. Yunnan 1984, 6, 453–456. [Google Scholar]
- Wan, Q.; Zhou, Z.P.; Li, A.Y. Advances in studies on pharmacological activities of Isodon. J. Mod. Med. Heal. 2008, 24, 362–364. [Google Scholar]
- Liang, H.J.; Liu, W.; Zhou, N.Q. Chemical constituents and bioactivity of Isodon japonica var glaucocalyx. J. Xinxiang Med. Coll. 2014, 31, 161–165. [Google Scholar]
- Jiao, K.; Li, H.Y.; Zhang, P.; Pi, H.F.; Ruan, H.L.; Wu, J.Z. Two new ent-kaurane diterpenoids from the aerial parts of Isodon excisoides. Chin. Chem. Lett. 2014, 25, 131–133. [Google Scholar] [CrossRef]
- Wu, Y.X.; Zhang, W.; Li, J.C.; Liu, N. Chemical constituents of flowers and fruits of Rabdosia excisa. Chin. J. Nat. Med. 2012, 10, 43–47. [Google Scholar] [CrossRef]
- Wang, T.; Tang, F.M.; Zhang, Y.H.; Chen, Z. A natural diterpenoid kamebacetal with anti-tumor activity: Theoretical and experimental study. J. Mol. Struct. 2010, 97, 317–322. [Google Scholar] [CrossRef]
- Wang, Z.M.; Cheng, P.Y. The structure elucidation of a new bis-ent-kaurane compound, isodopharicin E, isolated from Isodon pharicus (Prain) Murata. Chin. Chem. Lett. 1991, 2, 847–848. [Google Scholar]
- Liao, Y.J.; Bai, H.Y.; Li, Z.H.; Zou, J. Longikaurin A, a natural ent-kaurane, induces G2/M phasearrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells. Cell. Death Dis. 2014, 5, 1137–1148. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kim, J.Y.; Dong, S.P.; Wang, K.Y. Study on the immunomodulation effect of Isodon japonicus extract via splenocyte function and NK anti-tumor activity. Int. J. Mol. Sci. 2012, 13, 4880–4888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Feng, H.; Zhang, Q.; Liu, F.S.; Jin, W.S.; Mu, M.; Fang, Q.H.; Kong, M.; He, W.Y. The structures elucidation of isodopharicin D and F. Acta Pharm. Sin. 1998, 33, 207–211. [Google Scholar]
- Shen, Y.H.; Wen, Z.Y.; Xu, G. Cytotoxic ent-Kaurane Diterpenoids from Isodon eriocalyx. Chem. Biodivers. 2005, 2, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; Huang, B.; Xiao, C.J. Two new labdane diterpenoids from the rhizomes of Isodon yuennanensis. Nat. Prod. Res. 2015, 29, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.P.; Li, C.; Yang, H.Z.; Lu, Y.Q.; Yu, H.Y.; Gao, H.M.; Wang, Z.M. Three New Cytotoxic ent-Kaurane Diterpenes from Isodon excisoides. Molecules 2015, 20, 17544–17556. [Google Scholar] [CrossRef]
- Takahiro, M.; Seikou, N.; Naoto, K.; Tomohiro, H.; Masayuki, Y.; Tetsushi, W.; Hisashi, M. Antimutagenic activity of ent-kaurane diterpenoids from the aerial parts of Isodon japonicus. Tetrahedron Lett. 2017, 58, 3574–3578. [Google Scholar]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Liu, H.M.; Yan, X.; Kiuchi, F.; Liu, Z.Z. A new diterpene glycoside from rabdosia rubescens. Chem. Pharm. Bull. 2000, 48, 148–149. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, H.P.; Hu, H.P.; Sun, H.D.; Wang, S.Q.; Ueda, S.; Kuroda, Y.; Fujita, T. Structures of macrocalyxin B, F, G and H and maoyerabdosin from isodon macrocalyx. Phytochemistry 1995, 38, 921–926. [Google Scholar] [CrossRef]
- Dai, L.P.; Zhang, L.X.; Liu, Y.L.; Wu, H.; Liu, R.X.; Zhao, M.; Tian, S.S.; Jiang, X.; Chen, S.Q. Isolation and purification of diterpenoids from the aerial parts of Isodon excisoides target-guided by UPLC-LTQ- Orbitrap-MS. Nat. Prod. Res. 2018, 32, 2424–2430. [Google Scholar] [CrossRef]
- Kuo, L.M.; Kuo, C.Y.; Lin, C.Y.; Hung, M.F.; Shen, J.J.; Wang, T.L. Intracellular glutathione depletion by oridonin leads to apoptosis in hepatic stellate cells. Molecules 2014, 19, 3327–3344. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Xie, H.H.; Hao, J.; Jiang, Y.M.; Wei, X.Y. Eudesmane sesquiterpene glucosides from lychee seed and their cytotoxic activity. Food Chem. 2010, 123, 1123–1126. [Google Scholar] [CrossRef]
Sample Availability: Not available. |




| No. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 72.1 | 4.12, dd (11.7, 5.6) | 72.3 | 3.85, overlap | 85.4 | 3.57, dd (11.4, 5.6) | 32.2 | 1.58, m |
| 2 | 28.5 | 1.59, overlap | 31.4 | a 1.26, m b 1.83, overlap | 28.9 | 1.66, m 2.23, overlap | 19.6 | 1.49, overlap |
| 3 | 40.1 | a 1.33, m b 1.45, m | 42.2 | a 1.22, overlap b 1.43, overlap | 39.8 | a 1.27, overlap b 1.43, dt (13.5, 3.6) | 43.2 | a 1.19, m b 1.43, overlap |
| 4 | 34.7 | 34.8 | 34.2 | 35.2 | ||||
| 5 | 60.1 | 1.19, d (4.9) | 59.2 | 1.21, d (4.9) | 59.7 | 1.29, overlap | 57.8 | 1.33, d (5.3) |
| 6 | 74.7 | 3.65, d (4.9) | 73.6 | 3.63, d (4.9) | 75.1 | 3.69, d (6.0) | 76.2 | 4.22, d (5.3) |
| 7 | 97.4 | 100.1 | 97.4 | 101.2 | ||||
| 8 | 53.5 | 54.4 | 53.1 | 54.7 | ||||
| 9 | 49.2 | 2.33, d (8.7) | 50.3 | 2.47, overlap | 44.5 | 2.04, dd (13.0, 4.7) | 51.2 | 2.28, dd (9.4, 2.1) |
| 10 | 42.4 | 38.4 | 42.2 | 38.3 | ||||
| 11 | 75.1 | 4.52, overlap | 19.4 | 1.48, overlap | 19.7 | a 1.55, overlap b 2.04, qd (13.0, 8.2) | 61.7 | 3.88, overlap |
| 12 | 41.1 | a 1.82, dd (14.4, 8.7) b 2.76, dt (14.4, 9.3) | 44.0 | a 1.83, overlap b 2.98, dt (14.8, 9.0) | 33.2 | a 1.34, td (12.6, 7.1) b 2.21, overlap | 43.7 | a 1.51, overlap b 2.72, dt (13.9, 9.2) |
| 13 | 38.2 | 2.61, dd (9.8, 4.3) | 47.4 | 2.48, overlap | 38.0 | 2.56, dd (9.5, 4.5) | 46.8 | 2.49, dd (9.3) |
| 14 | 30.1 | 1.64, overlap | 75.8 | 4.29, s | 26.7 | a 1.52, overlap b 1.75, d (12.0) | 77.0 | 4.38, s |
| 15 | 75.1 | 4.54, overlap | 73.3 | 4.96, t (2.6) | 75.6 | 4.43, t (2.6) | 72.6 | 5.02, t (2.3) |
| 16 | 160.8 | 158.8 | 162.1 | 160.4 | ||||
| 17 | 107.2 | a 4.96, br s b 5.04, d (2.1) | 110.3 | a 5.19, d (2.6) b 5.21, br s | 107.3 | a 4.97, t (2.6) b 5.02, br d (2.6) | 110.5 | a 5.17, br d (2.3) b 5.29, br s |
| 18 | 32.8 | 0.99, s | 33.5 | 1.01, s | 33.1 | 1.01, s | 33.6 | 1.07, s |
| 19 | 22.1 | 1.09, s | 22.6 | 1.09, s | 22.1 | 1.12, s | 23.1 | 1.12, s |
| 20 | 64.9 | a 4.01, dd (10.2, 2.2) b 4.16, d (10.2); | 67.7 | a 3.83, overlap b 4.08, dd (9.9, 2.4) | 64.3 | a 3.97, dd (11.9, 1.4) b 4.32, overlap | 67.3 | a 3.85, d (9.9) b 4.10, dd (9.9, 2.1) |
| 1′ | 104.1 | 4.36, d (7.8) | 104.5 | 4.25, d (7.7) | 104.8 | 4.32, d (7.7) | 102.0 | 5.09, d (7.9) |
| 2′ | 75.7 | 3.19, t (7.8) | 75.5 | 3.12, dd (9.1, 7.7) | 75.6 | 3.13, dd (9.0, 7.7) | 76.0 | 3.11, dd (9.2, 7.9) |
| 3′ | 78.9 | 3.28, overlap | 78.1 | 3.29, overlap | 78.5 | 3.31, overlap | 78.5 | 3.35, overlap |
| 4′ | 71.4 | 3.26, t (10.1) | 71.8 | 3.23, overlap | 71.6 | 3.24, overlap | 77.0 | 3.24, overlap |
| 5′ | 77.8 | 3.23, overlap | 77.8 | 3.23, overlap | 77.7 | 3.23, overlap | 78.0 | 3.28, overlap |
| 6′ | 62.8 | a 3.65, m b 3.83, dd (11.9, 2.0) | 63.0 | a 3.64, overlap b 3.87, overlap | 62.8 | a 3.62, dd (11.9, 5.4) b 3.82, dd (11.9, 2.1) | 63.3 | a 3.68, dd (11.5, 5.7) b 3.91, overlap |
| Sample | A2780 | BGC-823 | HCT-116 | HepG2 |
|---|---|---|---|---|
| 1 | 0.53 ± 0.02 | 1.15 ± 0.04 | 0.38 ± 0.01 | 0.96 ± 0.06 |
| 2 | 0.53 ± 0.04 | 0.99 ± 0.05 | 0.35 ± 0.01 | 0.89 ± 0.03 |
| 3 | 0.27 ± 0.02 | 0.87 ± 0.03 | 0.26 ± 0.01 | 0.21 ± 0.04 |
| 4 | 0.60 ± 0.01 | 2.44 ± 0.08 | 0.29 ± 0.02 | 0.61 ± 0.11 |
| 5 | 0.24 ± 0.03 | 0.85 ± 0.07 | 0.28 ± 0.03 | 0.23 ± 0.05 |
| 6 | 0.68 ± 0.01 | 0.86 ± 0.02 | 0.40 ± 0.01 | 0.65 ± 0.05 |
| 7 | 0.28 ± 0.04 | 0.38 ± 2.11 | 0.29 ± 0.45 | 0.18 ± 4.42 |
| DDP | 0.002 ± 0.02 | 0.02 ± 0.14 | 0.01 ± 0.05 | 0.01 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.-L.; Zhang, L.-X.; Wu, H.; Chen, S.-Q.; Li, J.; Dai, L.-P.; Wang, Z.-M. Four New ent-Kaurane Diterpene Glycosides from Isodon henryi. Molecules 2019, 24, 2736. https://doi.org/10.3390/molecules24152736
Liu Y-L, Zhang L-X, Wu H, Chen S-Q, Li J, Dai L-P, Wang Z-M. Four New ent-Kaurane Diterpene Glycosides from Isodon henryi. Molecules. 2019; 24(15):2736. https://doi.org/10.3390/molecules24152736
Chicago/Turabian StyleLiu, Ya-Lin, Ling-Xia Zhang, Hong Wu, Sui-Qing Chen, Jun Li, Li-Ping Dai, and Zhi-Min Wang. 2019. "Four New ent-Kaurane Diterpene Glycosides from Isodon henryi" Molecules 24, no. 15: 2736. https://doi.org/10.3390/molecules24152736