Catalysis of Organic Pollutants Abatement Based on Pt-Decorated Ag@Cu2O Heterostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ag@Cu2O–Pt Nanocomposites
2.3. Catalytic Reduction
2.4. Characterization
3. Discussion
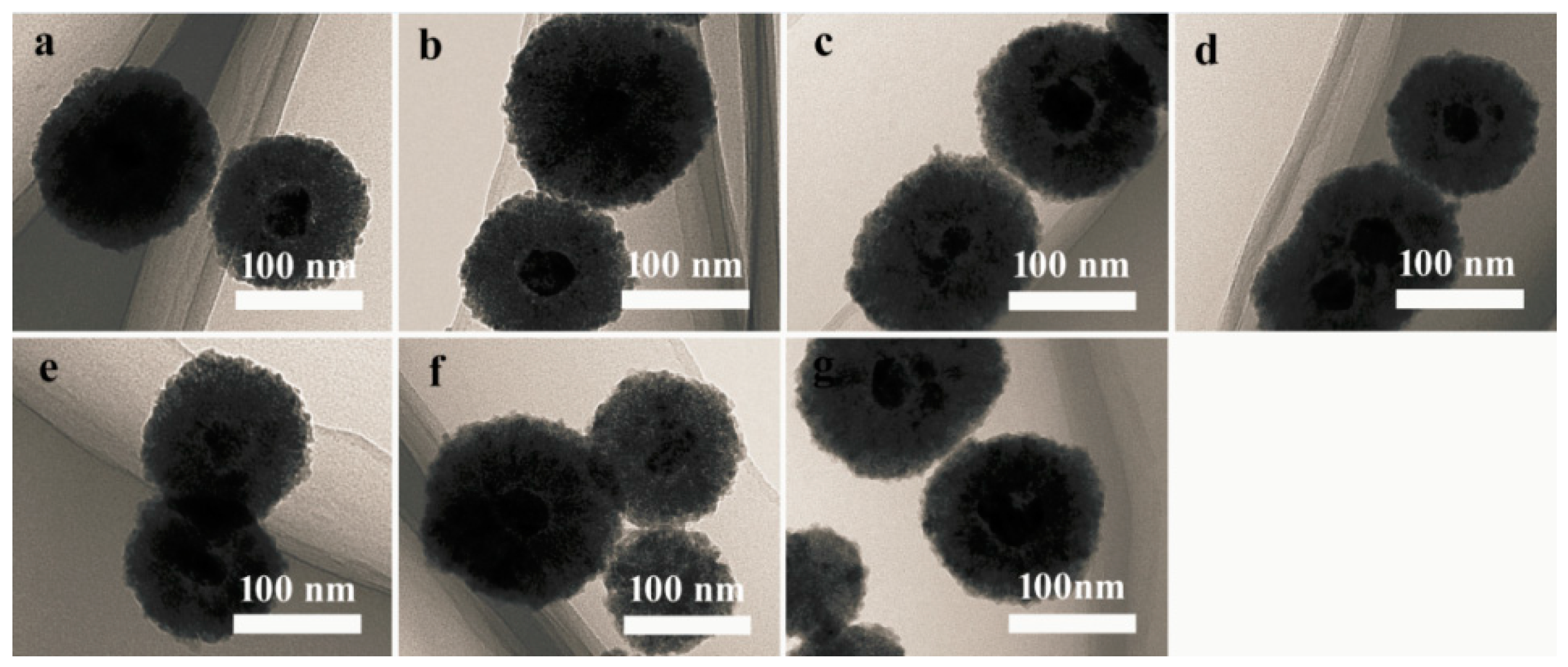
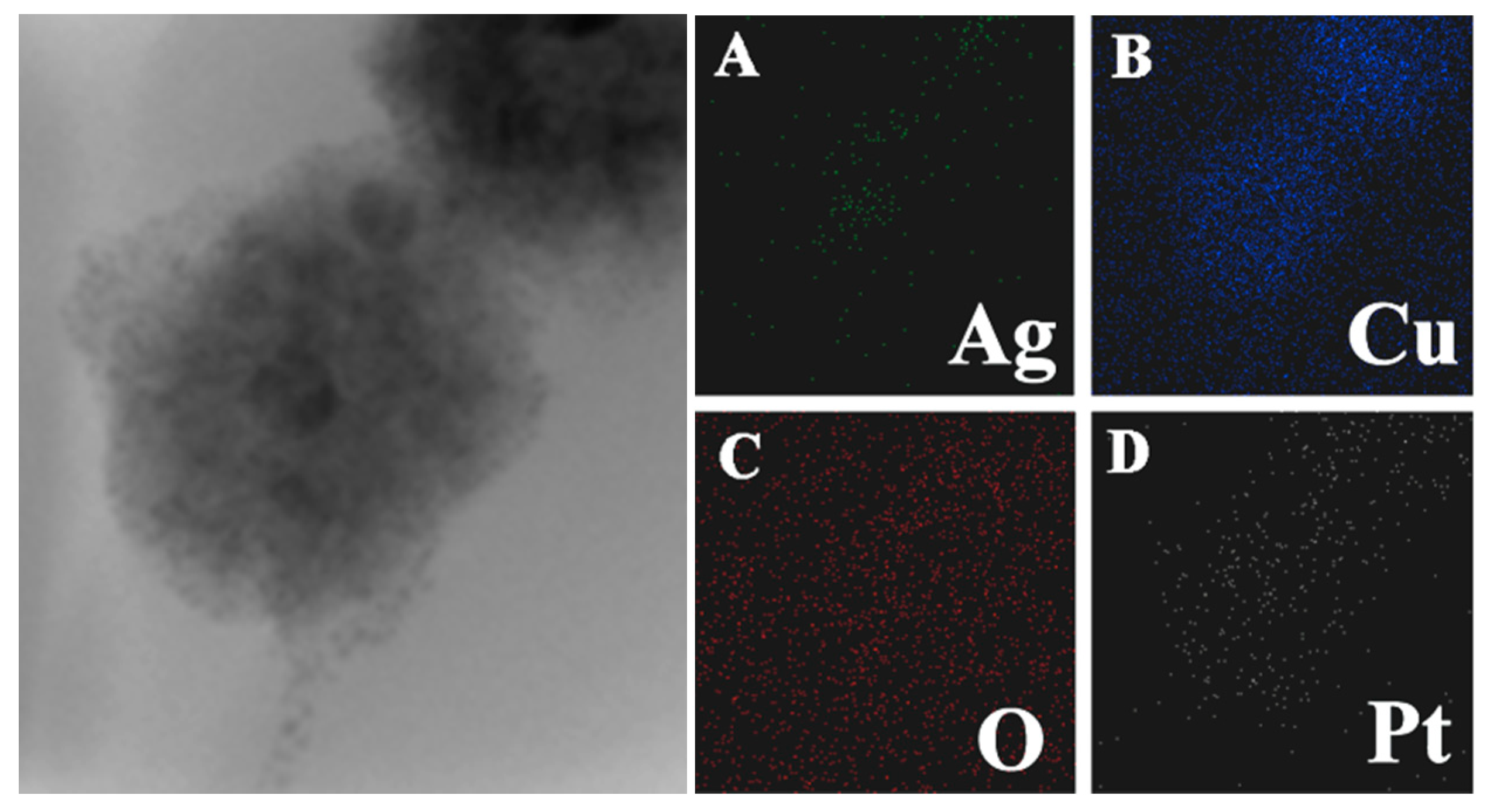
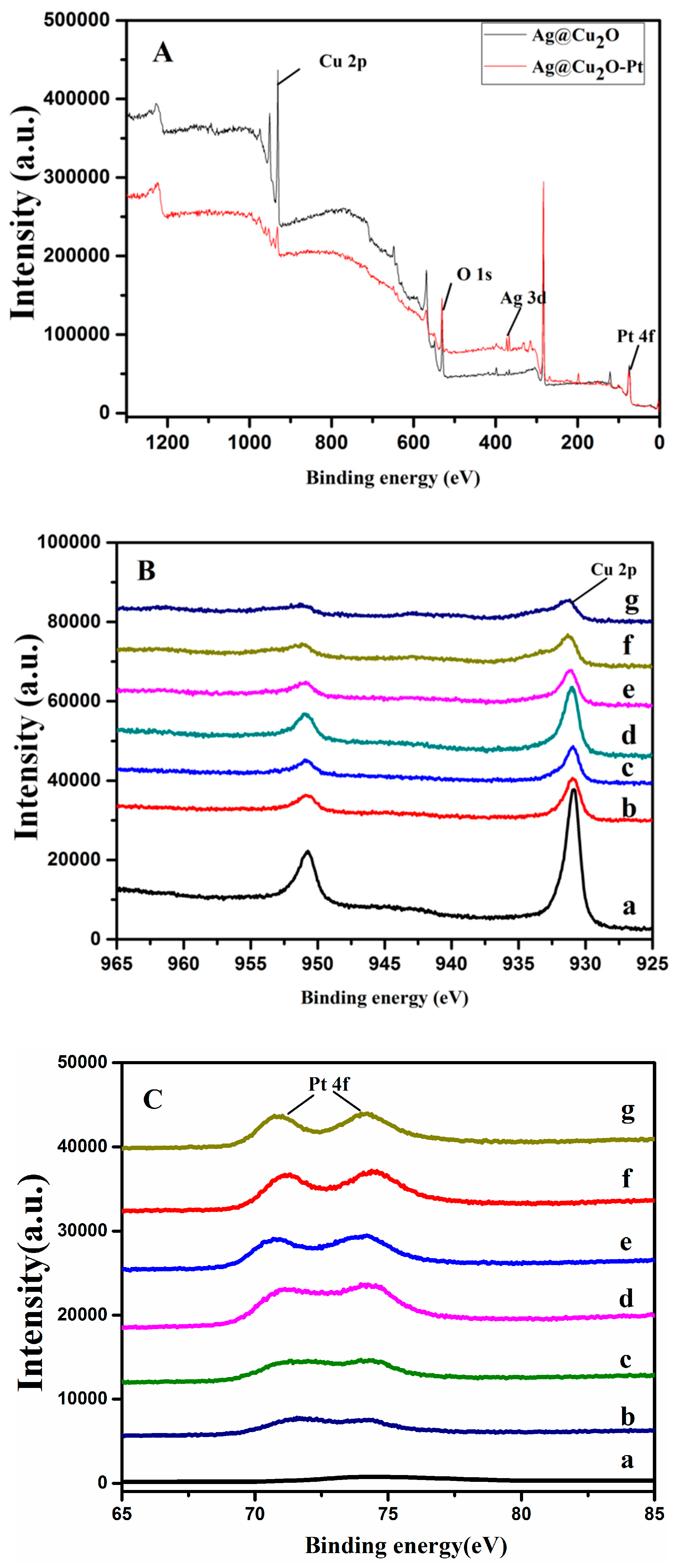
3.1. Properties of the Ag@Cu2O–Pt Heterostructure Nanocomposites
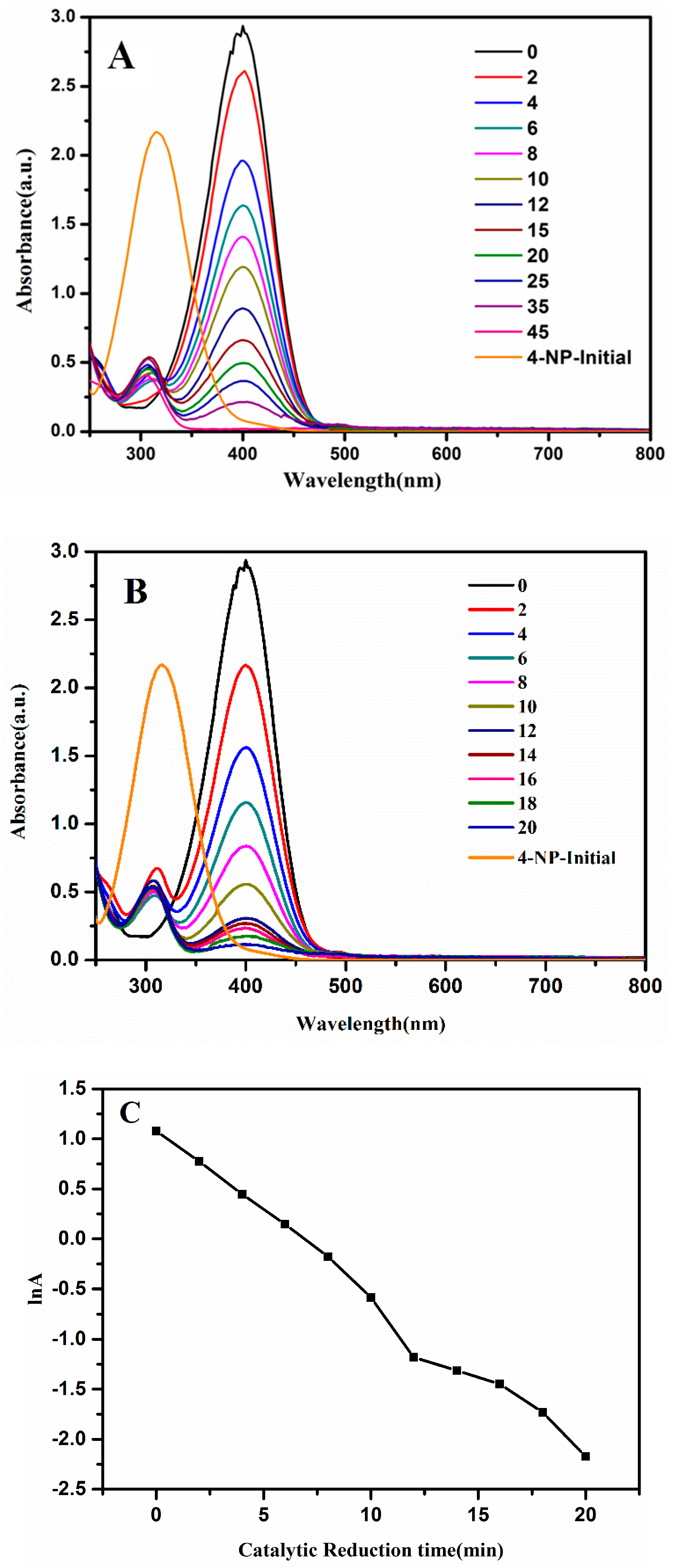
3.2. Catalytic Activity of the Ag@Cu2O–Pt Heterostructure
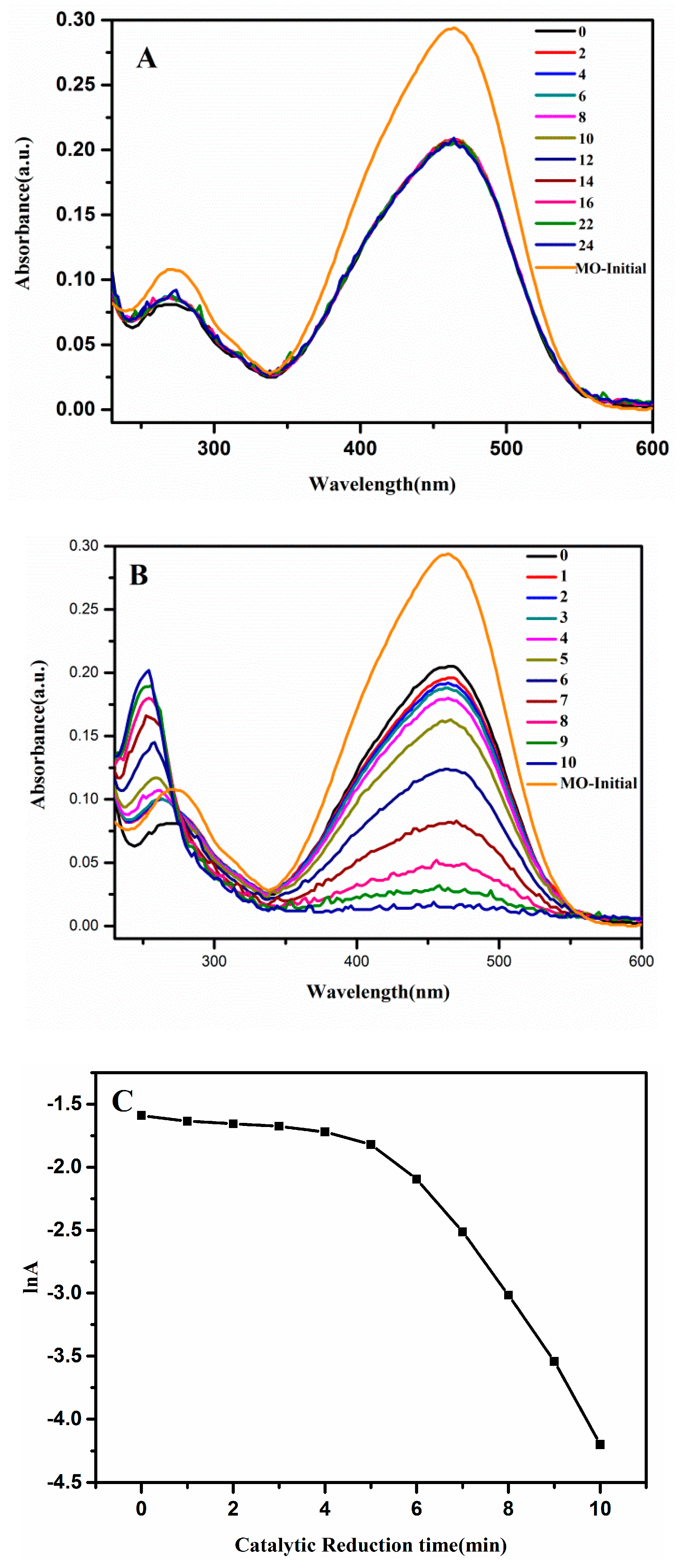
3.3. Catalytic Degradation of Organic Dyes (MO)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today. 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, Q.Q.; Sun, X.H. Nanogenerators for self-powered gas sensing. Nano-Micro Lett. 2017, 9, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yuan, L.; Liang, G.Z.; Gu, A.J. Aramid fibre-based wearable electrochemical capacitors with high energy density and mechanical properties through chemical synergistic combination of multi-coatings. Electrochim. Acta. 2018, 284, 149–158. [Google Scholar] [CrossRef]
- Belushkin, A.; Yesilköy, F.; Altug, H. Nanoparticle enhanced plasmonic biosensor for digital biomarker detection in a microarray. ACS Nano. 2018, 12, 4453–4461. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.Z.; Moehl, T.; Cui, W.; Wick-Joliat, R.; Zhu, L.P.; Tilley, S.D. Extended light harvesting with dual Cu2O-based photocathodes for high efficiency water splitting. Adv. Energy Mater. 2017, 8, 1702323–1702331. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Ma, X.W.; Jin, J.; Ji, W.; Wang, X.; Song, W.; Zhao, B.; He, C.Y. Fabrication of Ag-Cu2O/reduced graphene oxide nanocomposites as SERS substrates for in situ monitoring of peroxidase-like catalytic reaction and biosensing. ACS Appl. Mater. Inter. 2017, 9, 19074–19081. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.T.; Wang, B.Y.; Zhang, H.; Ding, P.; Wang, Q. Sandwiched ZnO@Au@Cu2O nanorod films as efficient visible-light-driven plasmonic photocatalysts. ACS Appl. Mater. Inter. 2015, 7, 4066–4074. [Google Scholar] [CrossRef]
- Chen, X.B.; Shen, S.H.; Guo, L.J.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Qu, Y.Q.; Duan, X.F. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef]
- Kuo, M.Y.; Hsiao, C.F.; Chiu, Y.H.; Lai, T.H.; Fang, M.J.; Wu, J.Y.; Chen, J.W.; Wu, C.L.; Wei, K.H.; Lin, H.C.; et al. Au@Cu2O core@shell nanocrystals as dual-functional catalysts for sustainable environmental applications. Appl. Catal. B Environ. 2019, 242, 499–506. [Google Scholar] [CrossRef]
- Chopra, R.; Kumar, M.; Bhalla, V. Development of a supramolecular ensemble of an AIEE active hexaphenyl benzene derivative and Ag@Cu2O core-shell NPs: An efficient photocatalytic system for C-H activation. Chem. Commun. 2016, 52, 10179–10182. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Páeza, C.A.; Navíoa, J.A.; Hidalgoa, M.C.; Macíasa, M. ZnO and Pt-ZnO photocatalysts: Characterization and photocatalytic activity assessing by means of three substrates. Catal. Today. 2018, 313, 12–19. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, W.; Chen, B.Y.; Hong, J.M. Deciphering acetaminophen electrical catalytic degradation using single-form S doped graphene/Pt/TiO2. Chem. Eng. J. 2018, 343, 662–675. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Mohammad, A.M.; El-Deab, M.S.; El-Anadouli, B.E. Propitious Dendritic Cu2O-Pt Nanostructured anodes for direct formic acid fuel cells. ACS Appl. Mater. Interfaces. 2017, 9, 19766–19772. [Google Scholar] [CrossRef] [PubMed]
- Manning, H.G.; Biswas, S.; Holmes, J.D.; Boland, J.J. Nonpolar Resistive Switching in Ag@TiO2 core-shell nanowires. ACS Appl. Mater. Interfaces. 2017, 9, 38959–38966. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S.M.; Naika, G.K.; Leea, H.J.; Song, H.G.; Leea, C.R.; Leec, I.H.; Yu, Y.T. Au@NiO core-shell nanoparticles as a p-type gas sensor: Novel synthesis, characterization, and their gas sensing properties with sensing mechanism. Sens. Actuators B Chem. 2018, 268, 223–231. [Google Scholar] [CrossRef]
- Liu, J.; Feng, J.W.; Gui, J.; Chen, T.; Xu, M.; Wang, H.Z.; Dong, H.F.; Chen, H.L.; Li, X.W.; Wang, L.; et al. Metal@semiconductor core-shell nanocrystals with atomically organized interfaces for efficient hot electron-mediated photocatalysis. Nano Energy 2018, 48, 44–52. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.Y.; Song, X.Y.; Yin, Z.L.; Bu, Y.X. Construction of reduced graphene oxide-supported Ag-Cu2O composites with hierarchical structures for enhanced photocatalytic activities and recyclability. ACS Appl. Mater. Interfaces 2015, 3, 5923–5933. [Google Scholar] [CrossRef]
- Cai, R.Q.; Zhang, B.G.; Shi, J.X.; Li, M.; Zhen, H. Photocatalytic decolorization of methyl orange under visible light using VS4/carbon powder nanocomposites. ACS Sustainable Chem. Eng. 2017, 5, 7690–7699. [Google Scholar] [CrossRef]
- Sasmal, A.K.; Pal, J.; Sahoo, R.; Kartikeya, P.; Dutta, S.; Pal, T. Superb DyeAdsorption and dye-sensitized change in Cu2O−Ag crystal faces in the dark. J. Phys. Chem. C. 2016, 120, 21580–21588. [Google Scholar] [CrossRef]
- Niu, H.Y.; Zheng, Y.; Wang, S.H.; Zhao, L.X.; Yang, S.P.; Cai, Y.Q. Continuous generation of hydroxyl radicals for highly efficient elimination of chlorophenols and phenols catalyzed by heterogeneous Fenton-like catalysts yolk/shell Pd@Fe3O4@metal organic frameworks. J. Hazard. Mater. 2018, 346, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Niu, R.; Zhang, Y. Ag-Au bimetallic nanocomposites stabilized with organic-inorganic hybrid microgels: Synthesis and their regulated optical and catalytic properties. RSC Adv. 2018, 8, 12428–12438. [Google Scholar] [CrossRef]
- Lee, C.; Shin, K.; Lee, Y.J.; Jung, C.; Lee, H.M. Effects of shell thickness on Ag-Cu2O core-shell nanoparticles with bumpy structures for enhancing photocatalytic activity and stability. Catal. Today. 2018, 303, 313–319. [Google Scholar] [CrossRef]
- Chen, J.C.; Xue, Z.T.; Feng, S.S.; Tu, B.; Zhao, D.Y. Synthesis of mesoporous silica hollow nanospheres with multiple gold cores and catalytic activity. J. Colloid Interface Sci. 2014, 429, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, Y.X.; Zhang, F.; Gao, R.X.; Liu, M.M.; Dong, L.Y.; Liu, Y.; Zhang, Y.J.; Chen, L. In situ synthesis of Ag@Cu2O-rGO architecture for strong light-matter interactions. Nanomaterials. 2018, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.H.; Bing, Q.M.; Qi, H.; Liu, J.Y.; Bai, T.Y.; Li, G.H.; Shi, Z.; Feng, S.H.; Xu, R.R. Rational design and functionalization of a Zn MOF for highly selective detection of TNP. ACS Appl. Mater. Interfaces. 2017, 9, 23828–23835. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, H.H.; Zhao, Y.; Zhang, Y.J.; Wang, Y.X.; Liu, Y.; Zhang, X.L.; Jiang, Y.H.; Hua, Z.; Yang, J.H. Plasmonic-induced SERS enhancement of shell dependent Ag@Cu2O core-shell nanoparticles. RSC Adv. 2017, 7, 16553–16560. [Google Scholar] [CrossRef]
- Chen, L.; Liu, M.M.; Zhao, Y.; Kou, Q.W.; Wang, Y.X.; Liu, Y.; Zhang, Y.J.; Yang, J.H.; Jung, Y.M. Enhanced catalyst activity by decorating of Au on Ag@Cu2O nanoshell. Appl. Surf. Sci. 2018, 435, 72–78. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, D.G.; Sun, H.Q.; Liang, P.; Duan, X.G.; Tian, W.J.; Tade, M.O.; Liu, S.M.; Wang, S.B. Oxygen vacancies in shape controlled Cu2O/reduced graphene oxide/In2O3 hybrid for promoted photocatalytic water oxidation and degradation of environmental pollutants. ACS Appl. Mater. Interfaces 2017, 9, 11678–11688. [Google Scholar] [CrossRef]
- Li, J.H.; Jiang, J.B.; Xu, Z.F.; Liu, M.Q.; Tang, S.P.; Yang, C.M.; Qian, D. Facile synthesis of Ag@Cu2O heterogeneous nanocrystals decorated N-doped reduced graphene oxide with enhanced electrocatalytic activity for ultrasensitive detection of H2O2. Sens. Actuat. B. 2018, 260, 529–540. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Han, B.; Wang, Y.; Liu, Y.; Chen, L.; Zhang, Y. Catalysis of Organic Pollutants Abatement Based on Pt-Decorated Ag@Cu2O Heterostructures. Molecules 2019, 24, 2721. https://doi.org/10.3390/molecules24152721
Zhang X, Han B, Wang Y, Liu Y, Chen L, Zhang Y. Catalysis of Organic Pollutants Abatement Based on Pt-Decorated Ag@Cu2O Heterostructures. Molecules. 2019; 24(15):2721. https://doi.org/10.3390/molecules24152721
Chicago/Turabian StyleZhang, Xiaolong, Bingbing Han, Yaxin Wang, Yang Liu, Lei Chen, and Yongjun Zhang. 2019. "Catalysis of Organic Pollutants Abatement Based on Pt-Decorated Ag@Cu2O Heterostructures" Molecules 24, no. 15: 2721. https://doi.org/10.3390/molecules24152721
APA StyleZhang, X., Han, B., Wang, Y., Liu, Y., Chen, L., & Zhang, Y. (2019). Catalysis of Organic Pollutants Abatement Based on Pt-Decorated Ag@Cu2O Heterostructures. Molecules, 24(15), 2721. https://doi.org/10.3390/molecules24152721




