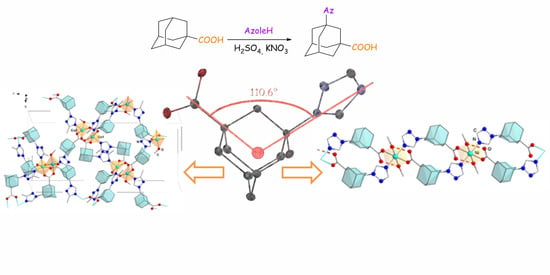
Facile Synthesis of 3-(Azol-1-yl)-1-adamantanecarboxylic Acids—New Bifunctional Angle-Shaped Building Blocks for Coordination Polymers
Abstract
:1. Introduction
2. Results and Discussion
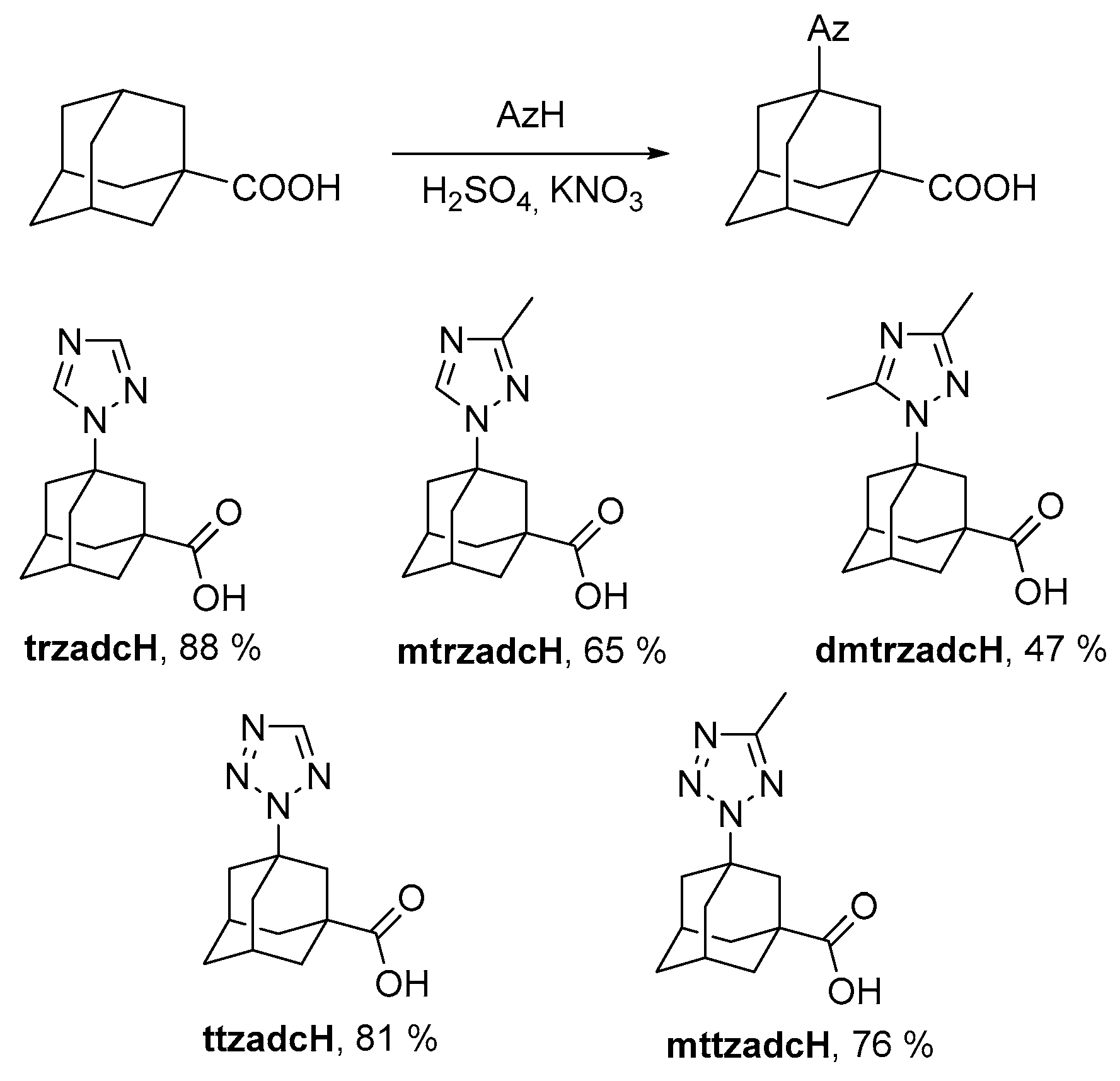
2.1. Symthetic Approach
2.2. Synthesis and IR Spectra of Coordination Polymers
2.3. Crystal Structure of 3-(1,2,4-Triazol-1-yl)-1-adamantanecarboxylic Acid
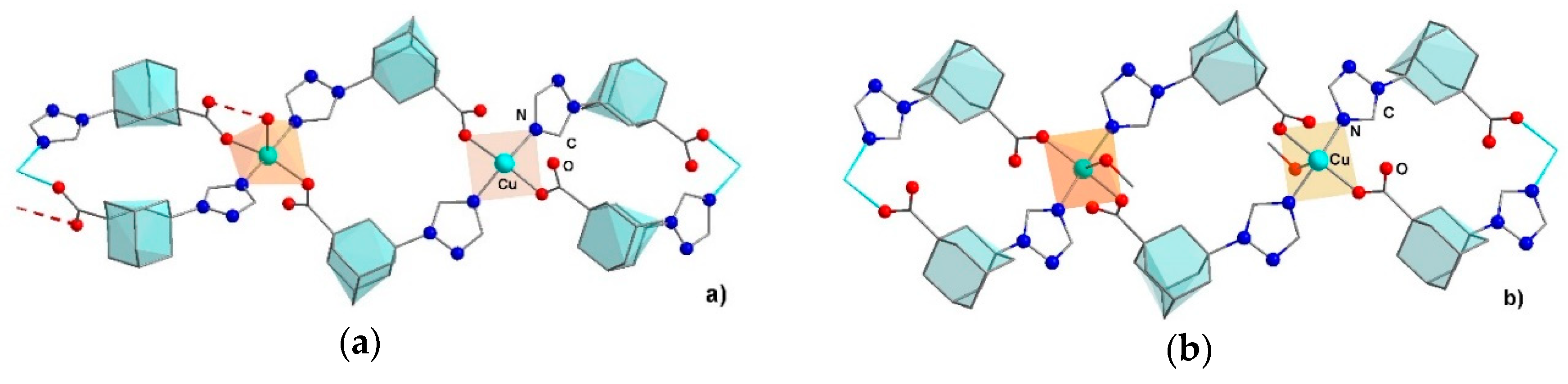
2.4. Crystal Structures of Coordination Polymers
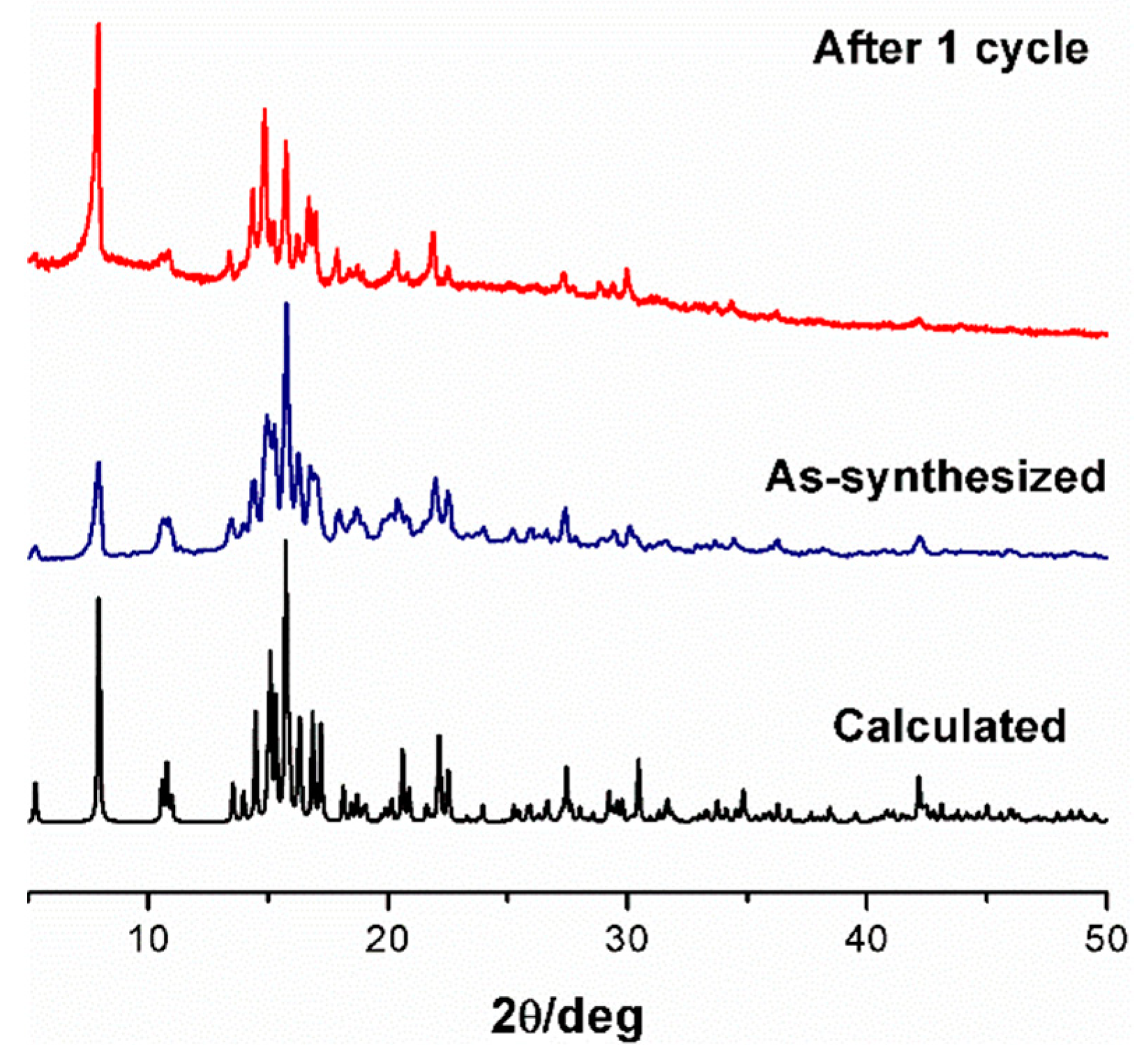
2.5. Powder X-Ray Diffraction and Thermal Studies
2.6. Catalytic Activity Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. X-ray Structure Determination
3.2.2. Ligand Synthesis
3.2.3. Preparation of Coordination Compounds
3.2.4. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Senchyk, G.A.; Lysenko, A.B.; Boldog, I.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K.V. 1,2,4-Triazole functionalized adamantanes: A new library of polydentate tectons for designing structures of coordination polymers. Dalt. Trans. 2012, 41, 8675. [Google Scholar] [CrossRef] [PubMed]
- Ermer, O. Fivefold-Diamond Structure of Adamantane-1,3,5,7-tetracarboxylic Acid. J. Am. Chem. Soc. 1988, 110, 3747–3754. [Google Scholar]
- Senchyk, G.A.; Lysenko, A.B.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K. V Polynuclear and polymeric metal complexes based upon 1,2,4-triazolyl functionalized adamantanes. Inorganica Chim. Acta 2009, 362, 4439–4448. [Google Scholar] [CrossRef]
- Chen, B.; Eddaoudi, M.; Reineke, T.M.; Kampf, J.W.; O’Keeffe, M.; Yaghi, O.M. Cu2(ATC)·6H2O: Design of open metal sites in porous metal-organic crystals (ATC: 1,3,5,7-Adamantane Tetracarboxylate). J. Am. Chem. Soc. 2000, 122, 11559–11560. [Google Scholar]
- Nasrallah, H.; Hierso, J.-C. Porous Materials Based on 3-Dimensional Td-Directing Functionalized Adamantane Scaffolds and Applied as Recyclable Catalysts. Chem. Mater. 2019, 31, 619–642. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Jaber, A.M.; AlDamen, M.A.; Al-Qawasmeh, R.A.; Khanfar, M.A.; Jaber, A.M.; AlDamen, M.A.; Al-Qawasmeh, R.A. Synthesis, Characterization, Crystal Structure, and DFT Study of a New Square Planar Cu(II) Complex Containing Bulky Adamantane Ligand. Molecules 2018, 23, 701. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Rusanov, E.B.; Chernega, A.N.; Jezierska, J.; Domasevitch, K.V.; Ozarowski, A. Structure and magnetic behavior of CuII MOFs supported by 1,2,4-triazolyl-bifunctionalized adamantane scaffold. Eur. J. Inorg. Chem. 2012, 5802–5813. [Google Scholar]
- Shimizu, G.K.H.; Vaidhyanathan, R.; Taylor, J.M. Phosphonate and sulfonate metal organic frameworks. Chem. Soc. Rev. 2009, 38, 1430–1449. [Google Scholar] [CrossRef]
- Mocanu, T.; Pop, L.; Hadade, N.D.; Shova, S.; Grosu, I.; Andruh, M. Coordination polymers constructed from tetrahedral-shaped adamantane tectons. CrystEngComm 2017, 19, 27–31. [Google Scholar]
- Yang, J.; Ma, J.-F.; Liu, Y.-Y.; Li, S.-L.; Zheng, G.-L. Four Novel 3D Copper(II) Coordination Polymers with Different Topologies. Eur. J. Inorg. Chem. 2005, 2005, 2174–2180. [Google Scholar]
- Hoffart, D.J.; Côté, A.P.; Shimizu, G.K.H. An Adamantane-Based Coordination Framework with the First Observation of Discrete Metal Sulfonate Clusters. Inorg. Chem. 2003, 42, 8603–8605. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Y.-N.; Shi, W.-J.; Liu, B.; Wang, Y.-Y.; Shi, Q.-Z. Structural diversity of coordination polymers assembled from adamantane dicarboxylates and conformational bis-triazole ligand. CrystEngComm 2011, 13, 5179. [Google Scholar] [CrossRef]
- Travis, J.Z.; Martinez, B.L.; LaDuca, R.L. Structurally Diverse Divalent Metal Adamantanedicarboxylate Coordination Polymers with Hydrogen-bonding Capable Dipyridyl Pillaring Ligands. Zeitschrift für Anorg. und Allg. Chemie 2018, 644, 33–42. [Google Scholar] [CrossRef]
- Travis, J.Z.; Pumford, S.R.; Martinez, B.L.; LaDuca, R.L. Nickel adamantanedicarboxylate and adamantanediacetate 2D and 3D coordination polymers with hydrogen-bonding capable dipyridyl ligands. Polyhedron 2018, 142, 25–37. [Google Scholar] [CrossRef]
- Qiu, L.; Yu, C.; Wang, X.; Xie, Y.; Kirillov, A.M.; Huang, W.; Li, J.; Gao, P.; Wu, T.; Gu, X.; et al. Tuning the Solid-State White Light Emission of Postsynthetic Lanthanide-Encapsulated Double-Layer MOFs for Three-Color Luminescent Thermometry Applications. Inorg. Chem. 2019, 58, 4524–4533. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Krautscheid, H.; Rusanov, E.B.; Chernega, A.N.; Krämer, K.W.; Liu, S.X.; Decurtins, S.; Domasevitch, K.V. Functionalized adamantane tectons used in the design of mixed-ligand copper(II) 1,2,4-triazolyl/carboxylate metal-organic frameworks. Inorg. Chem. 2013, 52, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, C.J.; Kryschenko, Y.K.; Radhakrishnan, U.; Seidel, S.R.; Huang, S.D.; Stang, P.J. Self-assembly of nanoscopic coordination cages of D(3h) symmetry. Proc. Natl. Acad. Sci. USA 2002, 99, 4932–4936. [Google Scholar] [CrossRef]
- Marchenko, R.; Potapov, A. 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molbank 2017, 2017, M968. [Google Scholar] [CrossRef]
- Fujimoto, H.; Kitagawa, Y.; Hao, H.; Fukui, K. Chemical Reactivity of Adamantane and Related Compounds. Bull. Chem. Soc. Jpn. 2006, 43, 52–56. [Google Scholar] [CrossRef]
- Fort, R.C.; Von Schleyer, P.R. Adamantane: Consequences of the diamondoid structure. Chem. Rev. 1964, 64, 277–300. [Google Scholar] [CrossRef]
- SMITH, G.W.; WILLIAMS, H.D. Some Reactions of Adamantane and Adamantane Derivatives. J. Org. Chem. 1961, 26, 2207–2212. [Google Scholar] [CrossRef]
- Tsypin, V.G.; Kachala, V.V.; Ugrak, B.I.; Golod, E.L. Adamantylation of indazole and its C-nitro derivatives. Russ. J. Org. Chem. 2002, 38, 90–94. [Google Scholar] [CrossRef]
- Amandurdyev, A.D.; Saraev, V.V.; Polyakova, I.N.; Golod, E.L. Adamantylazoles: IX. Acid-catalyzed Adamantylation of 1,2,4-Triazol-3-thione. Russ. J. Gen. Chem. 2005, 75, 130–133. [Google Scholar] [CrossRef]
- Saraev, V.V.; Kanakina, T.P.; Pevzner, M.S.; Golod, E.L.; Ugrak, B.I.; Kachala, V.V. Adamantylazoles. Acid-catalyzed adamantylation of 1,2,4-triazoles. Chem. Heterocycl. Compd. 1996, 32, 928–936. [Google Scholar] [CrossRef]
- Raenko, G.F.; Korotkikh, N.I.; Pekhtereva, T.M.; Shvaika, O.P. Adamantylation of Imidazoles and Benzimidazole. Russ. J. Org. Chem. 2001, 37, 1153–1157. [Google Scholar] [CrossRef]
- Gavrilov, A.S.; Golod, E.L.; Kachala, V.V.; Ugrak, B.I. Adamantylazoles: IV. Acid-Catalyzed Adamantylation of Pyrazoles. Russ. J. Org. Chem. 2001, 37, 1741–1746. [Google Scholar] [CrossRef]
- Terekhova, M.I.; Petrov, E.S.; Rokhlina, E.M.; Kravtsov, D.N.; Shatenshtein, A.I. Equilibrium NH acidity of nitrogen heterocycles. Chem. Heterocycl. Compd. 1979, 15, 904–908. [Google Scholar] [CrossRef]
- Nakamoto, K.; Fujita, J.; Tanaka, S.; Kobayashi, M. Infrared Spectra of Metallic Complexes. IV. Comparison of the Infrared Spectra of Unidentate and Bidentate Metallic Complexes. J. Am. Chem. Soc. 1957, 79, 4904–4908. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. In Handbook of Vibrational Spectroscopy; Griffiths, P.R., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2006. [Google Scholar]
- Aijaz, A.; Lama, P.; Sañudo, E.C.; Mishra, R.; Bharadwaj, P.K. Coordination polymers of various architectures built with mixed imidazole/benzimidazole and carboxylate donor ligands and different metal ions: Syntheses, structural features and magnetic properties. New J. Chem. 2010, 34, 2502. [Google Scholar] [CrossRef]
- Ben Kiran, A.; Mocanu, T.; Pöllnitz, A.; Shova, S.; Andruh, M.; Silvestru, C. Triphenylbismuth(v) di[(iso)nicotinates]-transmetallation agents or divergent organometalloligands? First organobismuth(v)-based silver(i) coordination polymers. Dalt. Trans. 2018, 47, 2531–2542. [Google Scholar] [CrossRef]
- Wang, D.-W.; Wang, T.; Du, L.; Zhou, J.; Yan, T.; Zhao, Q.-H. Four supramolecular transition metal(II) complexes based on triazole-benzoic acid derivatives: Crystal structure, Hirshfeld surface analysis, and spectroscopic and thermal properties. Struct. Chem. 2018, 29, 1013–1023. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Thirumoorthi, R. Reactions of 3,5-Pyrazoledicarboxylic Acid with Organotin Chlorides and Oxides. Coordination Polymers Containing Organotin Macrocycles. Organometallics 2009, 28, 2096–2106. [Google Scholar] [CrossRef]
- Feng, C.; Ma, Y.-H.; Zhang, D.; Li, X.-J.; Zhao, H. Highly efficient electrochemiluminescence based on pyrazolecarboxylic metal organic framework. Dalt. Trans. 2016, 45, 5081–5091. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, B.; Wang, X.; Yang, R.; Li, W. Synthesis, Crystal Structure and Magnetic Property of a New Three-Dimensional Mn–Na Heteronuclear Coordination Complex Based on 3,5-Pyrazoledicarboxylic Acid. J. Clust. Sci. 2016, 27, 1253–1261. [Google Scholar] [CrossRef]
- Kivi, C.E.; Gelfand, B.S.; Dureckova, H.; Ho, H.T.K.; Ma, C.; Shimizu, G.K.H.; Woo, T.K.; Song, D. 3D porous metal–organic framework for selective adsorption of methane over dinitrogen under ambient pressure. Chem. Commun. 2018, 54, 14104–14107. [Google Scholar] [CrossRef]
- Tu, B.; Pang, Q.; Xu, H.; Li, X.; Wang, Y.; Ma, Z.; Weng, L.; Li, Q. Reversible Redox Activity in Multicomponent Metal–Organic Frameworks Constructed from Trinuclear Copper Pyrazolate Building Blocks. J. Am. Chem. Soc. 2017, 139, 7998–8007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-H.; Chen, Q. A three-dimensional coordination polymer based on 1,2,3-triazole-4,5-dicarboxylic acid (H3 tda): {[Cd12(tda)8(H2O)11] · (H2O)6.25} n. Crystallogr. Reports 2017, 62, 238–241. [Google Scholar] [CrossRef]
- Leong, W.L.; Vittal, J.J. One-demensional coordination polymers: Complexity and diversity in structures, properties, and applications. Chem. Rev. 2011, 111, 688–764. [Google Scholar] [CrossRef] [PubMed]
- Loukopoulos, E.; Kostakis, G.E. Review: Recent advances of one-dimensional coordination polymers as catalysts. J. Coord. Chem. 2018, 71, 371–410. [Google Scholar] [CrossRef] [Green Version]
- Zu, W.; Liu, S.; Jia, X.; Xu, L. Chemoselective N -arylation of aminobenzene sulfonamides via copper catalysed Chan–Evans–Lam reactions. Org. Chem. Front. 2019, 6, 1356–1360. [Google Scholar] [CrossRef]
- Dar’in, D.; Krasavin, M. The Chan-Evans-Lam N-Arylation of 2-Imidazolines. J. Org. Chem. 2016, 81, 12514–12519. [Google Scholar] [CrossRef] [PubMed]
- Faridoon; Hussein, W.M.; Vella, P.; Islam, N.U.; Ollis, D.L.; Schenk, G.; McGeary, R.P. 3-Mercapto-1,2,4-triazoles and N-acylated thiosemicarbazides as metallo-β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, W.X.; Ye, B.H.; Lin, J.B.; Chen, X.M. In situ solvothermal generation of 1,2,4-triazolates and related compounds from organonitrile and hydrazine hydrate: A mechanism study. Inorg. Chem. 2007, 46, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Vereshchagina, T.N.; Lopyrev, V.A.; Pevzner, M.S.; Kogan, L.M. Synthesis of derivatives of 1, 2, 4-triazole-3, 5-dicarboxylic acid. Chem. Heterocycl. Compd. 1972, 5, 681–682. [Google Scholar] [CrossRef]
- APEX2 (Version 2.0); SAINT (Version 8.18c); SADABS (Version 2.11). Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2000–2012. [Google Scholar]
- Sheldrick, G.M. IUCr Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds reported are available from the authors. |
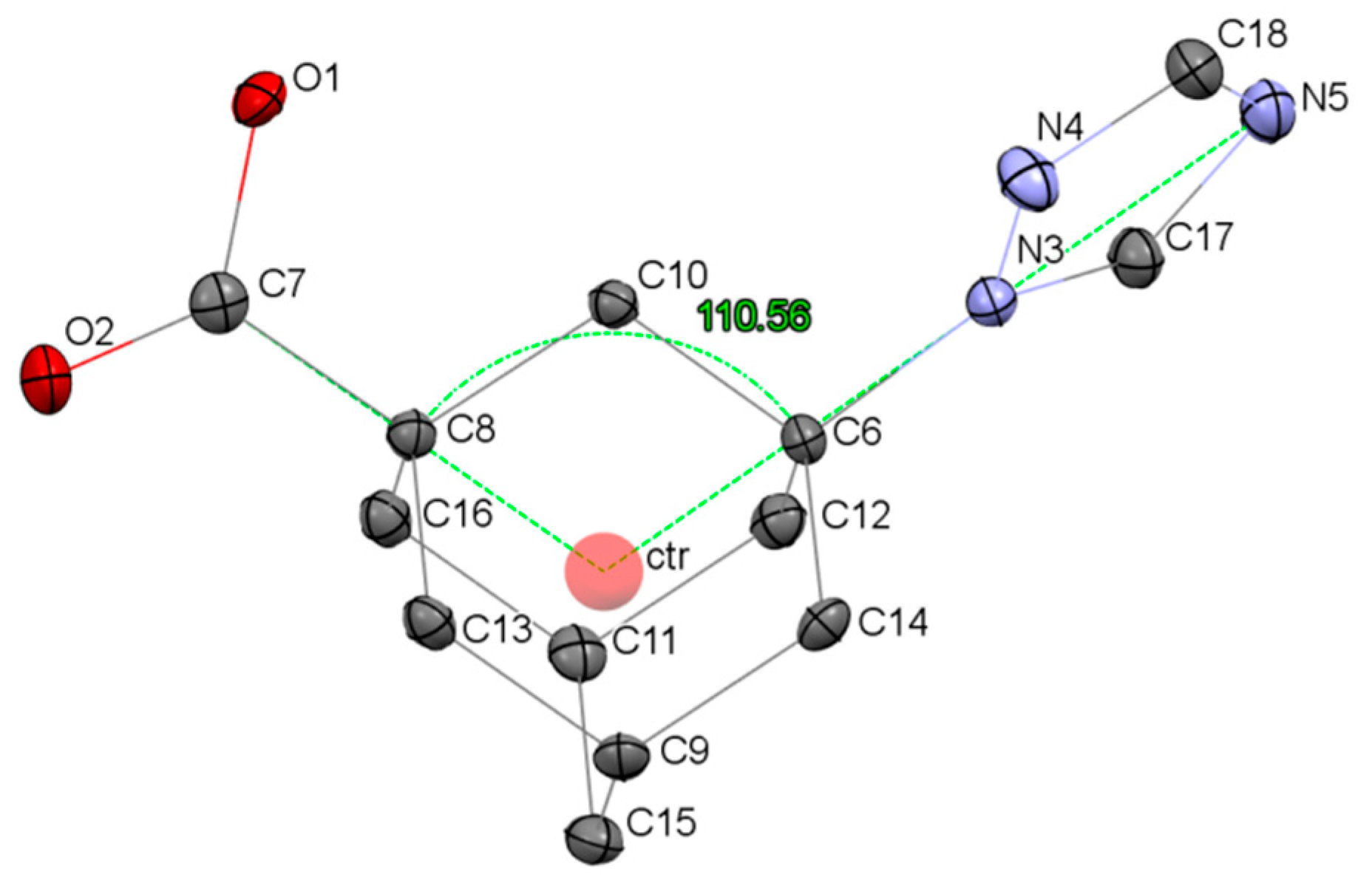


| Ligand | ϕCctrN, ° | CSD Code, Reference |
|---|---|---|
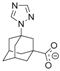 | 110.6 | this work |
 | 112.6 | NUYXAH [30] |
 | 119.4 | YEWFUE [31] |
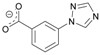 | 127.0 | CIJVUP [32] |
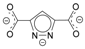 | 136.3 66.1 | BOZTAM [33] |
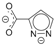 | 138.3 66.8 | LAGNOZ [34] |
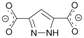 | 139.4 66.3 | ATAMEP [35] |
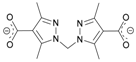 | 143.6 | RIQSOC [36] |
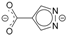 | 143.7 | JAZHOK [37] |
 | 144.8 65.5 | YEDBOB [38] |
| Entry | Solvent | Result |
|---|---|---|
| 1 | Methanol | 99% conversion |
| 2 | Methanol a | 60% conversion |
| 3 | Methanol b | 92% conversion |
| 4 | DMSO | - |
| 5 | Water | - |
| 6 | Dichloromethane | 13% conversion |
| 7 | Ethanol | 15% conversion |
| 8 | Methanol c | - |
| 9 | Methanol d | No reaction |
| Compound | trzadcH | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Empirical formula | C13H17N3O2 | C55H73Cu2N13O10 | C55H76Cu2N12O11 | C28H40N6NiO6 | C43H58Cu2N10O10 |
| Formula weight | 247.29 | 1203.34 | 1208.35 | 615.37 | 1002.07 |
| Space group | P21/n | P–1 | P–1 | P–1 | P21/c |
| a/Å | 11.1449(11) | 12.0509(5) | 6.6424(2) | 6.6392(5) | 10.1256(3) |
| b/Å | 6.7467(5) | 13.5356(5) | 12.0031(4) | 9.7528(7) | 14.5363(4) |
| c/Å | 15.3121(17) | 17.4158(8) | 17.8350(6) | 11.6021(8) | 30.4164(8) |
| α/° | 90 | 97.022(2) | 70.4510(10) | 66.060(2) | 90 |
| β/° | 100.142(10) | 108.9450(10) | 81.2370(10) | 81.403(2) | 95.9110(10) |
| γ/° | 90 | 95.3130(10) | 81.8090(10) | 74.214(2) | 90 |
| Volume/Å3 | 1133.35(19) | 2640.15(19) | 1317.96(7) | 660.07(8) | 4453.1(2) |
| Z | 4 | 2 | 1 | 1 | 4 |
| ρcalcg/cm3 | 1.449 | 1.514 | 1.522 | 1.548 | 1.495 |
| μ/mm−1 | 0.100 | 0.880 | 0.883 | 0.792 | 1.025 |
| F(000) | 528.0 | 1264.0 | 636.0 | 326.0 | 2096.0 |
| Crystal size/mm3 | 0.17 × 0.15 × 0.08 | 0.27 × 0.13 × 0.08 | 0.3 × 0.15 × 0.12 | 0.31 × 0.18 × 0.17 | 0.35 × 0.3 × 0.22 |
| 2Θ range for data collection/° | 6.52 to 62.93 | 3.61 to 52.746 | 3.66 to 57.612 | 3.844 to 57.49 | 3.108 to 57.488 |
| Index ranges | −14 ≤ h ≤ 12, −8 ≤ k ≤ 8, −19 ≤ l ≤ 19 | −15 ≤ h ≤ 15, −16 ≤ k ≤ 14, −21 ≤ l ≤ 21 | −8 ≤ h ≤ 8, −16 ≤ k ≤ 16, −24 ≤ l ≤ 24 | −8 ≤ h ≤ 8, −13 ≤ k ≤ 13, −15 ≤ l ≤ 15 | −13 ≤ h ≤ 13, −15 ≤ k ≤ 19, −38 ≤ l ≤ 41 |
| Reflections collected | 9394 | 30381 | 20394 | 12721 | 62483 |
| Independent reflections | 2601 [Rint = 0.0460, Rsigma = 0.0505] | 10753 [Rint = 0.0320, Rsigma = 0.0425] | 6840 [Rint = 0.0238, Rsigma = 0.0246] | 3415 [Rint = 0.0241, Rsigma = 0.0194] | 11544 [Rint = 0.0270, Rsigma = 0.0182] |
| parameters | 164 | 737 | 364 | 189 | 591 |
| Goodness-of-fit on F2 | 1.027 | 1.023 | 1.053 | 1.055 | 1.045 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0463, wR2 = 0.0923 | R1 = 0.0405, wR2 = 0.0971 | R1 = 0.0385, wR2 = 0.1029 | R1 = 0.0269, wR2 = 0.0732 | R1 = 0.0272, wR2 = 0.0704 |
| Final R indexes [all data] | R1 = 0.0733, wR2 = 0.1026 | R1 = 0.0558, wR2 = 0.1044 | R1 = 0.0427, wR2 = 0.1059 | R1 = 0.0280, wR2 = 0.0739 | R1 = 0.0312, wR2 = 0.0723 |
| Largest diff. peak/hole/e Å−3 | 0.34/−0.24 | 1.03/–0.53 | 1.51/–0.70 | 0.68/–0.46 | 0.48/–0.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlov, D.; Sukhikh, T.; Filatov, E.; Potapov, A. Facile Synthesis of 3-(Azol-1-yl)-1-adamantanecarboxylic Acids—New Bifunctional Angle-Shaped Building Blocks for Coordination Polymers. Molecules 2019, 24, 2717. https://doi.org/10.3390/molecules24152717
Pavlov D, Sukhikh T, Filatov E, Potapov A. Facile Synthesis of 3-(Azol-1-yl)-1-adamantanecarboxylic Acids—New Bifunctional Angle-Shaped Building Blocks for Coordination Polymers. Molecules. 2019; 24(15):2717. https://doi.org/10.3390/molecules24152717
Chicago/Turabian StylePavlov, Dmitry, Taisiya Sukhikh, Evgeny Filatov, and Andrei Potapov. 2019. "Facile Synthesis of 3-(Azol-1-yl)-1-adamantanecarboxylic Acids—New Bifunctional Angle-Shaped Building Blocks for Coordination Polymers" Molecules 24, no. 15: 2717. https://doi.org/10.3390/molecules24152717
APA StylePavlov, D., Sukhikh, T., Filatov, E., & Potapov, A. (2019). Facile Synthesis of 3-(Azol-1-yl)-1-adamantanecarboxylic Acids—New Bifunctional Angle-Shaped Building Blocks for Coordination Polymers. Molecules, 24(15), 2717. https://doi.org/10.3390/molecules24152717