Analysis of Proteins Associated with Quality Deterioration of Grouper Fillets Based on TMT Quantitative Proteomics during Refrigerated Storage
Abstract
:1. Introduction
2. Results and Discussion
2.1. Quality Change of Grouper Fillets
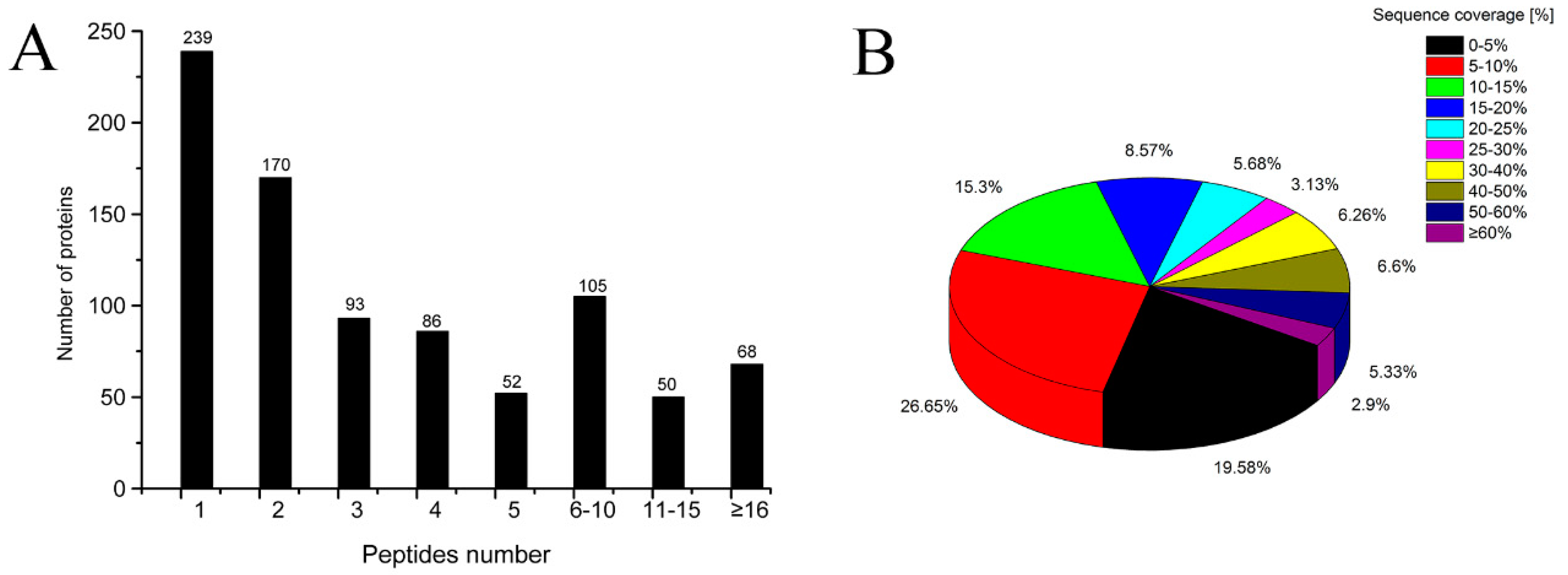
2.2. Identification of Proteins by Quantitative Proteomics Analysis
2.3. General Information for TMT-LC-MS/MS Analysis
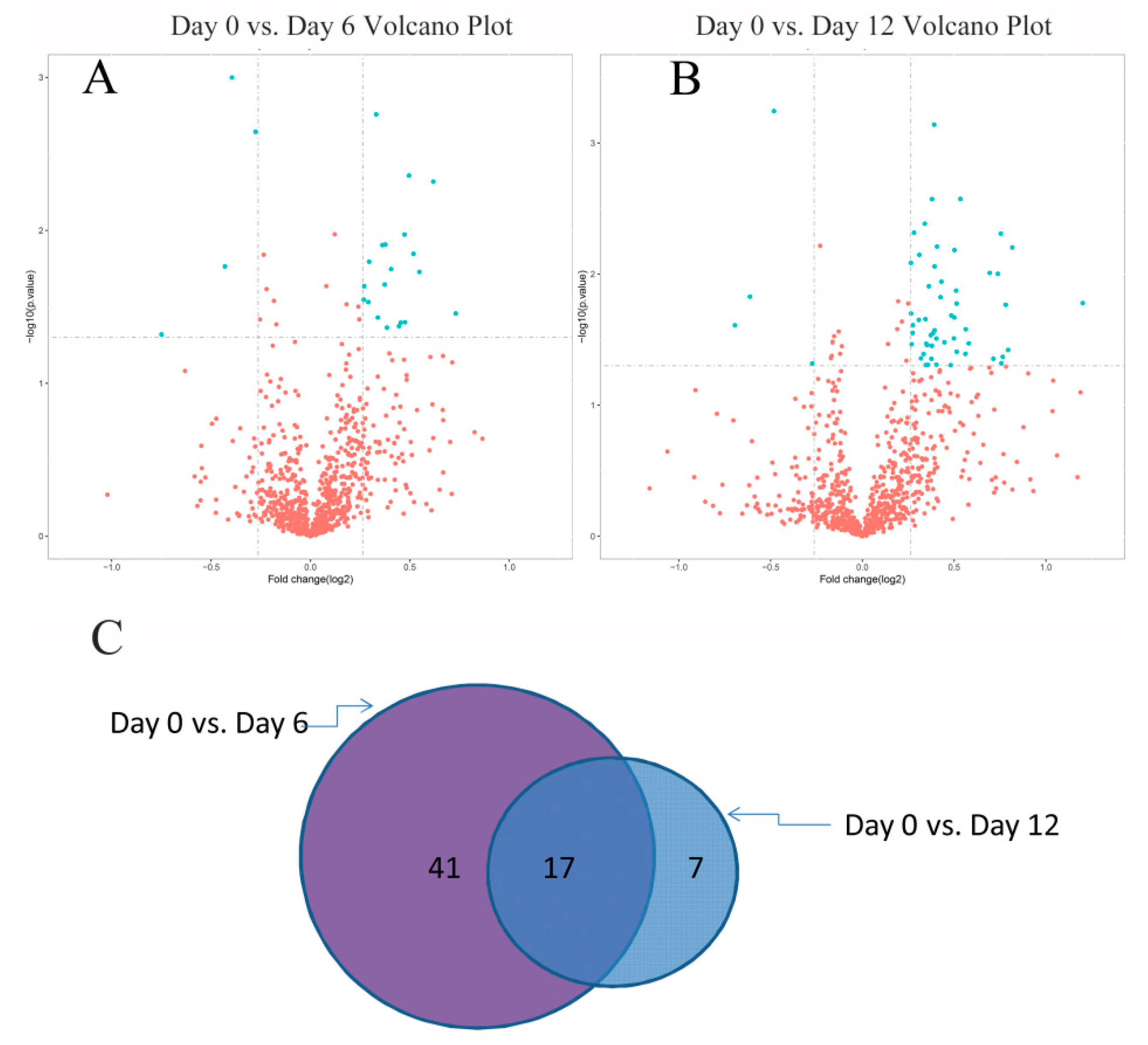
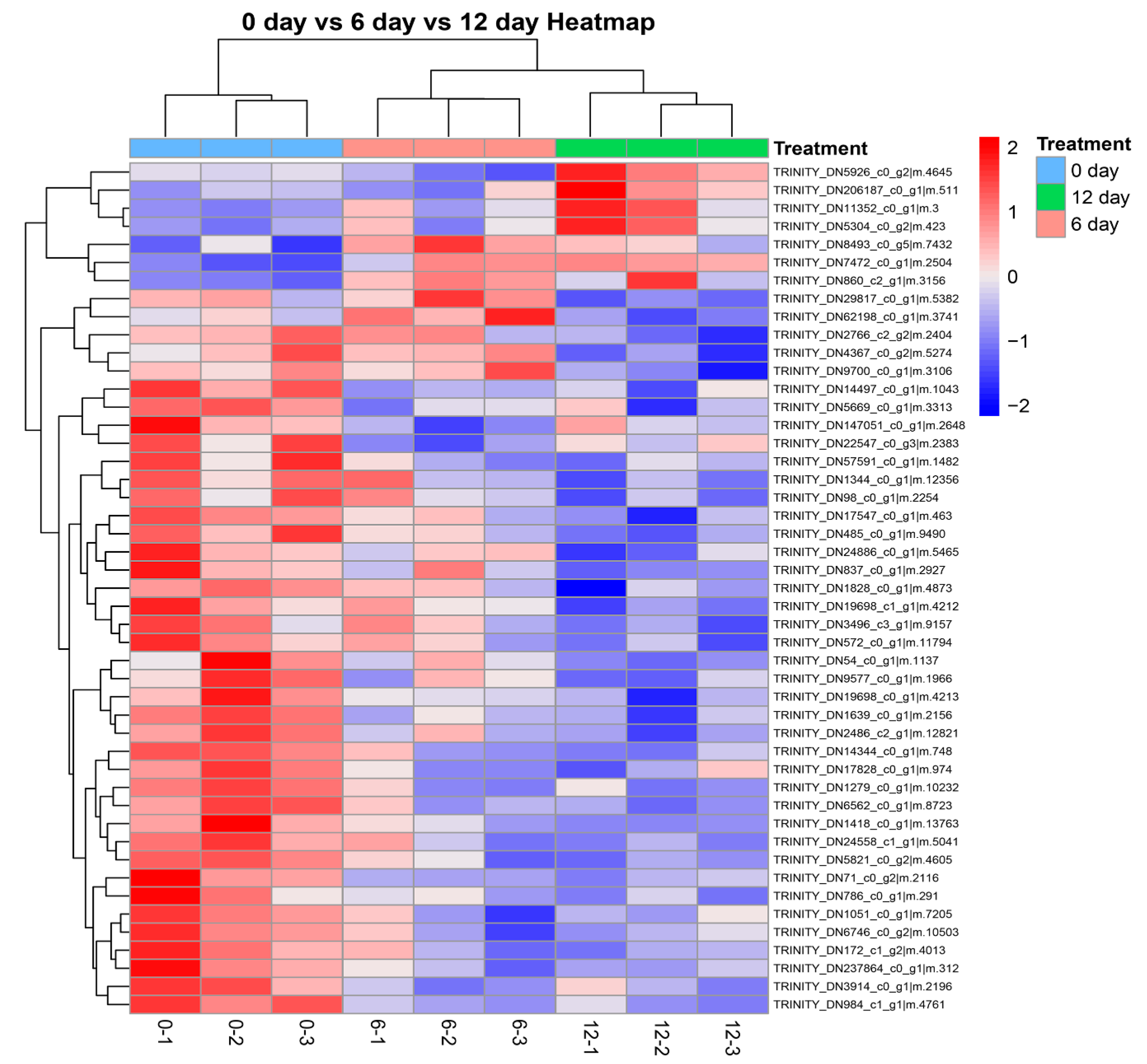
2.4. Identification of Differentially Significant Expressed Proteins (DSEPs)
2.5. Bioinformatic Analysis of Differentially Significant Expressed Proteins (DSEPs)
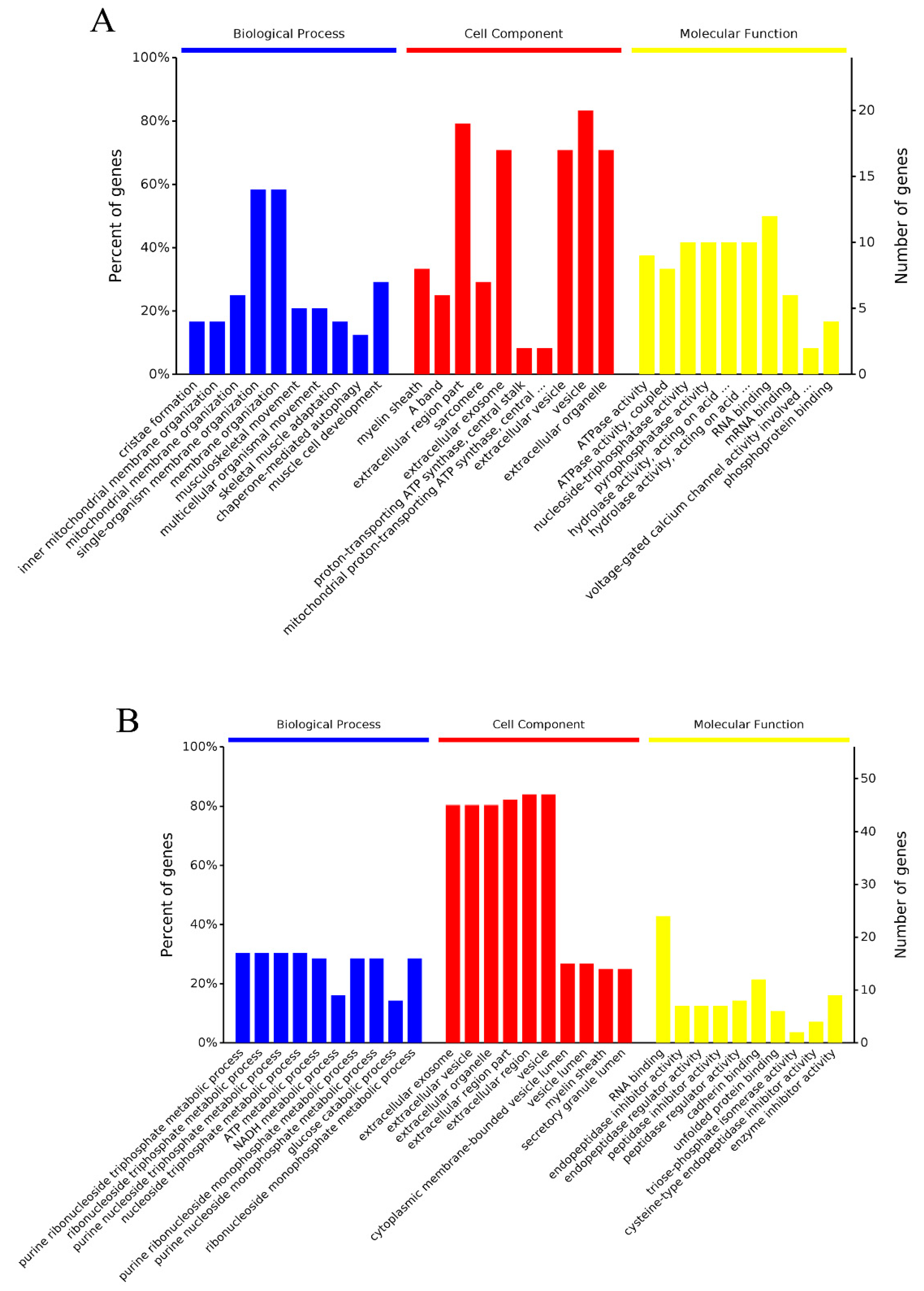
2.5.1. GO Enrichment Analysis
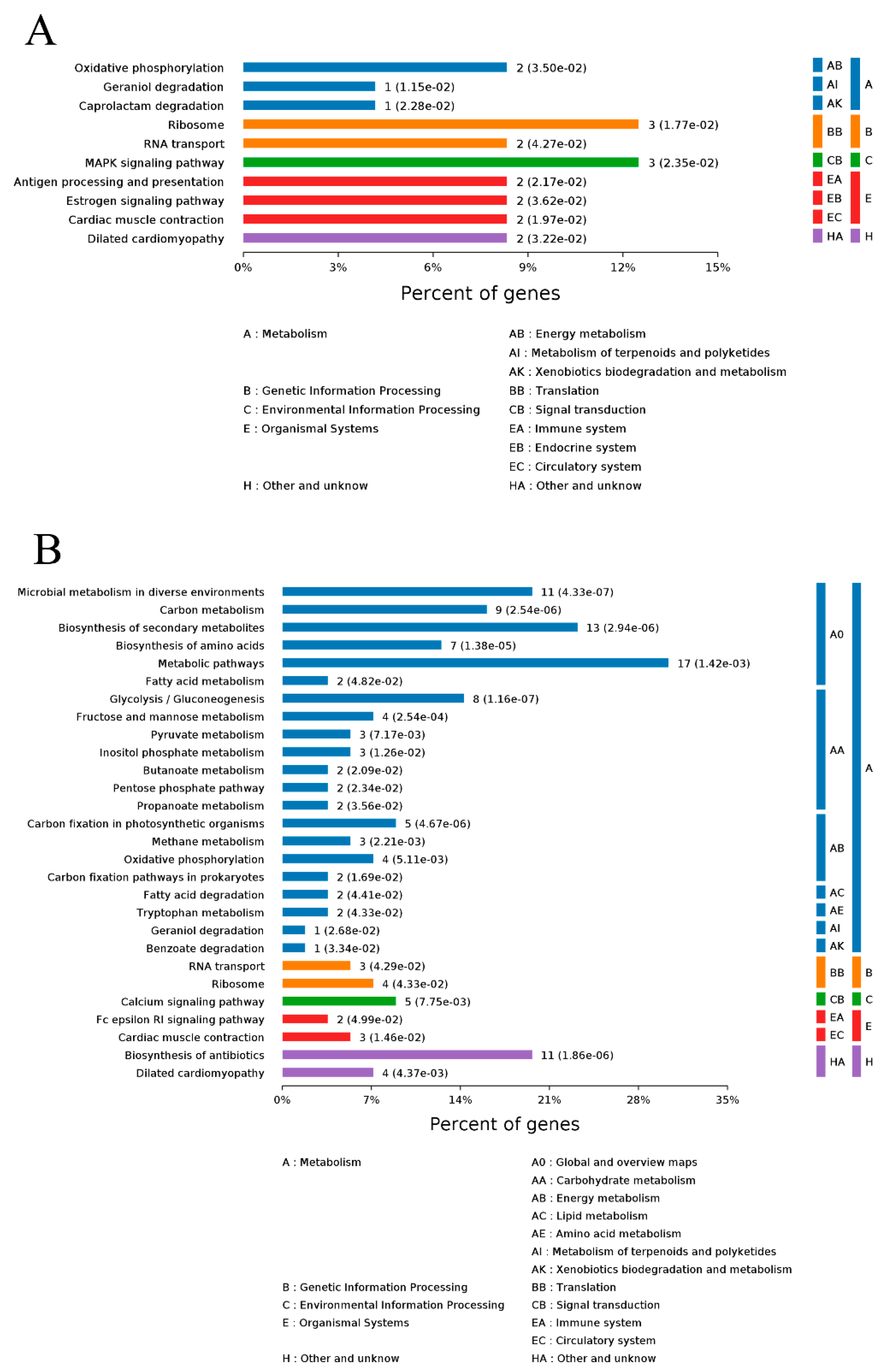
2.5.2. KEGG Pathway Analyses
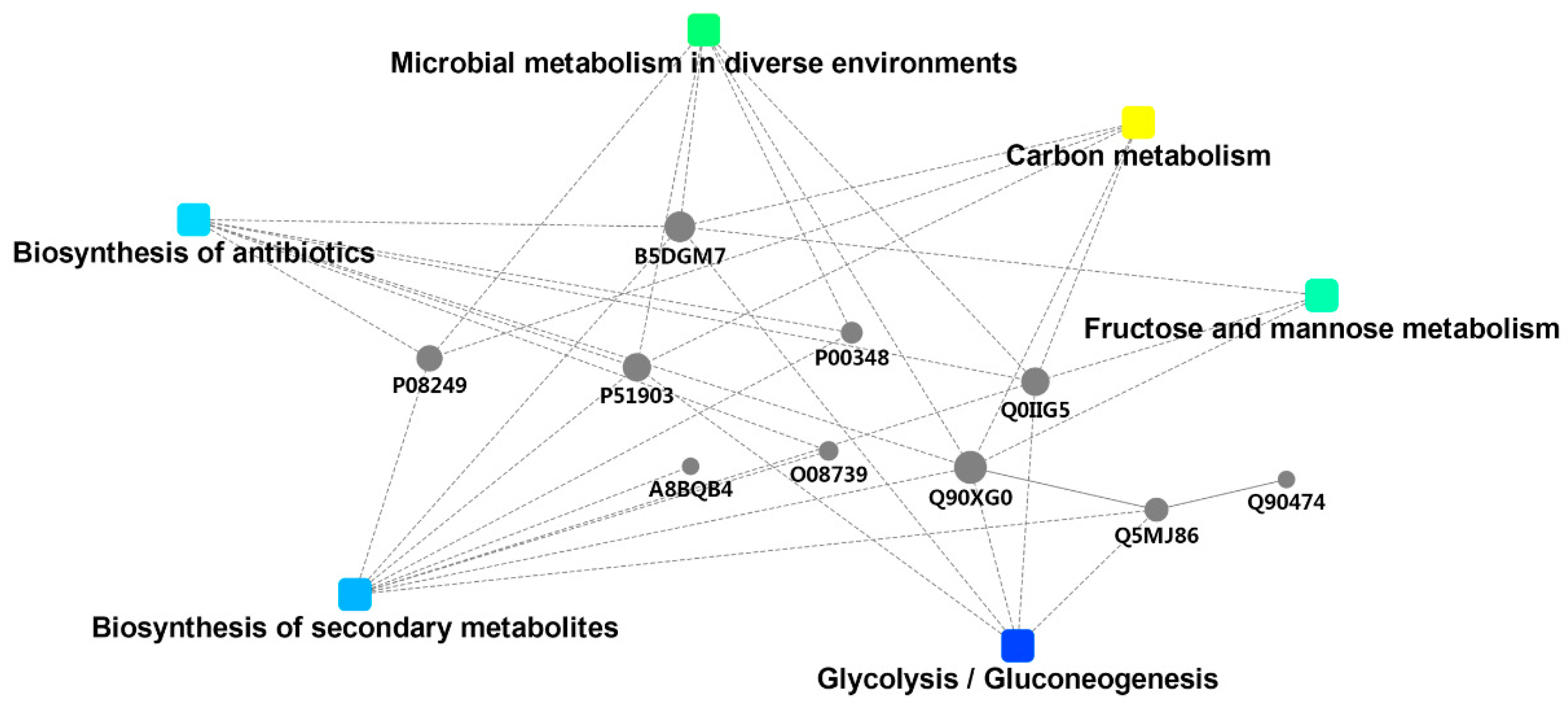
2.5.3. DSEP Interaction Network Analysis
2.6. Correlations between Differentially Significant Expressed Proteins (DSEPs) and Quality Index
2.7. Potential Protein Markers Associated with the Quality Change of Grouper Fillets Refrigerated Storage
2.7.1. Binding Proteins
2.7.2. Metabolic Enzyme
2.7.3. Heat Shock Protein (HSP) Family
2.7.4. Protein Turnover
2.7.5. Structural Proteins
3. Materials and Methods
3.1. Sample Preparation
3.2. Quality Index Analysis
3.3. Protein Extraction and Digestion
3.4. TMT Labeling of Peptides and High pH Reverse Phase Fractionation (HPRP)
3.5. LC-MS Analysis
3.6. Protein Database Searching and Analysis
3.7. Bioinformatics Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dong, J.H. Chinese Fishery Statistics Yearbook; China Agriculture Press: Beijing, China, 2017. [Google Scholar]
- Wang, L.; Tian, Y.; Tang, J. Analysis and quality evaluation of nutritional components in the muscle of epinehelus moara, E. lanceolatus and hybrid “yunlong grouper”. J. Fish. China 2018, 42, 1085–1093. [Google Scholar]
- Hassoun, A.; Karoui, R. Front-face fluorescence spectroscopy coupled with chemometric tools for monitoring fish freshness stored under different refrigerated conditions. Food Control 2015, 54, 240–249. [Google Scholar] [CrossRef]
- Suemitsu, L.; Cristianini, M. Effects of high pressure processing (hpp) on quality attributes of tilapia (Oreochromis niloticus) fillets during refrigerated storage. LWT-Food Sci. Technol. 2019, 101, 92–99. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2018; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Mora, L.; Gallego, M.; Toldrá, F. New approaches based on comparative proteomics for the assessment of food quality. Curr. Opin. Food Sci. 2018, 22, 22–27. [Google Scholar] [CrossRef]
- Josić, D.; Peršurić, Ž.; Rešetar, D.; Martinović, T.; Saftić, L.; Pavelić, S.K. Use of foodomics for control of food processing and assessing of food safety. Adv. Food Nutr. Res. 2017, 81, 187. [Google Scholar] [PubMed]
- Wu, W.; Gao, X.G.; Dai, Y.; Fu, Y.; Li, X.M.; Dai, R.T. Post-mortem changes in sarcoplasmic proteome and its relationship to meat color traits in M. semitendinosus of chinese luxi yellow cattle. Food Res. Int. 2015, 72, 98–105. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, H.; Rao, W.; Na, N.; Shen, Q.; Zhang, D. Proteomic analysis of goat longissimus dorsi muscles with different drip loss values related to meat quality traits. Food Sci. Biotechnol. 2016, 25, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Cai, L.; Xu, Y.; Yi, S.; Zhu, W.; Mi, H.; Li, J.; Lin, H. Freshness assessment of turbot (Scophthalmus maximus) by quality index method (qim), biochemical, and proteomic methods. LWT-Food Sci. Technol. 2017, 78, 172–180. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Lei, Y.; Shen, H.; Yu, X.; Luo, Y. Differential proteomic analysis to identify proteins associated with quality traits of frozen mud shrimp (Solenocera melantho) using an itraq-based strategy. Food Chem. 2018, 251, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Liu, Y.; Liu, Y.; Wang, Q.; Ye, H.-L.; Cheng, Y.-G.; Kuang, H.-X.; Jiang, X.-C.; Yang, B.-Y. Proteomics research on the protective effect of mangiferin on h9c2 cell injury induced by h2o2. Molecules 2019, 24, 1911. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-Y.; Xu, Y.-P.; Cai, X.-Z. Tmt-based quantitative proteomics analyses reveal novel defense mechanisms of brassica napus against the devastating necrotrophic pathogen sclerotinia sclerotiorum. J. Proteom. 2016, 143, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Stryiński, R.; Mateos, J.; Pascual, S.; González, Á.F.; Gallardo, J.M.; Łopieńska-Biernat, E.; Medina, I.; Carrera, M. Proteome profiling of l3 and l4 anisakis simplex development stages by tmt-based quantitative proteomics. J. Proteom. 2019, 201, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, L.; Zeng, Q.; Liu, J.; Yang, Y.; Ren, D. Quantitative differences in whey proteins among murrah, nili-ravi and mediterranean buffaloes using a tmt proteomic approach. Food Chem. 2018, 269, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, J.; Jia, R.; Bao, S.; Chen, X. Physiological and tmt-based proteomic analysis of oat early seedlings in response to alkali stress. J. Proteom. 2019, 193, 10–26. [Google Scholar] [CrossRef]
- Li, D.; Qin, N.; Zhang, L.; Li, Q.; Prinyawiwatkul, W.; Luo, Y. Degradation of adenosine triphosphate, water loss and textural changes in frozen common carp (Cyprinus carpio) fillets during storage at different temperatures. Int. J. Refrig. 2019, 98, 294–301. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with cur/βcd emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef]
- Richards, M.P.; Modra, A.M.; Li, R. Role of deoxyhemoglobin in lipid oxidation of washed cod muscle mediated by trout, poultry and beef hemoglobins. Meat Sci. 2002, 62, 157–163. [Google Scholar] [CrossRef]
- Cao, M.; Cao, A.; Li, Y.; Wang, W.; Wang, Y.; Cai, L. Effects of magnetic nanoparticles plus microwave on the thawing of largemouth bass (Micropterus salmoides) fillets based on itraq quantitative proteomics. Food Chem. 2019, 286, 506–514. [Google Scholar] [CrossRef]
- Zhang, G.; Xue, W.; Dai, J.; Xu, Q.; Wang, Y.; Yuan, H.; Yang, K.; Qi, Y.; Zeng, X.; Nyima, T. Quantitative proteomics analysis reveals proteins and pathways associated with anthocyanin accumulation in barley. Food Chem. 2019, 298, 124973. [Google Scholar] [CrossRef]
- Wang, C.; Chu, J.; Fu, L.; Wang, Y.; Zhao, F.; Zhou, D. Itraq-based quantitative proteomics reveals the biochemical mechanism of cold stress adaption of razor clam during controlled freezing-point storage. Food Chem. 2018, 247, 73–80. [Google Scholar] [CrossRef]
- Chauhan, S.S.; England, E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons. Meat Sci. 2018, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Goyal, N. Cloning, characterization and sub-cellular localization of gamma subunit of t-complex protein-1 (chaperonin) from leishmania donovani. Biochem. Biophys. Res. Commun. 2012, 429, 70–74. [Google Scholar]
- Chuang, W.-H.; Lee, K.-K.; Liu, P.-C. Characterization of alpha-2-macroglobulin from groupers. Fish Shellfish Immunol. 2013, 35, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Zia, M.K.; Siddiqui, T.; Ahsan, H.; Khan, F.H. Biophysical analysis of interaction between curcumin and alpha-2-macroglobulin. Int. J. Biol. Macromol. 2019, 128, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Donkor, O.; Street, W.A.; Vasiljevic, T. Calpains- and cathepsins-induced myofibrillar changes in post-mortem fish: Impact on structural softening and release of bioactive peptides. Trends Food Sci. Technol. 2015, 45, 130–146. [Google Scholar] [CrossRef]
- Gaarder, M.Ø.; Bahuaud, D.; Veiseth-Kent, E.; Mørkøre, T.; Thomassen, M.S. Relevance of calpain and calpastatin activity for texture in super-chilled and ice-stored atlantic salmon (Salmo salar L.) fillets. Food Chem. 2012, 132, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Yao, J.; Rexroad, C.E.; Kenney, P.B.; Semmens, K.; Killefer, J.; Nath, J. Characterization of calpastatin gene in fish: Its potential role in muscle growth and fillet quality. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 141, 488–497. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; De Koning, L.; Picard, B. Reverse phase protein array for the quantification and validation of protein biomarkers of beef qualities: The case of meat color from charolais breed. Meat Sci. 2018, 145, 308–319. [Google Scholar] [CrossRef]
- Veiseth-Kent, E.; Grove, H.; Færgestad, E.M.; Fjæra, S.O. Changes in muscle and blood plasma proteomes of atlantic salmon (Salmo salar) induced by crowding. Aquaculture 2010, 309, 272–279. [Google Scholar] [CrossRef]
- Grunwald, E.W.; Richards, M.P. Mechanisms of heme protein-mediated lipid oxidation using hemoglobin and myoglobin variants in raw and heated washed muscle. J. Agric. Food Chem. 2006, 54, 8271–8280. [Google Scholar] [CrossRef]
- Grunwald, E.W.; Richards, M.P. Effects of hemopexin on hemin and hemoglobin-mediated lipid oxidation in washed fish muscle. LWT-Food Sci. Technol. 2012, 46, 412–418. [Google Scholar] [CrossRef]
- Fonvielle, M.; Mariano, S.; Therisod, M. New inhibitors of rabbit muscle triose-phosphate isomerase. Bioorg. Med. Chem. Lett. 2005, 15, 2906–2909. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yu, Q.-Q.; Fu, Y.; Tian, X.-J.; Jia, F.; Li, X.-M.; Dai, R.-T. Towards muscle-specific meat color stability of chinese luxi yellow cattle: A proteomic insight into post-mortem storage. J. Proteom. 2016, 147, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wei, W.; Ma, C.; Li, X.; Dai, R. Postmortem changes in sarcoplasmic proteins associated with color stability in lamb muscle analyzed by proteomics. Eur. Food Res. Technol. 2016, 242, 527–535. [Google Scholar] [CrossRef]
- Brüser, A.; Kirchberger, J.; Schöneberg, T. Altered allosteric regulation of muscle 6-phosphofructokinase causes tarui disease. Biochem. Biophys. Res. Commun. 2012, 427, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, R.D.; King, D.A.; Jenschke, B.E.; Behrends, J.M.; Hively, T.S.; Smith, S.B. Postmortem regulation of glycolysis by 6-phosphofructokinase in bovine M. sternocephalicus pars mandibularis. Meat Sci. 2005, 70, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.M.; Hofmann, G.E. Expression of 70 kda heat shock proteins in antarctic and new zealand notothenioid fish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 125, 229–238. [Google Scholar] [CrossRef]
- Carvalho, M.E.; Gasparin, G.; Poleti, M.D.; Rosa, A.F.; Balieiro, J.C.C.; Labate, C.A.; Nassu, R.T.; Tullio, R.R.; Mourão, G.B. Heat shock and structural proteins associated with meat tenderness in nellore beef cattle, a bos indicus breed. Meat Sci. 2014, 96, 1318–1324. [Google Scholar] [CrossRef]
- de Oliveira, L.G.; Delgado, E.F.; Steadham, E.M.; Huff-Lonergan, E.; Lonergan, S.M. Association of calpain and calpastatin activity to postmortem myofibrillar protein degradation and sarcoplasmic proteome changes in bovine longissiumus lumborum and triceps brachii. Meat Sci. 2019, 155, 50–60. [Google Scholar] [CrossRef]
- Lomiwes, D.; Farouk, M.M.; Frost, D.A.; Dobbie, P.M.; Young, O.A. Small heat shock proteins and toughness in intermediate ph u beef. Meat Sci. 2013, 95, 472–479. [Google Scholar] [CrossRef]
- Fan, L.; Wang, A.; Miao, Y.; Liao, S.; Ye, C.; Lin, Q. Comparative proteomic identification of the hepatopancreas response to cold stress in white shrimp, litopenaeus vannamei. Aquaculture 2016, 454, 27–34. [Google Scholar] [CrossRef]
- Seo, J.-K.; Kim, D.-G.; Oh, R.; Park, K.-S.; Lee, I.-A.; Cho, S.-M.; Lee, K.Y.; Nam, B.-H. Antimicrobial effect of the 60 s ribosomal protein l29 (cgrpl29), purified from the gill of pacific oyster, crassostrea gigas. Fish Shellfish Immunol. 2017, 67, 675–683. [Google Scholar] [CrossRef]
- Veronique, S.L.; Laurent, A.; Philippe, G. Effect of oxidation on In Vitro digestibility of skeletal muscle myofibrillar proteins. J. Agric. Food Chem. 2007, 55, 5343–5348. [Google Scholar]
- Wojtysiak, D.; Połtowicz, K. Carcass quality, physico-chemical parameters, muscle fibre traits and myosin heavy chain composition of M. longissimus lumborum from puławska and polish large white pigs. Meat Sci. 2014, 97, 395–403. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, W.; Tian, X.; Jia, F.; Xu, L.; Dai, R.; Li, X. Comparative proteomics to reveal muscle-specific beef color stability of holstein cattle during post-mortem storage. Food Chem. 2017, 229, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Richardson, E.; Bohrer, B.M.; Arkfeld, E.K.; Boler, D.D.; Dilger, A.C. A comparison of intact and degraded desmin in cooked and uncooked pork longissimus thoracis and their relationship to pork quality. Meat Sci. 2017, 129, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Starkey, C.P.; Geesink, G.H.; van de Ven, R.; Hopkins, D.L. The relationship between shear force, compression, collagen characteristics, desmin degradation and sarcomere length in lamb biceps femoris. Meat Sci. 2017, 126, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Starkey, C.P.; Geesink, G.H.; Collins, D.; Hutton Oddy, V.; Hopkins, D.L. Do sarcomere length, collagen content, ph, intramuscular fat and desmin degradation explain variation in the tenderness of three ovine muscles? Meat Sci. 2016, 113, 51–58. [Google Scholar] [CrossRef]
- Li, Q.; Gui, P.; Huang, Z.; Feng, L.; Luo, Y. Effect of transglutaminase on quality and gel properties of pork and fish mince mixtures. J. Texture Stud. 2018, 49, 56–64. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Wisniewski, J.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Boutet, E.; Lieberherr, D.; Tognolli, M.; Schneider, M.; Bansal, P.; Bridge, A.J.; Poux, S.; Bougueleret, L.; Xenarios, I. Uniprotkb/swiss-prot, the manually annotated section of the uniprot knowledgebase: How to use the entry view. In Plant Bioinformatics; Humana Press: New York, NY, USA, 2016. [Google Scholar]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


| Quality Index | Refrigerated Storage Time (Day) | ||
|---|---|---|---|
| 0 | 6 | 12 | |
| pH | 6.79 ± 0.002 a | 6.81 ± 0.04 a | 6.94 ± 0.04 b |
| Centrifugal loss% | 9.94 ± 1.42 a | 17.46 ± 0.86 b | 22.76 ± 1.57 c |
| L * | 60.85 ± 0.73 a | 55.87 ± 1.49 b | 54.43 ± 5.46 b |
| a * | 1.67 ± 0.38 a | 2.75 ± 0.70 b | 3.77 ± 0.90 b |
| b * | 1.42 ± 0.21 a | 3.25 ± 0.67 b | 4.05 ± 1.23 c |
| Hardness | 2326.09 ± 152.52 a | 1873.28 ± 119.25 b | 1245.71 ± 60.28 c |
| Chewiness | 584.74 ± 64.14 a | 354.02 ± 33.87 b | 163.52 ± 21.33 c |
| Gumminess | 1470.42 ± 78.50 a | 738.671 ± 31.97 b | 345.27 ± 24.67 c |
| Uniprot ID | Description | Classification | pH | Centrifugal Loss | L * | a * | b * | Hardness | Chewiness | Gumminess |
|---|---|---|---|---|---|---|---|---|---|---|
| P50143 | T-complex protein 1 subunit gamma | Binding proteins | −0.769 | −0.981 | 1.000 ** | −0.962 | −0.997 * | 0.926 | 0.971 | 0.993 |
| P06238 | Alpha-2-macroglobulin | Binding proteins | −0.999 * | −0.985 | 0.999 * | −0.967 | −0.999 * | 0.934 | 0.976 | 0.820 |
| P20810 | Calpastatin | Binding proteins | −0.929 | −0.993 | 0.946 | −0.999 * | −0.970 | 0.997 * | 0.997 * | 0.981 |
| Q6PHG2 | Hemopexin | Binding proteins | −0.827 | −0.995 | 0.994 | −0.984 | −1.000 * | 0.959 | 0.990 | 0.985 |
| Q90XG0 | Triose phosphate isomerase B | Enzymes | −0.955 | −0.980 | 0.918 | −0.993 | −0.949 | 1.000** | 0.988 | 0.963 |
| P08249 | Malate dehydrogenase, mitochondrial | Enzymes | −0.862 | −0.974 | 0.984 | −0.999 * | −0.999 * | 0.975 | 0.997 * | 0.999 * |
| Q0IIG5 | ATP-dependent 6-phosphofructokinase, muscle type | Enzymes | −0.999 * | −0.817 | 0.679 | −0.862 | −0.740 | 0.912 | 0.842 | 0.773 |
| P51903 | Phosphoglycerate kinase | Enzymes | −0.904 | −0.998 * | 0.964 | −0.801 | −0.983 | 0.991 | 0.890 | 0.991 |
| Q3SYR3 | Uncharacterized protein | Enzymes | −1.000 * | −0.884 | 0.768 | −0.920 | −0.820 | 0.957 | 0.904 | 0.848 |
| Q589R5 | Triosephosphate isomerase | Enzymes | −0.868 | −1.000 ** | 0.982 | −0.995 | −0.935 | 0.978 | 0.998 * | 0.999 * |
| Q9I8F9 | Heat shock 70 kDa protein 1 | Heat shock protein family | 0.620 | −0.920 | 0.981 | −0.885 | −0.961 | 0.828 | 0.902 | 0.996 * |
| Q08699 | 40S ribosomal protein S14 | Protein turnover | −0.712 | −0.962 | 0.998 * | −0.935 | −0.988 | 0.891 | 0.948 | 0.979 |
| Q90YT6 | 60S ribosomal protein L32 | Protein turnover | −0.998 * | −0.903 | 0.795 | −0.936 | −0.844 | 0.969 | 0.921 | 0.870 |
| A2ASS6 | Titin | Structural proteins | −0.792 | −0.988 | 0.972 | −0.972 | −0.909 | 0.999 * | 0.999 * | 0.996 |
| Q90339 | Myosin heavy chain | Structural proteins | −0.892 | −1.000 ** | 0.999 | −0.909 | −0.999 * | 0.998 * | 1.000 ** | 0.995 |
| P23239 | Desmin | Structural proteins | −0.933 | −0.991 | 0.942 | −0.999 * | −0.968 | 0.999 * | 0.996 | 0.979 |
| A7E2Y1 | Myosin-7B | Structural proteins | −0.946 | −0.985 | 0.929 | −0.996 | −0.957 | 1.000 * | 0.992 | 0.971 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xie, J. Analysis of Proteins Associated with Quality Deterioration of Grouper Fillets Based on TMT Quantitative Proteomics during Refrigerated Storage. Molecules 2019, 24, 2641. https://doi.org/10.3390/molecules24142641
Zhang X, Xie J. Analysis of Proteins Associated with Quality Deterioration of Grouper Fillets Based on TMT Quantitative Proteomics during Refrigerated Storage. Molecules. 2019; 24(14):2641. https://doi.org/10.3390/molecules24142641
Chicago/Turabian StyleZhang, Xicai, and Jing Xie. 2019. "Analysis of Proteins Associated with Quality Deterioration of Grouper Fillets Based on TMT Quantitative Proteomics during Refrigerated Storage" Molecules 24, no. 14: 2641. https://doi.org/10.3390/molecules24142641
APA StyleZhang, X., & Xie, J. (2019). Analysis of Proteins Associated with Quality Deterioration of Grouper Fillets Based on TMT Quantitative Proteomics during Refrigerated Storage. Molecules, 24(14), 2641. https://doi.org/10.3390/molecules24142641







