The Amphoteric and Hydrophilic Properties of Cartilage Surface in Mammalian Joints: Interfacial Tension and Molecular Dynamics Simulation Studies
Abstract
:1. Introduction
2. Results and Discussion
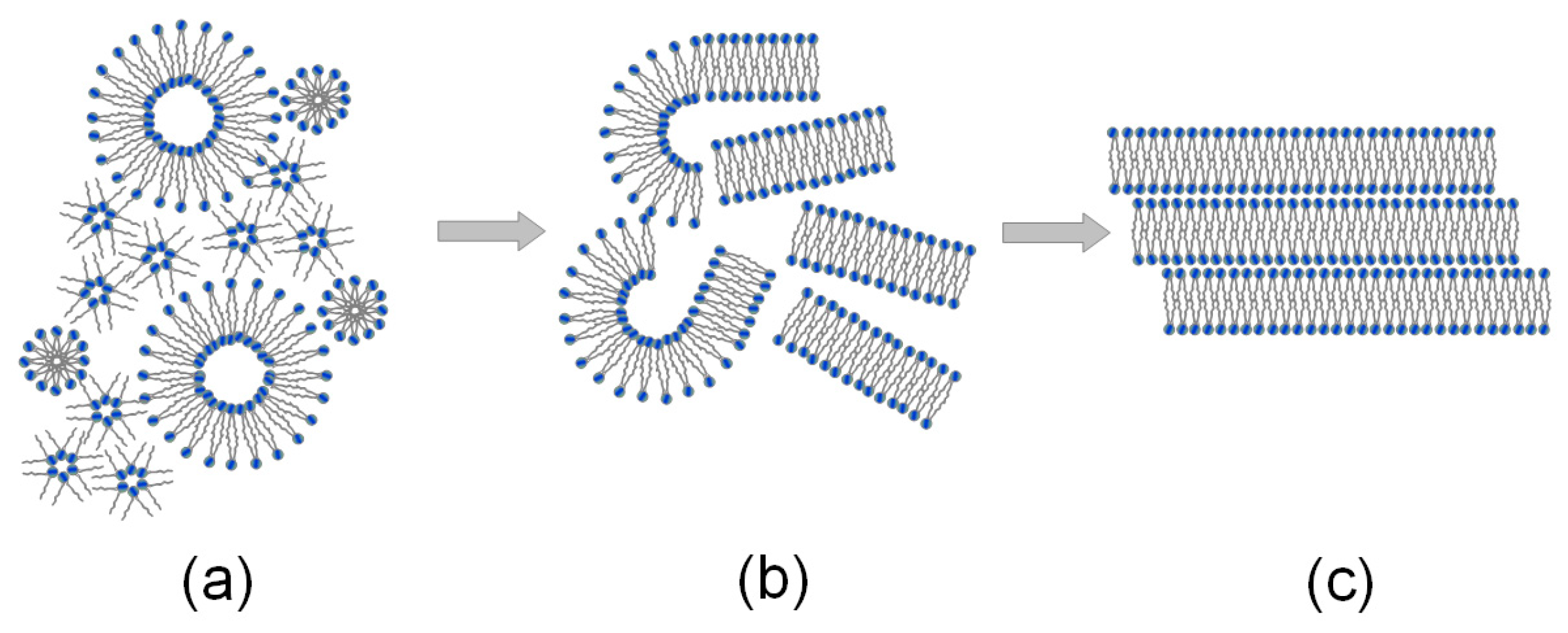
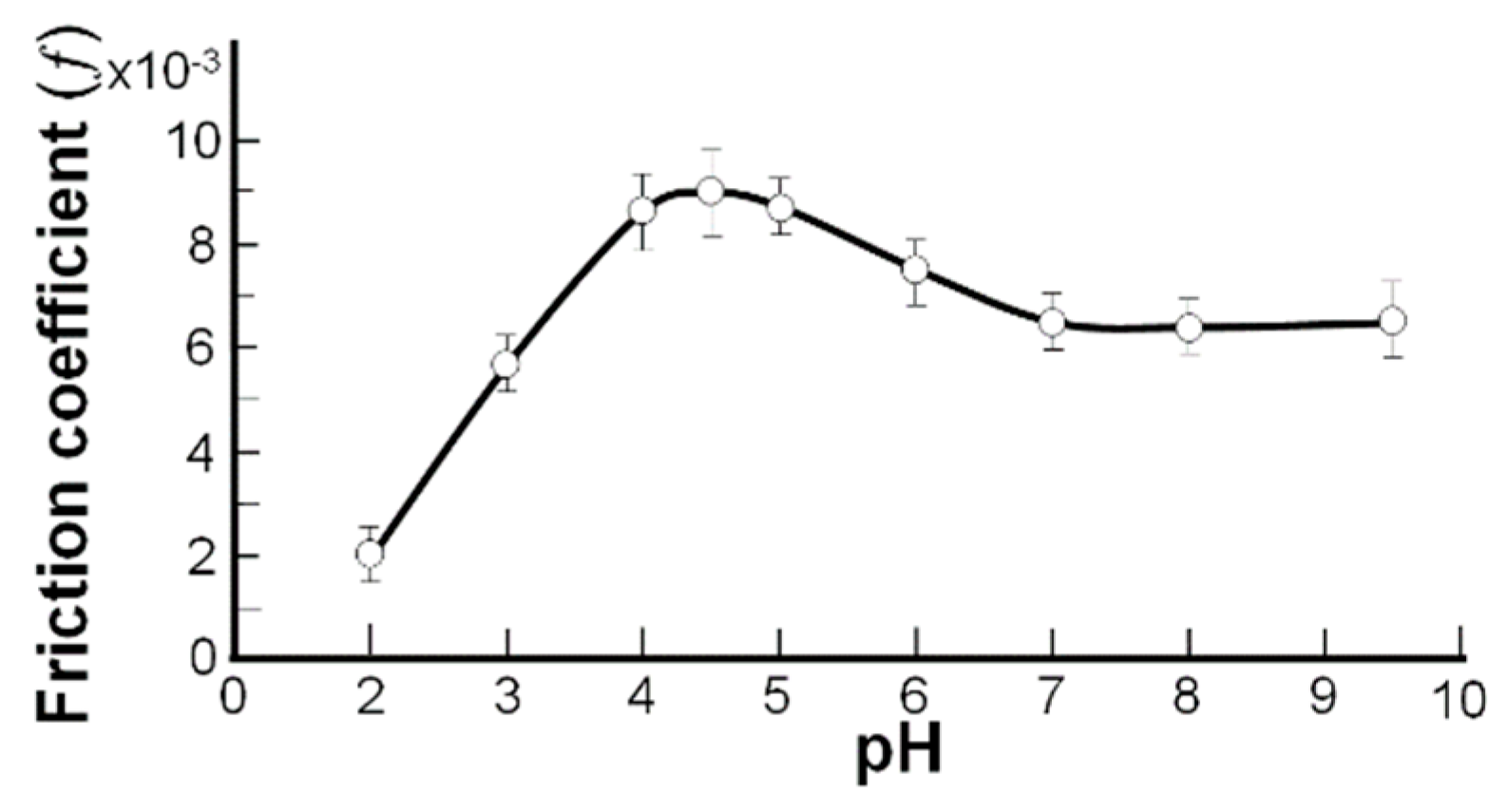
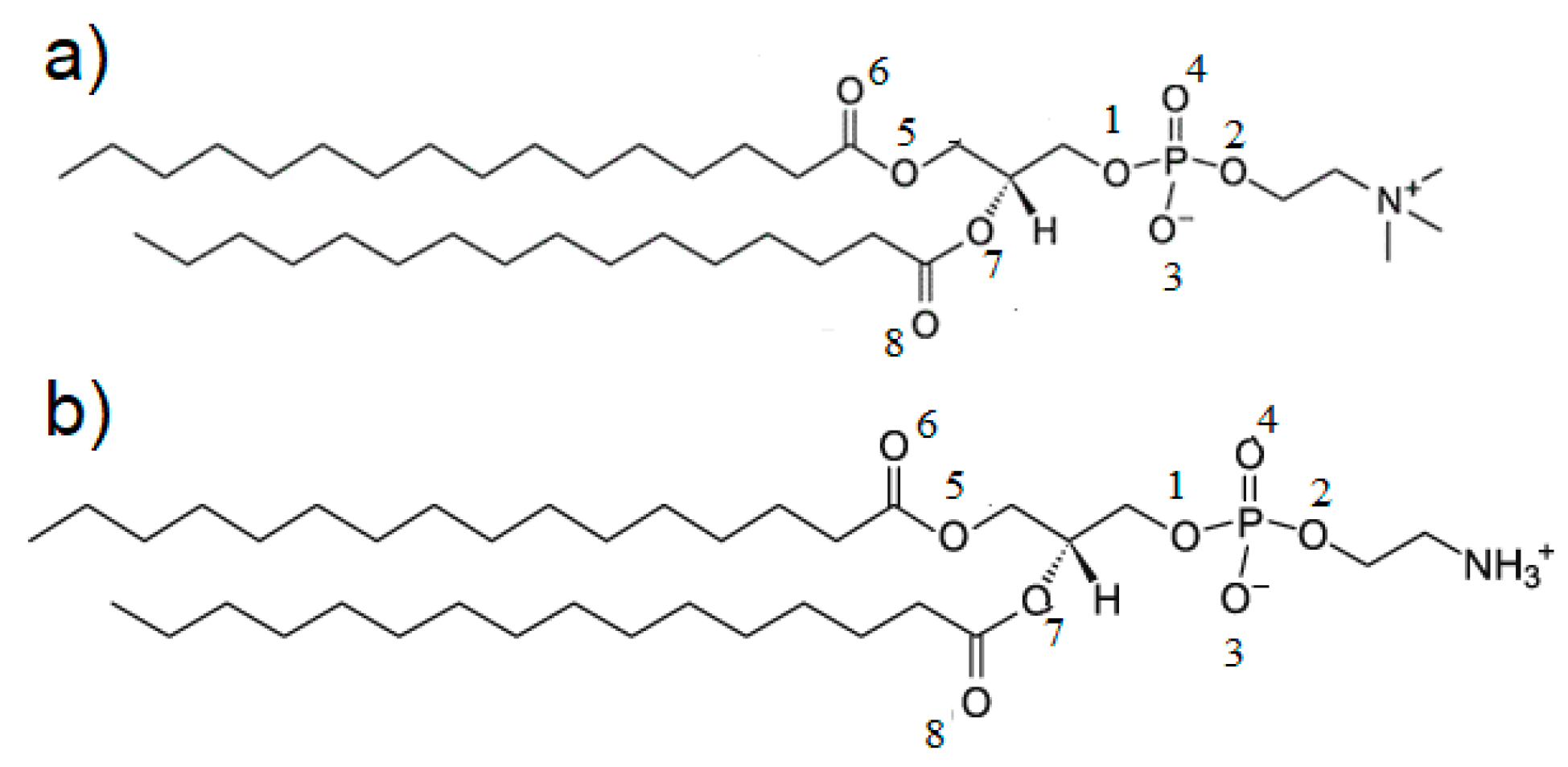
2.1. The Interfacial Tension of the Phospholipid Membrane
- pI (pH 4.20); H2N (CH2)n PO4H–R1R2 ⇆ H3N+(CH2)n PO4−–R1R2
- pH 1 to 4.20 (–NH3+ → –NH2) (pH 4.20 to 6.5) (–PO4H → –PO4−)
- (Left side of the curve) (Right side of the curve)
2.2. Molecular Dynamics Analysis
3. Materials and Methods
3.1. Methods of Measuring the Interfacial Tension
3.2. Molecular Dynamics Simulations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, P.F.; Powell, G.L.; LaBerge, M. Sliding friction analysis of phosphatidylcholine as a boundary lubricant for articular cartilage. Proc. Inst. Mech. Eng. Part H. J. Eng. Med. 1993, 207, 59–66. [Google Scholar] [CrossRef]
- Hills, B.A.; Butler, B.D.; Barrow, R.E. Boundary lubrication imparted by pleural surfactants and their identification. J. Appl. Physiol. 1982, 53, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.A.; Butler, B.D. Surfactants identified in synovial fluid and their ability to act as boundary lubricants. Ann. Rheum. Dis. 1984, 43, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.A. The Biology of Surfactant; Cambridge University Press: London, UK, 1988. [Google Scholar]
- Hills, B.A. Graphite like lubrication of mesothelium by oligolamellar pleural surfactant. J. Appl. Physiol. 1992, 73, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.A. Boundary lubrication in vivo. Proc. Inst. Mech. Eng. 2000, 214, 94. [Google Scholar] [CrossRef] [PubMed]
- Hills, B.A. Surface-active phospholipid: A Pandora’s box of clinical applications. Part II Barrier and lubricating properties. Int. Med. J. 2002, 32, 242–251. [Google Scholar] [CrossRef]
- Hills, B.A.; Crawford, R.W. Normal and prosthetic synovial joints are lubricated by surface-active phospholipids: A hypothesis. J. Arthroplast. 2003, 18, 499–505. [Google Scholar] [CrossRef]
- Petelska, A.D.; Kazimierska-Drobny, K.; Janicka, K.; Majewski, T.; Urbaniak, W. Understanding the Unique Role of Phospholipids in the Lubrication of Natural Joints: An Interfacial Tension Study. Coatings 2019, 9, 264. [Google Scholar] [CrossRef]
- Pawlak, Z.; Petelska, A.D.; Urbaniak, W.; Yusuf, A.Q.; Oloyede, A. Relationship between wettability and lubrication characteristics of the surfaces of contacting phospholipid-based membranes. Cell Biochem. Biophys. 2013, 6, 335–345. [Google Scholar] [CrossRef]
- McCutchen, C.W. Mechanism of animal joints: Sponge-hydrostatic and weeping bearings. Nature 1959, 184, 1284–1285. [Google Scholar] [CrossRef]
- Ballantine, G.C.; Stachowiak, G.W. The effects of lipid depletion on osteoarthritic wear. Wear 2002, 253, 385–393. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Liebisch, G.; Lochni, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum. 2013, 65, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.V.; Powell, G.L.; LaBerg, M. Phospholipid composition of articular cartilage boundary lubricant. J. Orthoped. Res. 2001, 19, 671–676. [Google Scholar] [CrossRef]
- Bayer, I.S. Advances in tribology of lubricin and lubricin-like synthetic polymer nanostructures. Lubricants 2018, 6, 30. [Google Scholar] [CrossRef]
- Mocny, P.; Klok, H.A. Tribology of surface-grafted polymer brushes. Mol. Syst. Des. Eng. 2016, 1, 141–154. [Google Scholar] [CrossRef]
- Samaroo, K.J.; Tan, M.; Eguiluz, R.C.A.; Gourdon, D.; Putnam, D.; Bonassar, L.J. Tunable Lubricin-mimetics for boundary lubrication of cartilage. Biotribology 2017, 9, 18–23. [Google Scholar] [CrossRef]
- Singh, A.; Corvelli, M.; Unterman, S.A.; Wepasnick, K.A.; McDonnell, P.; Elisseeff, J.H. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 2014, 13, 988–995. [Google Scholar] [CrossRef]
- Chang, D.P.; Abu-Lail, N.I.; Guilak, F.; Jay, G.D.; Zauscher, S. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir 2008, 24, 1183–1193. [Google Scholar] [CrossRef]
- Chang, D.P.; Abu-Lail, N.I.; Coles, J.M.; Guilak, F.; Jay, G.D.; Zauscher, S. Friction force microscopy of lubricin and hyaluronic acid between hydrophobic and hydrophilic surfaces. Soft Matter 2009, 5, 3438–3445. [Google Scholar] [CrossRef] [Green Version]
- Petelska, A.D.; Figaszewski, Z.A. Effect of pH on the interfacial tension of bilayer lipid membrane. Biophys. J. 2000, 78, 812–817. [Google Scholar] [CrossRef]
- Petelska, A.D.; Figaszewski, Z.A. Effect of pH on the interfacial tension of bilayer lipid membrane formed from phosphatidylcholine or phosphatidylserine. Biochim. Biophys. Acta 2002, 1561, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Petelska, A.D.; Figaszewski, Z.A. Acid–base equilibria at interface separating electrolyte solution and lipid bilayer formed from phosphatidylcholine. Biophys. Chem. 2003, 104, 13–19. [Google Scholar] [CrossRef]
- Petelska, A.D.; Figaszewski, Z.A. Effect of pH on the interfacial tension of bilayer lipid membrane formed from phosphatidylethanolamine. Biochim. Biophys. Acta 2002, 1567, 79–86. [Google Scholar] [CrossRef]
- Petelska, A.D. Interfacial tension of bilayer lipid membrane. Cent. Eur. J. Chem. 2012, 10, 16–26. [Google Scholar] [CrossRef]
- Pawlak, Z.; Urbaniak, W.; Gadomski, A.; Yusuf, K.Q.; Afara, I.O.; Oloyede, A. The role of lamellate phospholipid bilayers in lubrication of joints. Acta Bioeng. Biomech. 2012, 14, 101–106. [Google Scholar] [PubMed]
- Pawlak, Z.; Gadomski, A.; Sojka, M.; Urbaniak, W.; Bełdowski, P. The amphoteric effect on friction between the bovine cartilage/cartilage surfaces under slightly sheared hydration lubrication mode. Colloids Surf. B 2016, 146, 452–458. [Google Scholar] [CrossRef]
- Marti, A.; Hahner, G.; Spencer, N.D. Sensitivity of frictional forces to pH on a nanometer scale: A lateral force microscopy study. Langmuir 1995, 11, 4632–4635. [Google Scholar] [CrossRef]
- Krieger, E.; Koraimann, G.; Vriend, G. Increasing the precision of comparative models with YASARA NOVA—a self-parameterizing force field. Proteins 2002, 47, 393–402. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. Models@Home: Distributed computing in bioinformatics using a screensaver based approach. Bioinformatics 2002, 18, 315–318. [Google Scholar] [CrossRef]
- Krieger, E.; Darden, T.; Nabuurs, S.B.; Finkelstein, A.; Vriend, G. Making optimal use of empirical energy functions: Force-field parameterization in crystal space. Proteins. 2004, 57, 678–683. [Google Scholar] [CrossRef]
- Bełdowski, P.; Weber, P.; Dédinaité, A.; Claesson, P.M.A.; Gadomski, A. Physical crosslinking of hyaluronic acid in the presence of phospholipids in an aqueous nano-environment. Soft Matters 2018, 14, 8997–9004. [Google Scholar]
- Mueller, P.; Rudin, D.O.; Tien, H.T.; Wescott, W.C. Methods for the formation of single bimolecular lipid membranes in aqueous solution. J. Phys. Chem. 1963, 67, 534–535. [Google Scholar] [CrossRef]
- Britton, H.T.K.; Robinson, R.A. Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462. [Google Scholar] [CrossRef]
- Petelska, A.D.; Figaszewski, Z.A. Interfacial tension of the two-component bilayer lipid membrane modelling of cell membrane. Bioelectrochem. Bioenerg. 1998, 46, 199–204. [Google Scholar] [CrossRef]
- Adamson, A. Physical Chemistry of Surfaces; Interscience Publishers, Inc.: New York, NY, USA, 1960. [Google Scholar]
- Duan, Y.; Wu, C.; Chowdhury, S.; Lee, M.C.; Xiong, G.; Zhang, W.; Yang, R.; Cieplak, P.; Luo, R.; Lee, T.; et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J. Comput. Chem. 2003, 24, 1999–2012. [Google Scholar] [CrossRef]
- Yeghiazaryan, G.A.; Poghosyan, A.H.; Shahinyan, A.A. Structural and Dynamical Features of hydrocarbon chains of Dipalmitoylphosphatidylcholine (DPPC) Molecules in Phospholipid Bilayers: A Molecular Dynamics Study. Electron. J. Nat. Sci. 2005, 4, 44–49. [Google Scholar]
- Neria, E.; Fischer, S.; Karplus, M. Simulation of activation free energies in molecular systems. J. Chem. Phys. 1996, 105, 1902. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are not available or not from the authors. |



| Interfacial Tension Values at pI γmax (N/m) | Isoelectric Point (pI) Values | Acid–Base Constant Values [21,24] | Surface Energy [J/mol] | |
|---|---|---|---|---|
| pKX | pKY | |||
| DPPS [23] | ||||
| 2.9 × 10−3 | 3.80 | 3.36 | 9.55 | 1824 |
| DPPC | ||||
| 3.5 × 10−3 | 4.14 | 2.58 | 5.69 | 1800 |
| DPPE | ||||
| 4.0 × 10−3 | 4.20 | 2.42 | 5.98 | 1854 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janicka, K.; Beldowski, P.; Majewski, T.; Urbaniak, W.; Petelska, A.D. The Amphoteric and Hydrophilic Properties of Cartilage Surface in Mammalian Joints: Interfacial Tension and Molecular Dynamics Simulation Studies. Molecules 2019, 24, 2248. https://doi.org/10.3390/molecules24122248
Janicka K, Beldowski P, Majewski T, Urbaniak W, Petelska AD. The Amphoteric and Hydrophilic Properties of Cartilage Surface in Mammalian Joints: Interfacial Tension and Molecular Dynamics Simulation Studies. Molecules. 2019; 24(12):2248. https://doi.org/10.3390/molecules24122248
Chicago/Turabian StyleJanicka, Katarzyna, Piotr Beldowski, Tomasz Majewski, Wieslaw Urbaniak, and Aneta D. Petelska. 2019. "The Amphoteric and Hydrophilic Properties of Cartilage Surface in Mammalian Joints: Interfacial Tension and Molecular Dynamics Simulation Studies" Molecules 24, no. 12: 2248. https://doi.org/10.3390/molecules24122248






