Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars
Abstract
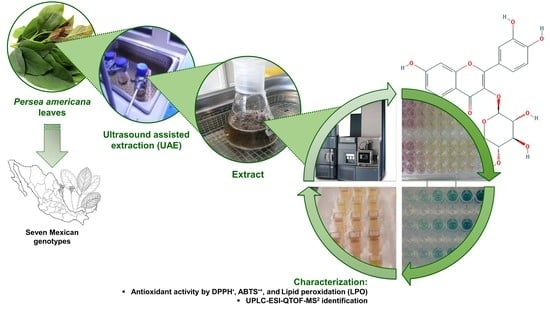
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Capacities from the Different P. americana Cultivars
2.2. Phytochemical Constituents Profile of P. americana Cultivars
2.2.1. Phenolic Acids
2.2.2. Flavonoids
2.2.3. Other Compounds
2.3. Possible Genotypic Effect on Antioxidant Activity and Phytochemical Profile
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction of P. americana Polyphenolic Compounds
3.3.1. Ultrasound-Assisted Extraction (UAE)
3.3.2. Purification of the Extracts
3.4. Antioxidant Activities Evaluation
3.4.1. DPPH• Radical Scavenging Activity
3.4.2. ABTS•+ Radical Scavenging Activity
3.4.3. Linoleic Acid Peroxidation Inhibition Assay
3.5. UPLC-ESI-Q/TOF-MS2 Analysis for Compounds
3.6. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chanderbali, A.S.; Albert, V.A.; Ashworth, V.E.T.M.; Clegg, M.T.; Litz, R.E.; Soltis, D.E.; Soltis, P.S. Persea americana (avocado): Bringing ancient flowers to fruit in the genomics era. BioEssays 2008, 30, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C.R.F. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crops Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef]
- Fonseca-Duarte, P.; Alves-Chaves, M.; Dellinghausen-Borges, C.; Barboza-Mendonça, C.R. Avocado: Characteristics, health benefits and uses. Ciênc. Rural. 2016, 46, 747–754. [Google Scholar] [CrossRef]
- Sánchez-González, E.I.; Hernández-Delgado, S.; Aguirre-Arzola, V.E.; Torres-Castillo, J.A.; Gutiérrez-Díez, A. Etiquetas de secuencias expresadas diferenciales de frutos de aguacate raza mexicana (Persea americana Mill. var. drymifolia). Rev. Bras. Frutic. 2017, 40, e184. [Google Scholar]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability–A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Kaur-Kala, H.; Mehta, R.; Tandey, R.; Sen, K.K.; Mandal, V. Ten years of research on phenolics (2005–2015): A status report. PSRA 2016, 18, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bost, J.B.; Smith, N.J.H.; Crane, J.H. History, distribution and uses. In The Avocado: Botany, Production and Uses; Schaffer, B.A., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI: Wallingford, UK, 2013; pp. 10–30. [Google Scholar]
- Kosińska, A.; Karamác, M.; Estrella, I.; Hernández, T.; Bartolomé, B.; Dykes, G.A. Phenolic compound profiles and antioxidant capacity of Persea americana Mill. peels and seeds of two varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Kristanty, R.E.; Suriawati, J.; Sulistiyo, J. Cytotoxicity activity of avocado seeds extracts (Persea Americana Mill.) on T47D cell lines. IRJP 2014, 5, 557–559. [Google Scholar] [CrossRef]
- Rodríguez-Carpena, J.; Morcuende, D.; Andrade, M.J.; Kylli, P.; Estévez, M. Avocado (Persea americana Mill.) Phenolics, in vitro antioxidant and antimicrobial activities, and inhibition of lipid and protein oxidation in porcine patties. J. Agric. Food Chem. 2011, 59, 5625–5635. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Tech. 2017, 80, 51–56. [Google Scholar] [CrossRef]
- Viera, V.B.; Piovesan, N.; Rodrigues, J.B.; Mello, R.O.; Prestes, R.C.; Santos, R.C.V.; Vaucher, R.A.; Hautrive, T.P.; Kubota, E.H. Extraction of phenolic compounds and evaluation of the antioxidant and antimicrobial capacity of red onion skin (Allium cepa L.). Int. Food Res. J. 2017, 24, 990–999. [Google Scholar]
- Kumar, B.; Cumbal, L. UV-Vis, FTIR and antioxidant study of Persea americana (avocado) leaf and fruit: A comparison. RFCQ 2016, 14, 13–20. [Google Scholar]
- Wang, W.; Terrell, R.; Bostic, L.G. Antioxidant capacities, procyanidins and pigments in avocados of different strains and cultivars. Food Chem. 2010, 122, 1193–1198. [Google Scholar] [CrossRef]
- López-Yerena, A.; Guerra-Ramírez, D.; Jácome-Rincón, J.; Espinosa-Solares, T.; Reyes-Trejo, B.; Famiani, F.; Cruz-Castillo, J.G. Initial evaluation of fruit of accessions of Persea schiedeana Nees for nutritional value, quality and oil extraction. Food Chem. 2018, 245, 879–884. [Google Scholar] [CrossRef]
- Oboh, G.; Odubanjo, V.O.; Bello, F.; Ademosun, A.O.; Oyeleye, S.I.; Nwanna, E.E.; Ademiluyi, A.O. Aqueous extracts of avocado pear (Persea americana Mill.) leaves and seeds exhibit anticholinesterases and antioxidant activities in vitro. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 131–140. [Google Scholar] [CrossRef]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional characterization and phenolic profiling of Moringa oleifera leaves grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2015, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Gutiérrez, P.K.; Hurtado-Fernández, E.; Gómez-Romero, M.; Hormaza, J.I.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Determination of changes in the metabolic profile of avocado fruits (Persea americana) by two CE-MS approaches (targeted and non-targeted). Electrophoresis 2013, 34, 2928–2942. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodríguez, J.A.; Molina-Corral, F.J.; Ayala-Zavala, J.F.; Olivas, G.I.; Gonzalez-Aguilar, G.A. Effect of maturity stage on the content of fatty acids and antioxidant activity of “Hass” avocado. Food Res. Int. 2011, 44, 1231–1237. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gomez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernandez-Gutierrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as valuable tools for the determination of phenolic and other polar compounds in the edible part and by-products of avocado. LWT-Food Sci. Technol. 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Pinto, J.; Spínola, V.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Molina-García, L.; Castilho, P.C. Polyphenolic profile and antioxidant activities of Madeiran elderberry (Sambucus lanceolata) as affected by simulated in vitro digestion. Food Res. Int. 2017, 100, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Spínola, V.; Castilho, P.C. Phenolic profiles of Lauraceae plant species endemic to Laurisilva forest: A chemotaxonomic survey. Ind. Crops Prod. 2017, 107, 1–12. [Google Scholar] [CrossRef]
- Jia, X.; Luo, H.; Xu, M.; Zhai, M.; Guo, Z.; Qiao, Y.; Wang, L. Dynamic changes in phenolics and antioxidant capacity during pecan (Carya illinoinensis) kernel ripening and its phenolics profiles. Molecules 2018, 23, 435. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Fernández, E.; Pacchiarotta, T.; Gómez-Romero, M.; Schoenmaker, B.; Derks, R.; Deelder, A.M.; Mayboroda, O.A.; Carrasco-Pancorbo, A.; Fernández-Gutiérrez, A. Ultra high-performance liquid chromatography-time of flight mass spectrometry for analysis of avocado fruit metabolites: Method evaluation and applicability to the analysis of ripening degrees. J. Chromatogr. A 2011, 1218, 7723–7738. [Google Scholar] [CrossRef]
- Santos, A.; Barros, L.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Leaves and decoction of Juglans regia L.: Different performances regarding bioactive compounds and in vitro antioxidant and antitumor effects. Ind. Crops Prod. 2013, 51, 430–436. [Google Scholar] [CrossRef]
- Beltrame, F.L.; Rodrigues-Filho, E.; Barros, F.A.P.; Cortez, D.A.G.; Cass, Q.B. A validated higher-performance liquid chromatography method for quantification of cinchonain Ib in bark and phytopharmaceuticals of Trichilia catigua used as Catuaba. J. Chromatogr. A 2006, 1119, 257–263. [Google Scholar] [CrossRef]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural characterization of flavonoid glycosides from leaves of wheat (Triticum aestivum L.) using LC/MS/MS profiling of the target compounds. J. Mass Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef]
- Jaiswal, J.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Zucolotto, S.M.; Fagundes, C.; Reginatto, F.H.; Ramos, F.A.; Castellanos, L.; Duque, C. Analysis of C-glycosyl flavonoids from South American Passiflora species by HPLC-DAD and HPLC-MS. Phytochem. Anal. 2012, 23, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive identification of bioactive compounds of avocado peel by liquid chromatography coupled to ultra-high-definition accurate-mass QTOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Inbaraj, B.S.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum Kitam by liquid chromatography-tandem mass spectrometry coupled with a post-column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Junqueira-Gonçalves, M.P.; Yáñez, L.; Morales, C.; Navarro, M.; Contreras, R.A.; Zúñiga, G.E. Isolation and characterization of phenolic compounds and anthocyanins from Murta (Ugni molinae Turcz.) fruits. Assessment of Antioxidant and Antibacterial Activity. Molecules 2015, 20, 5698–5713. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Isolation of Dihydroquercetin and Its Glycoside Dihydroquercetin Rhamnoside from Catha edulis Forsk. Available online: https://www.researchgate.net/publication/215662172_Dihydroquercetin_and_its_glycoside_isolated_from_Catha_edulis (accessed on 7 March 2018).
- Zhang, J.Y.; Zhang, Q.; Zhang, H.X.; Ma, Q.; Lu, J.Q.; Qiao, Y.J. Characterization of polymethoxylated flavonoids (PMFs) in the peels of ‘Shatangju’ mandarin (Citrus reticulata Blanco) by online High-Performance Liquid Chromatography coupled to Photodiode Array Detection and Electrospray Tandem Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 9023–9034. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.Y.; Jin, J.S.; Cho, Y.A.; Lee, J.H.; Park, S.; Jeong, S.W.; Kim, Y.; Lim, C.C.; Abd El-Aty, A.M.; Kim, G.S.; et al. Determination of polyphenols in three Capsicum annuum L. (bell pepper) varieties using high-performance liquid chromatography-tandem masss spectrometry: Their contribution to overall antioxidant and anticancer activity. J. Sep. Sci. 2011, 34, 2967–2974. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.D.; Yahia, E.M.; Ramirez-Bustamante, N.; Perez-Martinez, J.D.; Escalante-Minakata, M.D.; Ibarra-Junquera, V.; Acosta-Muñiz, C.; Guerrero-Prieto, V.; Ochoa-Reyes, E. Physical attributes and chemical composition of organic strawberry fruit (Fragaria x ananassa Duch, Cv. Albion) at six stages of ripening. Food Chem. 2013, 138, 372–381. [Google Scholar] [CrossRef]
- Williams, J.D.; Wojcińska, M.; Calabria, L.M.; Linse, K.; Clevinger, J.A.; Mabry, T.J. The flavonoids and phenolic acids of the genus Silphium and their chemosystematic and medicinal value. Nat. Prod. Commun. 2009, 4, 435–446. [Google Scholar]
- García-Salas, P.; Gómez-Caravaca, A.M.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification and quantification of phenolic and other polar compounds in the edible part of Annona cherimola and its by-products by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2015, 78, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Tasioula-Margari, M.; Tsabolatidou, E. Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS. Antioxidants 2015, 4, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signport: Trivandrum, India, 2006; Volume 1, pp. 23–67. [Google Scholar]
- Alfaro, S.; Mutis, A.; Palma, R.; Quiroz, A.; Seguel, I.; Scheuermann, E. Influence of genotype and harvest year on polyphenol content and antioxidant activity in murtilla (Ugni molinae Turcz) fruit. J. Soil Sci. Plant Nutr. 2013, 13, 67–78. [Google Scholar] [CrossRef]
- Margraf, T.; Taborda-Santos, E.N.; Forvillede Andrade, E.; van Ruth, S.M.; Granato, D. Effects of geographical origin, variety and farming system on the chemical markers and in vitro antioxidant capacity of Brazilian purple grape juices. Food Res. Int. 2016, 82, 145–155. [Google Scholar] [CrossRef]
- Mitsopoulos, G.; Papageorgiou, V.; Komaitis, M.; Hagidimitriou, M. Total phenolic content and antioxidant activity of leaves and drupes in major greek olive varieties. Not. Bot. Horti. Agrobot. Cluj. Napoca. 2016, 44, 155–161. [Google Scholar] [CrossRef]
- Daayf, F.; Ongena, M.; Boulanger, R.; El Hadrami, I.; Bélanger, R.R. Induction of phenolic compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extracts of Reynoutria sachalinensis. J. Chem. Ecol. 2000, 26, 1579–1593. [Google Scholar] [CrossRef]
- Yan, K.; Zhao, S.; Bian, L.; Chen, X. Saline stress enhanced accumulation of leaf phenolics in honeysuckle (Lonicera japonica Thunb.) without induction of oxidative stress. Plant. Physiol. Biochem. 2017, 112, 326–334. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; Rubio-Wilhelmi, M.M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: Changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Mayoral, O.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant 2016, 38. [Google Scholar] [CrossRef]
- Ariestya-Arlene, A.; Prima, K.A.; Utama, L.; Anggraini, S.A. The preliminary study of the dye extraction from the avocado seed using ultrasonic assisted extraction. Procedia Chem. 2015, 16, 334–340. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Van den Berg, R.; Haenen, G.R.; Van den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Ventura-Sobrevilla, J.M.; González-Hernández, M.D.; Rojas, R.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Martínez-Ávila, G.C.G. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


| Cultivar | Antioxidant Activity (IC50 Value µg·mL−1) | ||
|---|---|---|---|
| DPPH• | ABTS•+ | LPO | |
| Platano | 1514.24 (±20.02) b | 416.20 (±11.65) b | 1708.18 (±12.39) b |
| Criollo 6 | 308.43 (±27.67) e | 269.56 (±6.52) f | 810.34 (±7.63) g |
| PD | 271.86 (±13.69) f | 287.52 (±6.63) e | 1829.60 (±14.01) a |
| Criollo 1 | 1029.71 (±6.33) c | 415.21 (±1.61) b | 1255.37 (±4.16) c |
| PT | 1618.71 (±23.96) a | 442.72 (±9.62) a | 1229.66 (±13.12) d |
| Campeon | 984.97 (±5.34) d | 318.32 (±19.62) d | 837.17 (±6.53) f |
| TA | 1026.89 (±7.88) c | 388.95 (±8.70) c | 1048.88 (±28.30) e |
| Peak N° | Rt (min) | m/z Experimental [M − H]− | m/z Calculated [M − H]− | Tentative Assignment | Molecular Formula | Error (ppm) | MS2 Fragment Ion | Cultivar | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platano | Criollo 6 | PD | Criollo 1 | PT | Campeon | TA | |||||||||
| Phenolic acids | |||||||||||||||
| 5 | 1.66 | 209.1988 | 209.0533 | 5-Hydroxyferulic acid | C10H10O5 | 695.99 | 137.2726 | x | [20] | ||||||
| 7 | 1.83 | 163.0793 | 163.0478 | p-Coumaric acid | C9H8O3 | 193.19 | 163.0441 | x | [21] | ||||||
| 8 | 1.83 | 353.1204 | 353.0956 | 5-O-Caffeoylquinic acid | C16H18O9 | 70.23 | 191.1986 | x | [22] | ||||||
| 9 | 1.96 | 147.0825 | 147.0529 | trans-Cinnamic acid | C9H8O2 | 201.28 | 103.0221 | x | [23] | ||||||
| 10 | 1.96 | 325.1297 | 325.0928 | p-Coumaroyl hexose | C15H17O8 | 113.50 | 163.1347 | x | [24] | ||||||
| 18 | 2.47 | 337.1829 | 337.1007 | 3-O-p-Coumaroylquinic acid | C16H18O8 | 243.84 | 163.1162 | x | [25] | ||||||
| 19 | 2.47 | 431.2072 | 431.1922 | Roseoside (formate adduct) | C20H31O10 | 34.78 | 385.1421 | x | [26] | ||||||
| 26 | 3.82/4.16 | 551.2521 | 551.2497 | Dihydroxybenzoic acid derivative | C28H39O11 | 4.35 | 443.2231 | x | x | x | [27] | ||||
| 35 | 5.51 | 491.0785 | 491.1065 | Dimethyl ellagic acid hexoside | C22H22O13 | −57.01 | 328.0980 | x | x | x | [28] | ||||
| 37 | 5.68 | 487.1827 | 487.1821 | Caffeoyl hexose-deoxyhexoside | C22H31O12 | 1.23 | 179.2602 | x | x | [29] | |||||
| 39 | 5.99 | 341.1605 | 341.0956 | Caffeic acid-hexoside | C15H18O9 | 190.26 | 179.0185 | x | x | x | x | [29] | |||
| Flavonoids | |||||||||||||||
| 4 | 1.49 | 863.1945 | 863.190713 | Procyanidin trimer A | C45H36O18 | 4.38 | 411.1457 | x | [30] | ||||||
| 6 | 1.66 | 451.2266 | 451.1112 | Cinchonain | C24H20O9 | 255.81 | 341.1636 | x | x | x | x | x | x | [31] | |
| 11 | 1.96 | 563.2106 | 563.1484 | Apigenin-C-hexoside-C-pentoside | C26H28O14 | 110.45 | 353.1074 | x | x | [32] | |||||
| 13 | 2.16/2.23 | 435.2368 | 435.1011 | Taxifolin O-pentoside | C20H20O11 | 311.88 | 285.1166 | x | x | x | x | x | [33] | ||
| 14 | 2.16 | 577.1094 | 577.1351 | Procyanidin dimer B | C30H25O12 | −44.53 | 425.1052 | x | [30] | ||||||
| 15 | 2.30 | 431.2063 | 431.1061 | Vitexin | C21H20O10 | 232.42 | 311.1196 | x | [34] | ||||||
| 16 | 2.37/4.74 | 433.2227 | 433.1089 | Peonidin 3-O-pentoside | C21H21O11 | 262.75 | 300.9112 | x | x | x | x | x | [35] | ||
| 17 | 2.40 | 289.1178 | 289.0717 | Catechin | C15H13O6 | 159.47 | 245.1362 | x | [36] | ||||||
| 20 | 2.71 | 625.0995 | 625.1488 | Quercetin 3,4′-O-diglucoside | C27H30O17 | −78.86 | 300.1967 | x | x | x | x | x | [37] | ||
| 21 | 2.94/3.32 | 433.2232 | 433.1140 | Pelargonidin 3-O-glucoside | C21H21O10 | 252.12 | 271.1117 | x | x | x | x | x | x | [38] | |
| 22 | 3.35/3.42/4.63 | 609.1123 | 609.1539 | Rutin | C27H30O16 | −68.29 | 301.0183 | x | x | x | x | x | [25] | ||
| 23 | 3.62 | 595.0945 | 595.1382 | Quercetin-O-arabinosyl-glucoside | C26H28O16 | −73.42 | 300.0086 | x | x | x | x | x | [25] | ||
| 27 | 3.89/4.02 | 463.0943 | 463.0960 | Quercetin-3-glucoside | C21H20O12 | −3.67 | 300.0722 | x | x | x | x | x | x | [39] | |
| 28 | 3.96 | 477.0708 | 477.0752 | Quercetin glucuronide | C21H18O13 | −9.22 | 301.0646 | x | x | x | [39] | ||||
| 29 | 4.19 | 521.1903 | 521.1875 | Polymethoxylated flavonoid-O-pentoside | C22H33O14 | 5.37 | 389.1792 | x | x | x | [40] | ||||
| 30 | 4.47 | 419.2465 | 419.0983 | Cyanidin 3-O-arabinoside | C20H19O10 | 353.61 | 287.1895 | x | x | [33] | |||||
| 31 | 4.47/4.80 | 579.1083 | 579.1433 | Luteolin 7-O-(2″-O-pentosyl) hexoside | C26H28O15 | −60.43 | 285.2477 | x | x | x | x | x | [41] | ||
| 32 | 4.67/4.97 | 447.1046 | 447.0932 | Kaempferol-O-hexoside | C21H19O11 | 25.49 | 285.0909 | x | x | x | x | x | [42] | ||
| 33 | 4.97 | 461.0767 | 461.1167 | Isorhamnetin-O-coumaroyl | C22H22O11 | −86.74 | 314.1016 | x | x | x | [21] | ||||
| 34 | 5.01/5.14 | 447.1041 | 447.1011 | Quercetin-O-deoxyhesoxide | C21H20O11 | 6.70 | 301.0722 | x | x | x | x | x | x | x | [21] |
| 36 | 5.51 | 417.1019 | 417.0905 | Kaempferol-O-pentoside | C20H18O10 | 27.33 | 284.0728 | x | x | x | x | [42] | |||
| 38 | 5.68 | 593.1145 | 593.1511 | Catechin diglucopyranoside | C27H29O15 | −61.70 | 284.0763 | x | x | x | x | x | [43] | ||
| 40 | 6.43 | 431.1131 | 431.0983 | Kaempferol-O-coumaroyl | C21H19O10 | 34.16 | 285.0876 | x | x | x | x | x | x | x | [42] |
| 41 | 6.63 | 449.1195 | 449.1167 | Dihydroquercetin-3,5-rhamnoside | C21H22O11 | 6.23 | 303.0182 | x | [44] | ||||||
| Other compounds | |||||||||||||||
| 1 | 0.78 | 211.1406 | 211.0901 | Perseitol | C7H16O7 | 239.23 | 101.0183 | x | x | x | x | x | x | x | [25] |
| 2 | 1.01 | 375.1574 | 375.1508 | Unknown | C13H27O12 | 17.59 | 241.1546 | x | x | x | x | x | x | x | ------- |
| 3 | 1.29 | 315.1134 | 315.1163 | Hydroxytyrosol glucoside | C14H20O8 | −9.20 | 135.1637 | x | x | x | [25,45] | ||||
| 12 | 2.16 | 413.2761 | 413.2756 | Unknown | C19H41O9 | 1.20 | 175.1897 | x | x | ------- | |||||
| 24 | 3.72 | 401.1310 | 401.1300 | Unknown | C14H25O13 | 2.49 | 269.0166 | x | ------- | ||||||
| 25 | 3.79 | 551.2495 | 551.2497 | Unknown | C28H39O11 | −0.54 | 505.2192 | x | x | ------- | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-López, C.; Bautista-Hernández, I.; González-Hernández, M.D.; Martínez-Ávila, G.C.G.; Rojas, R.; Gutiérrez-Díez, A.; Medina-Herrera, N.; Aguirre-Arzola, V.E. Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars. Molecules 2019, 24, 173. https://doi.org/10.3390/molecules24010173
Castro-López C, Bautista-Hernández I, González-Hernández MD, Martínez-Ávila GCG, Rojas R, Gutiérrez-Díez A, Medina-Herrera N, Aguirre-Arzola VE. Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars. Molecules. 2019; 24(1):173. https://doi.org/10.3390/molecules24010173
Chicago/Turabian StyleCastro-López, Cecilia, Israel Bautista-Hernández, María D. González-Hernández, Guillermo C. G. Martínez-Ávila, Romeo Rojas, Adriana Gutiérrez-Díez, Nancy Medina-Herrera, and Víctor E. Aguirre-Arzola. 2019. "Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars" Molecules 24, no. 1: 173. https://doi.org/10.3390/molecules24010173