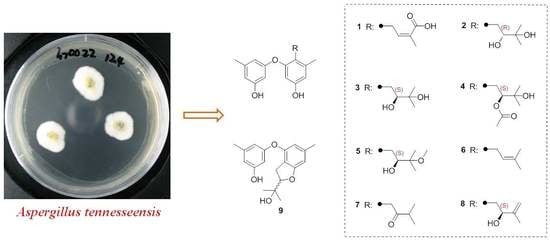
Prenylated Diphenyl Ethers from the Marine Algal-Derived Endophytic Fungus Aspergillus tennesseensis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Elucidation of the New Compounds
2.2. Biological Activities of the Isolated Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction and Isolation
3.4. Antimicrobial Assay
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, S.S.; Jiang, N.; Wang, X.L.; Chen, C.J.; Fan, J.Y.; Wurin, G.; Ge, H.M.; Tan, R.X.; Jiao, R.H. Prenylated diphenyl ethers from the mantis-associated fungus Aspergillus versicolor GH-2. Tetrahedron Lett. 2015, 56, 3894–3897. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, L.; Cai, S.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Diorcinols B–E, new prenylated diphenyl ethers from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Antibiot. 2013, 66, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, H.; Xu, H.; Yin, L.; Chen, Z.; Shen, H. Diphenyl ethers from a marine-derived isolate of Aspergillus sp. CUGB-F046. Nat. Prod. Res. 2018, 32, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.M.; Shang, Z.; Li, C.S.; Ji, N.Y.; Wang, B.G. Meroterpenoid and diphenyl ether derivatives from Penicillium sp. MA-37, a fungus isolated from marine mangrove rhizospheric soil. J. Nat. Prod. 2012, 75, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kanoh, K.; Adachi, K.; Shizuri, Y. New dihydrobenzofuran derivative, awajanoran, from marine-derived Acremonium sp. AWA16-1. J. Antibiot. 2006, 59, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.L.; Shao, C.L.; Wang, C.Y.; Wang, M.; Yang, L.J.; Wang, C.Y. Naphthalenones and depsidones from a sponge-derived strain of the fungus Corynespora cassiicola. Molecules 2016, 21, 160. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yun, K.; Nenkep, V.N.; Choi, H.D.; Kang, J.S.; Son, B.W. Induced production of halogenated diphenyl ethers from the marine derived fungus Penicillium chrysogenum. Chem. Biodivers. 2010, 7, 2766–2770. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Schmitz, F.J.; Govindan, M.; Abbas, S.A.; Hanson, K.M.; Horton, P.A.; Crews, P.; Laney, M.; Schatzman, R.C. Enzyme inhibitors: new and known polybrominated phenols and diphenyl ethers from four Indo-Pacific Dysidea sponges. J. Nat. Prod. 1995, 58, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Bunyapaiboonsri, T.; Yoiprommarat, S.; Intereya, K.; Kocharin, K. New diphenyl ethers from the insect pathogenic fungus Cordyceps sp. BCC 1861. Chem. Pharm. Bull. 2007, 55, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, N.Y.; Wang, B.G. Mycochemistry of marine algicolous fungi. Fungal Divers. 2016, 80, 301–342. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Li, X.M.; Teuscher, F.; Li, D.L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, G.Q.; Tong, X.P.; Chen, G.D.; Huang, Y.F.; Cui, J.Y.; Kong, M.Z.; Guo, L.D.; Zheng, Y.Z.; Yao, X.S.; et al. Diphenyl ethers from Aspergillus sp. and their anti-Aβ42 aggregation activities. Fitoterapia 2014, 98, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Yurchenko, E.A.; Min’ko, E.M.; Ivanets, E.V.; Afiyatullov, S.S. New diorcinol J produced by co-cultivation of marine fungi Aspergillus sulphureus and Isaria feline. Chem. Nat. Compd. 2016, 52, 227–280. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Yurchenko, E.A.; Denisenko, V.A.; Kirichuk, N.N.; Dmitrenok, P.S. New metabolites from the algal associated marine-derived fungus Aspergillus carneus. Nat. Prod. Comm. 2013, 8, 1071–1074. [Google Scholar]
- Itabashi, T.; Nozawa, K.; Nakajima, S.; Kawai, K. A new azaphilone, falconensin H, from Emericella falconensis. Chem. Pharm. Bull. 1993, 41, 2040–2041. [Google Scholar] [CrossRef]
- Al-Burtamani, S.K.S.; Fatope, M.O.; Marwah, R.G.; Onifade, A.K.; Al-Saidi, S.H. Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman. J. Ethnopharmacol. 2005, 96, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.L.; Zhang, P.; Liu, X.M.; Du, Y.M.; Hou, X.D.; Cheng, S.; Zhang, Z.F. Cytological assessments and transcriptome profiling demonstrate that evodiamine inhibits growth and induces apoptosis in a renal carcinoma cell line. Sci. Rep. 2017, 7, 12572–12583. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–9 are available from the authors. |


| Compound 1 | Compound 2 | |||
|---|---|---|---|---|
| No. | δH (mult, J in Hz) | δC, Type | δH (mult, J in Hz) | δC, Type |
| 1 | 154.8, C | 156.1, C | ||
| 2 | 121.0, C | 119.3, C | ||
| 3 | 139.1, C | 139.8, C | ||
| 4 | 6.39, d (2.1) | 113.5, CH | 6.37, d (2.2) | 113.1, CH |
| 5 | 156.5, C | 156.6, C | ||
| 6 | 6.10, d (2.1) | 104.8, CH | 6.08, d (2.2) | 104.1, CH |
| 7 | 2.13, s | 19.8, CH3 | 2.25, s | 20.5, CH3 |
| 8 | 3.17, d (7.0) | 25.6, CH2 | 3.05, dd (14.5, 2.0) 2.80, dd (14.5, 11.4) | 29.3, CH2 |
| 9 | 6.17, t (7.0) | 132.5, CH | 4.04, dd (11.4, 2.0) | 73.2, CH |
| 10 | 133.8, C | 72.1, C | ||
| 11 | 172.5, C | 1.19, s | 27.2, CH3 | |
| 12 | 1.73, s | 14.1, CH3 | 1.20, s | 26.2, CH3 |
| 1’ | 159.1, C | 158.9, C | ||
| 2’ | 6.26, br s | 111.0, CH | 6.30, br s | 111.2, CH |
| 3’ | 140.2, C | 140.4, C | ||
| 4’ | 6.11, br s | 109.0, CH | 6.20, br s | 109.9, CH |
| 5’ | 158.9, C | 158.4, C | ||
| 6’ | 6.08, br s | 102.3, CH | 6.10, br s | 102.7, CH |
| 7’ | 2.14, s | 21.6, CH3 | 2.16, s | 21.6, CH3 |
| 10-OH | 4.77, s | |||
| Compound | Bacteria a | Fungi b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. s. | E. c. | P. a. | R. s. | A. a. | C. h. | G. g. | G. c. | M. h. | T. b. | |
| 1 | − c | 8 | 16 | 8 | 16 | 32 | 16 | 32 | − | − |
| 2 | 8 | 4 | 16 | 16 | 64 | 4 | 16 | 64 | 32 | 64 |
| 3 | − | − | 16 | 32 | − | − | 32 | 64 | 64 | 64 |
| 4 | 16 | 16 | 32 | 8 | − | − | 32 | − | 32 | 64 |
| 5 | 8 | − | 32 | − | − | − | − | 32 | 16 | − |
| 6 | 32 | 8 | 64 | − | − | − | 64 | 32 | − | − |
| 7 | 8 | 64 | − | 32 | − | − | 2 | − | 16 | − |
| 8 | 4 | − | 64 | 16 | − | − | − | − | 8 | − |
| 9 | 2 | 64 | 32 | − | − | − | − | 16 | 8 | 8 |
| Ch d | 0.5 | 2 | 16 | 16 | ||||||
| Pr e | 16 | 8 | 8 | 32 | 8 | 64 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-X.; Wang, X.-F.; Ren, G.-W.; Yuan, X.-L.; Deng, N.; Ji, G.-X.; Li, W.; Zhang, P. Prenylated Diphenyl Ethers from the Marine Algal-Derived Endophytic Fungus Aspergillus tennesseensis. Molecules 2018, 23, 2368. https://doi.org/10.3390/molecules23092368
Li Z-X, Wang X-F, Ren G-W, Yuan X-L, Deng N, Ji G-X, Li W, Zhang P. Prenylated Diphenyl Ethers from the Marine Algal-Derived Endophytic Fungus Aspergillus tennesseensis. Molecules. 2018; 23(9):2368. https://doi.org/10.3390/molecules23092368
Chicago/Turabian StyleLi, Zhao-Xia, Xiu-Fang Wang, Guang-Wei Ren, Xiao-Long Yuan, Ning Deng, Gui-Xia Ji, Wei Li, and Peng Zhang. 2018. "Prenylated Diphenyl Ethers from the Marine Algal-Derived Endophytic Fungus Aspergillus tennesseensis" Molecules 23, no. 9: 2368. https://doi.org/10.3390/molecules23092368
APA StyleLi, Z.-X., Wang, X.-F., Ren, G.-W., Yuan, X.-L., Deng, N., Ji, G.-X., Li, W., & Zhang, P. (2018). Prenylated Diphenyl Ethers from the Marine Algal-Derived Endophytic Fungus Aspergillus tennesseensis. Molecules, 23(9), 2368. https://doi.org/10.3390/molecules23092368