Three New Iridoid Derivatives Have Been Isolated from the Stems of Neonauclea reticulata (Havil.) Merr. with Cytotoxic Activity on Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results and Discussions
2.1. Isolation and Structural Elucidation
2.2. Structural Identification of Known Isolates
2.3. In-Vitro Cytotoxic Activity Against Hep3B Cells
3. Materials and Methods
3.1. General
3.2. Chemicals
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Cell Culture
3.6. Cytotoxic Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, T.Y.A. Neonauclea merr. In Flora of Taiwan, 2nd ed.; The College of Science and Agriculture, National Taiwan University: Taipei, Taiwan, 1998; Volume 4, pp. 304–306. [Google Scholar]
- Rahman, A.U.; Vohra, I.I.; Choudhary, M.I.; de Silva, L.B.; Herath, W.H.M.W.; Navaratne, K.M. Neozeylanicine: A novel alkaloid from the timber of neonauclea zeylanica. Planta Med. 1988, 54, 461–462. [Google Scholar]
- Johns, S.; Lamberton, J.; Sioumis, A. The identification of a leaf alkaloid of neonauclea schlechteri (rubiaceae) as gambirine (9-hydroxydihydrocorynantheine). Aust. J. Chem. 1970, 23, 1285–1286. [Google Scholar] [CrossRef]
- Tosa, H.; Iinuma, M.; Asai, F.; Tanaka, T.; Nozaki, H.; Ikeda, S.; Tsutsui, K.; Tsutsui, K.; Yamada, M.; Fujimori, S. Anthraquinones from neonauclea calycina and their inhibitory activity against DNA topoisomerase ii. Biol. Pharm. Bull. 1998, 21, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Nishi, T. Two chromone-secoiridoid glycosides and three indole alkaloid glycosides from neonauclea sessilifolia. Phytochemistry 2003, 62, 359–369. [Google Scholar] [CrossRef]
- Kang, W.Y.; Zhang, B.R.; Hao, X.J. Isolation and identification of a new triterpene from neonauclea sessilifolia. Chem. J. Chin. Univ. 2007, 28, 2096–2098. [Google Scholar]
- Itoh, A.; Tanahashi, T.; Nagakura, N.; Nishi, T. Two triterpenoid saponins from neonauclea sessilifolia. Chem. Pharm. Bull. 2003, 51, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Abdulah, R.; Milanda, T.; Sugijanto, M.; Barliana, M.I.; Diantini, A.; Supratman, U.; Subarnas, A. Antibacterial properties of selected plants consumed by primates against escherichia coli and bacillus subtilis. Southeast. Asian J. Trop. Med. Public Health 2017, 48, 109–116. [Google Scholar] [PubMed]
- Kaur, R.; Kaur, H. Plant derived antimalarial agents. J. Med. Plants Stud. 2017, 5, 346–363. [Google Scholar]
- Chiang, H.M.; Chen, H.C.; Chiu, H.H.; Chen, C.W.; Wang, S.M.; Wen, K.C. Neonauclea reticulata (havil.) merr. stimulates skin regeneration after uvb exposure via ros scavenging and modulation of the mapk/mmps/collagen pathway. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA A Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Cancer Registry Annual Report, 2015 Taiwan. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=269&pid=8084 (accessed on 8 August 2018).
- Qiu, G.H.; Xie, X.; Xu, F.; Shi, X.; Wang, Y.; Deng, L. Distinctive pharmacological differences between liver cancer cell lines hepg2 and hep3b. Cytotechnology 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, E.K.; Stuart, K.E. Overview of treatment approaches for hepatocellular carcinoma. In Uptodate; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 12 February 2018. [Google Scholar]
- Lee, H.Z.; Bau, D.T.; Kuo, C.L.; Tsai, R.Y.; Chen, Y.C.; Chang, Y.H. Clarification of the phenotypic characteristics and anti-tumor activity of hedyotis diffusa. Am. J. Chin. Med. 2011, 39, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Qiu, X.Q.; Shu, Z.H.; Liu, Q.C.; Hu, M.B.; Han, T.; Rahman, K.; Qin, L.P.; Zheng, C.J. Hepatoprotective activity of total iridoid glycosides isolated from paederia scandens (lour.) merr. Var. Tomentosa. J. Ethnopharm. 2015, 174, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Hart, N.; Johns, S.; Lamberton, J. Alkaloids of jasminum species (family oleaceae). Ii. Isolation of a new monoterpenoid alkaloid and other constituents. Aust. J. Chem. 1969, 22, 1283–1290. [Google Scholar] [CrossRef]
- Topcu, G.; Che, C.T.; Cordell, G.A.; Ruangrungsi, N. Iridolactones from alyxia reinwardti. Phytochemistry 1990, 29, 3197–3199. [Google Scholar] [CrossRef]
- Horn, M.M.; Drewes, S.E.; Brown, N.J.; Munro, O.Q.; Meyer, J.J.M.; Mathekga, A.D.M. Transformation of naturally-occurring 1,9-trans-9,5-cis sweroside to all trans sweroside during acetylation of sweroside aglycone. Phytochemistry 2001, 57, 51–56. [Google Scholar] [CrossRef]
- Mitsunaga, K.; Koike, K.; Fukuda, H.; Ishii, K.; Ohmoto, T. Ligustrinoside, a new bisiridoid glucoside from strychnos ligustrina. Chem. Pharm. Bull. 1991, 39, 2737–2738. [Google Scholar] [CrossRef]
- Umezawa, T.; Nakatsubo, F.; Higuchi, T. Lignin degradation by phanerochaete chrysosporium: Metabolism of a phenolic phenylcoumaran substructure model compound. Arch. Microbiol. 1982, 131, 124–128. [Google Scholar] [CrossRef]
- Yang, J.H.; Liu, W.Y.; Li, S.C.; Ye, H.Y.; Tang, H.; Chen, L.J.; Peng, A.H. Coumarinolignans isolated from the seeds of brucea javanica. Helv. Chim. Acta 2014, 97, 278–282. [Google Scholar] [CrossRef]
- Li, Y.C.; Kuo, Y.H. Four new compounds, ficusal, ficusesquilignan a, b, and ficusolide diacetate from the heartwood of ficus microcarpa. Chem. Pharm. Bull. 2000, 48, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Zhang, H.J.; Niu, X.M.; Yao, P.; Sun, H.D.; Fong, H.H.S. Chemical constituents from amentotaxus yunnanensis and torreya yunnanensis. J. Nat. Prod. 2003, 66, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.; Makhmoor, T.; Choudhary, M.I. Radical scavenging potential of compounds isolated from Vitex agnus-castus. Turk. J. Chem. 2010, 34, 119–126. [Google Scholar]
- Lee, C.K.; Lu, C.K.; Kuo, Y.H.; Chen, J.Z.; Sun, G.Z. New prenylated flavones from the roots of ficus beecheyana. J. Chin. Chem. Soc. 2004, 51, 437–441. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural occurrence of abscisic acid in heather honey and floral nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Siems, W.G. Lipid peroxidation and pharmaceutical drugs. Free Radic. Biol. Med. 2018, 124, 565. [Google Scholar] [CrossRef]
- Lou, L.L.; Yao, G.D.; Wang, J.; Zhao, W.Y.; Wang, X.B.; Huang, X.X.; Song, S.J. Enantiomeric neolignans from picrasma quassioides exhibit distinctive cytotoxicity on hepatic carcinoma cells through ros generation and apoptosis induction. Bioorg. Med. Chem. Lett. 2018, 28, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–15 are available from the authors. |

 ) correlations for compounds 1–3 (b). Significant NOESY (
) correlations for compounds 1–3 (b). Significant NOESY (  ) correlations of compounds 1–3.
) correlations of compounds 1–3.
 ) correlations for compounds 1–3 (b). Significant NOESY (
) correlations for compounds 1–3 (b). Significant NOESY (  ) correlations of compounds 1–3.
) correlations of compounds 1–3.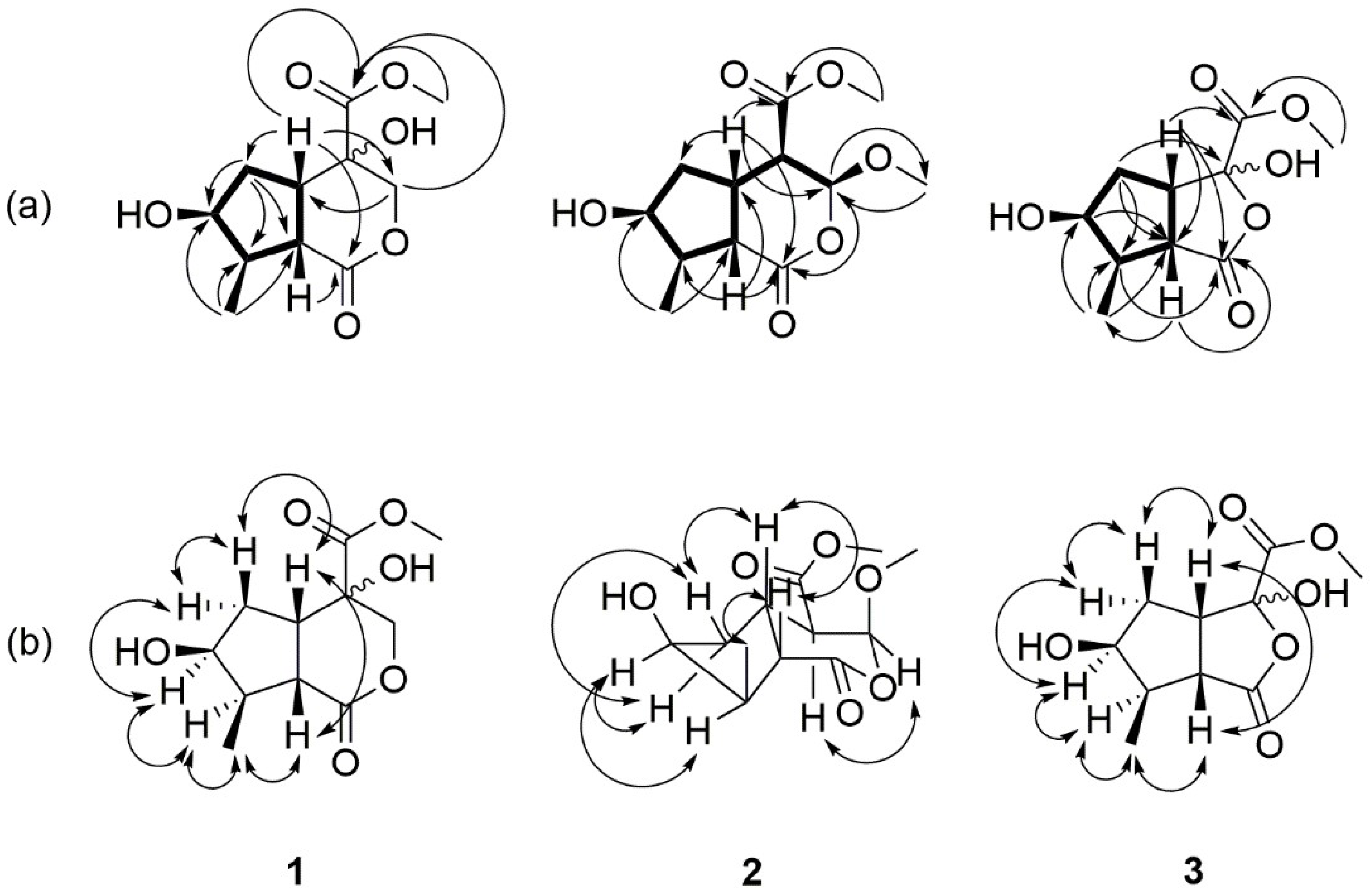

| Compound 1 | Compound 2 | Compound 3 | |||||
|---|---|---|---|---|---|---|---|
| Position | δH | δC | δH | δC | Position | δH | δC |
| 1 | 178.5 | 173.9 | 1 | 176.7 | |||
| 2 | 2 | ||||||
| 3α | 3.87 (d, J = 12.0) | 67.5 | 5.42 (d, J = 2.4) | 101.9 | 3 | 100.3 | |
| 3β | 3.97 (d, J = 12.0) | 4 | 3.47 (q, J = 8.5) | 44.8 | |||
| 4 | 88.4 | 2.55 (dd, J = 11.4, 2.4) | 49.8 | 5α | 2.25 (m) | 34.6 | |
| 5 | 3.31 (ddd, J = 9.9, 8.0) | 43.9 | 3.28 (m) | 31.9 | 5β | 1.88 (m) | |
| 6α | 1.53 (m) | 37.8 | 1.38 (m) | 42.1 | 6 | 4.29 (m) | 76.0 |
| 6β | 2.01 (m) | 2.34 (m) | 7 | 2.36 (m) | 44.3 | ||
| 7 | 4.22 (m) | 76.9 | 4.12 (m) | 74.8 | 8 | 2.99 (t, J = 8.5) | 51.6 |
| 8 | 2.29 (m) | 44.4 | 2.27 (m) | 43.1 | 9 | 1.23 (d, J = 7.0) | 13.6 |
| 9 | 2.88 (dd, J = 9.9, 7.1) | 50.6 | 2.79 (dd, J = 11.4, 9.1) | 46.0 | 10 | 170.1 | |
| 10 | 1.23 (d, J = 7.1) | 14.1 | 1.28 (d, J = 6.9) | 14.3 | COOMe | 3.88 (s) | 54.1 |
| 11 | 169.1 | 169.8 | |||||
| COOMe | 3.81 (s) | 52.8 | 3.51 (s) | 57.1 | |||
| 3-OMe | 3.76 (s) | 52.4 | |||||
| Compounds | EC50 (µg/mL) in 48 h |
|---|---|
| neonanin A (1) | >100 |
| neonanin B (2) | >100 |
| neoretinin A (3) | >100 |
| 6-hydroxy-7-methyl-1-oxo-4-carbomethoxyoctahydrocyclopenta[c]pyran (4) | >100 |
| 4-epi-alyxialactone (5) | >100 |
| loganetin (6) | >100 |
| loganin (7) | >100 |
| phenylcoumaran-α′-aldehyde (8) | >100 |
| cleomiscosin A (9) | >100 |
| ficusal (10) | 85.36 ± 4.36 |
| balanophonin (11) | 92.63 ± 1.41 |
| vanillic acid (12) | >100 |
| p-coumaric acid (13) | 29.18 ± 3.48 |
| Doxorubicin | 0.31 ± 0.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, F.-P.; Chao, W.; Wang, S.-Y.; Huang, H.-C.; Sung, P.-J.; Chen, J.-J.; Cheng, M.-J.; Huang, G.-J.; Kuo, Y.-H. Three New Iridoid Derivatives Have Been Isolated from the Stems of Neonauclea reticulata (Havil.) Merr. with Cytotoxic Activity on Hepatocellular Carcinoma Cells. Molecules 2018, 23, 2297. https://doi.org/10.3390/molecules23092297
Chang F-P, Chao W, Wang S-Y, Huang H-C, Sung P-J, Chen J-J, Cheng M-J, Huang G-J, Kuo Y-H. Three New Iridoid Derivatives Have Been Isolated from the Stems of Neonauclea reticulata (Havil.) Merr. with Cytotoxic Activity on Hepatocellular Carcinoma Cells. Molecules. 2018; 23(9):2297. https://doi.org/10.3390/molecules23092297
Chicago/Turabian StyleChang, Fang-Pin, Wei Chao, Sheng-Yang Wang, Hui-Chi Huang, Ping-Jyun Sung, Jih-Jung Chen, Ming-Jen Cheng, Guan-Jhong Huang, and Yueh-Hsiung Kuo. 2018. "Three New Iridoid Derivatives Have Been Isolated from the Stems of Neonauclea reticulata (Havil.) Merr. with Cytotoxic Activity on Hepatocellular Carcinoma Cells" Molecules 23, no. 9: 2297. https://doi.org/10.3390/molecules23092297






