Molybdenum Trioxide: Efficient Nanosorbent for Removal of Methylene Blue Dye from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Molybdenum Trioxide Nanosorbent
2.2. Adsorption Experiments
2.3. Adsorbent Regeneration Method
2.4. Characterization
3. Results and Discussion
3.1. Removal of MB
3.1.1. Effect of Initial Dye Concentration and Contact Time
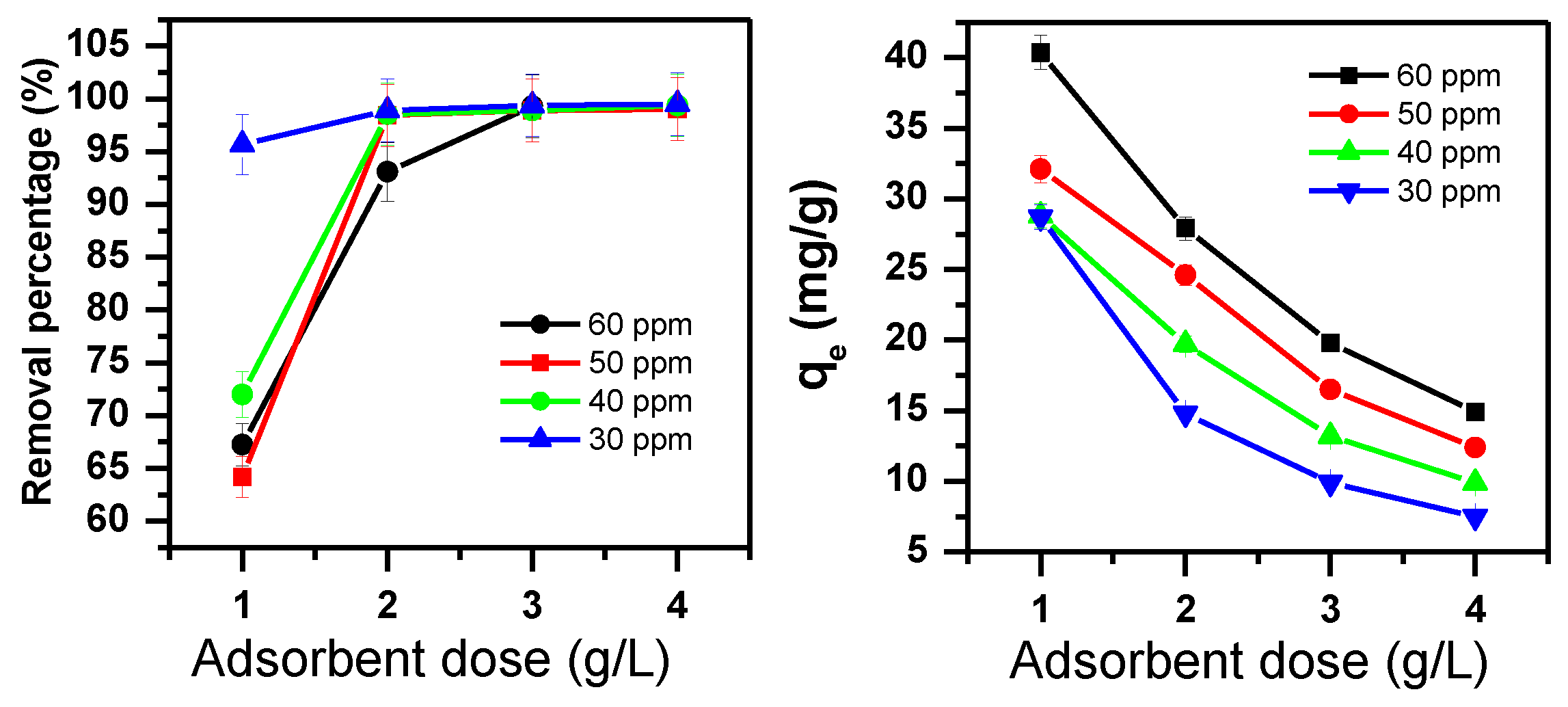
3.1.2. Effect of Adsorbent Dose and Initial Dye Concentration
3.1.3. Temperature Effect
3.1.4. Effect of pH
3.1.5. Effect of MB Initial Dye Concentration and Contact Time after pH Adjustment
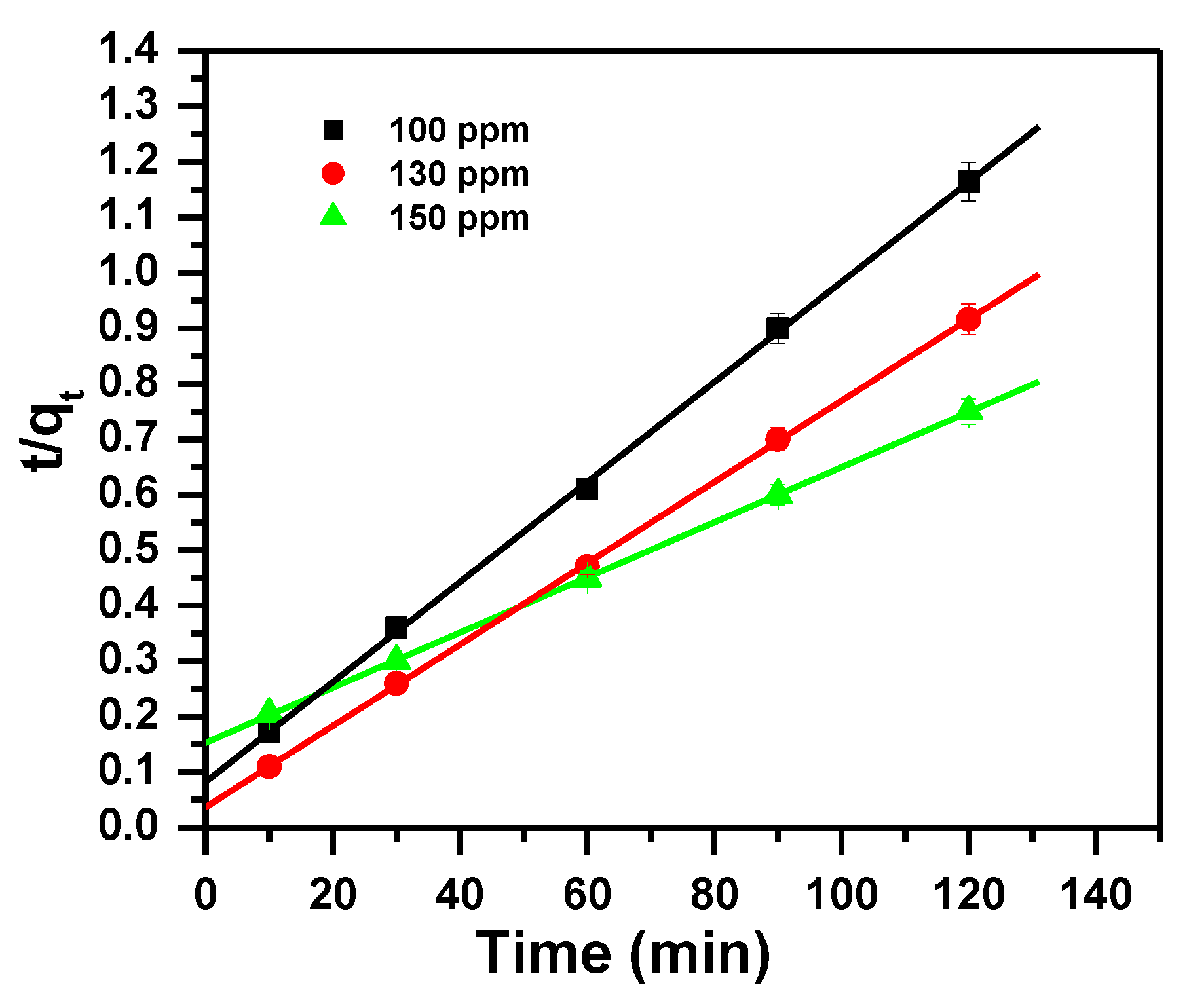
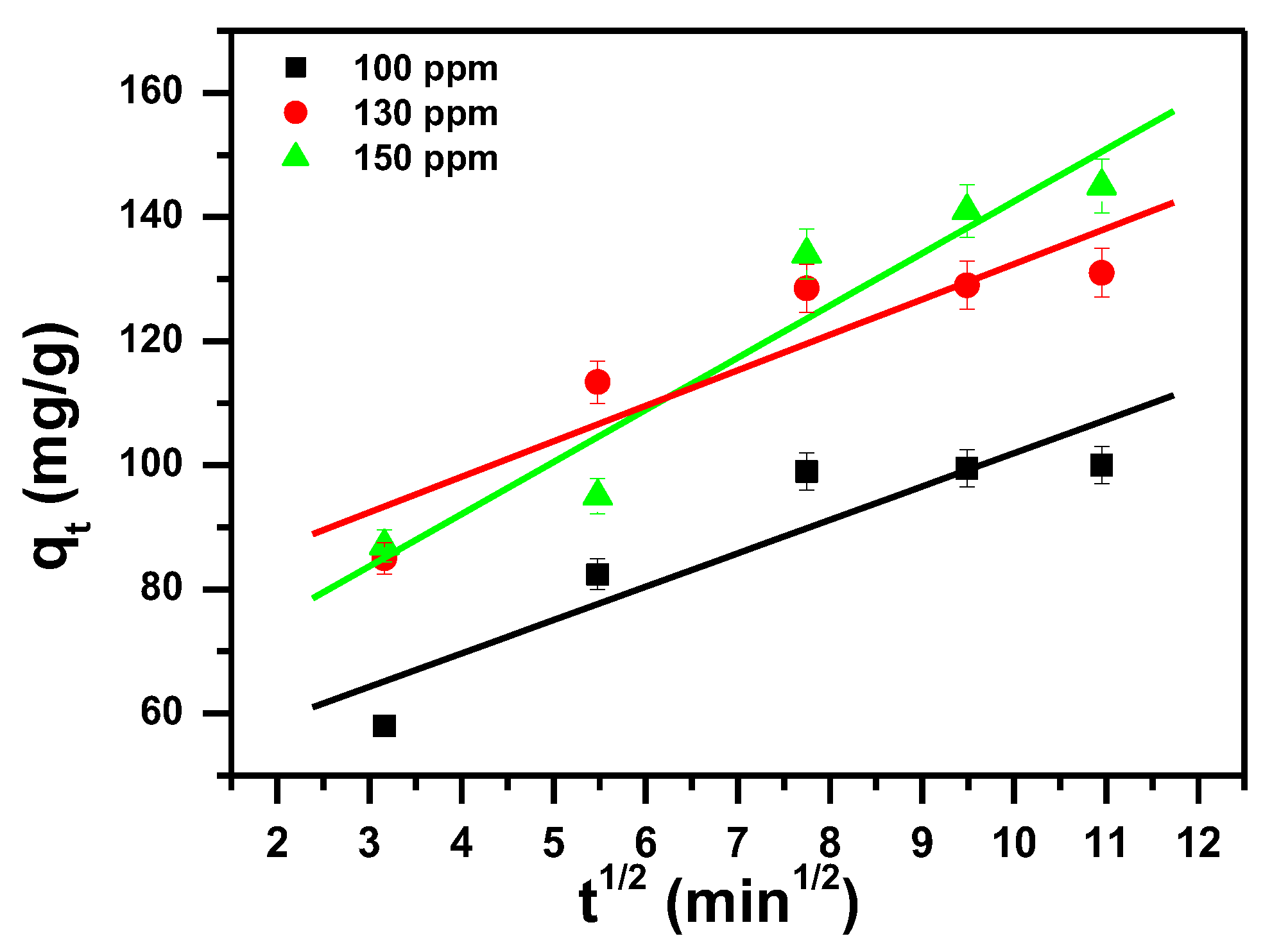
3.2. Kinetic Study
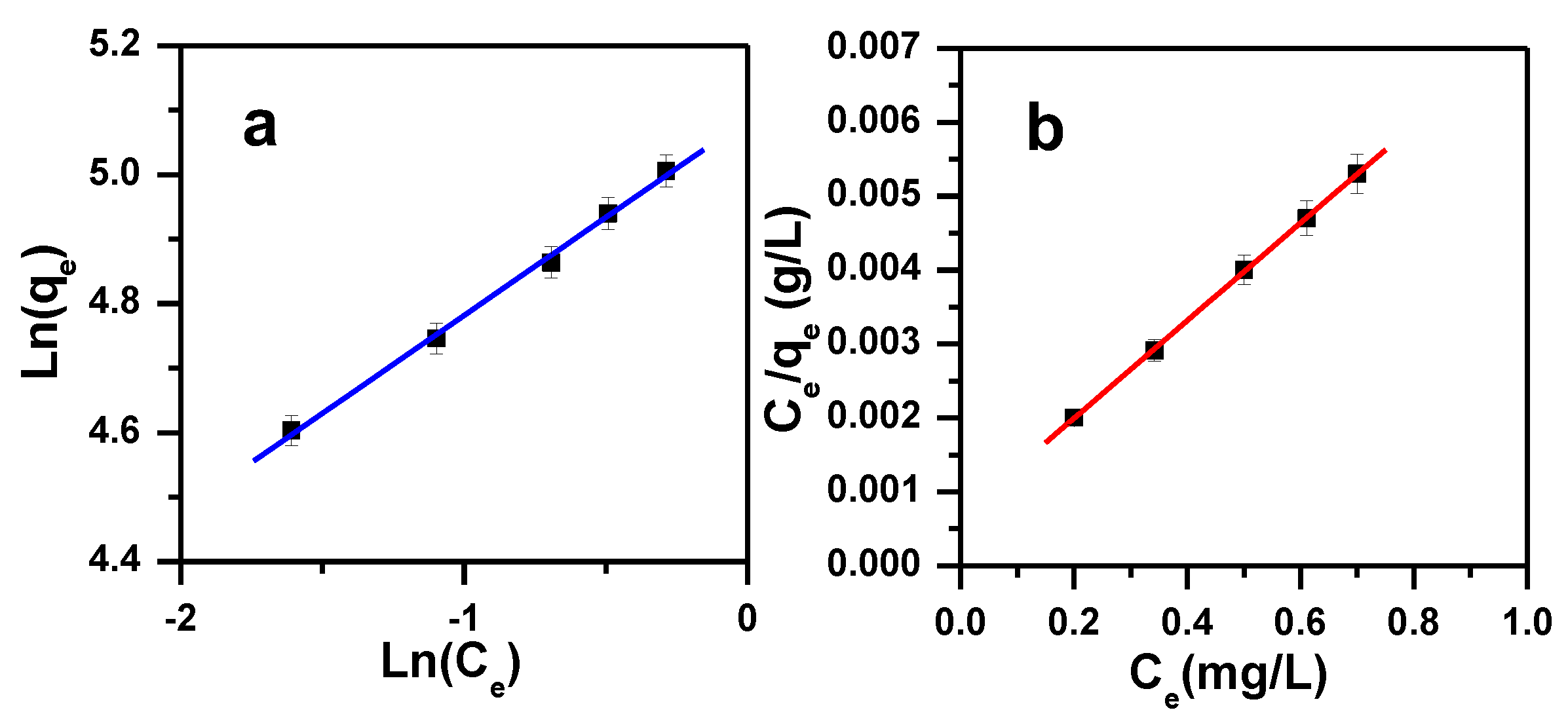
3.3. Adsorption Isotherms
3.4. Regeneration and Characterization of the Nanosorbent
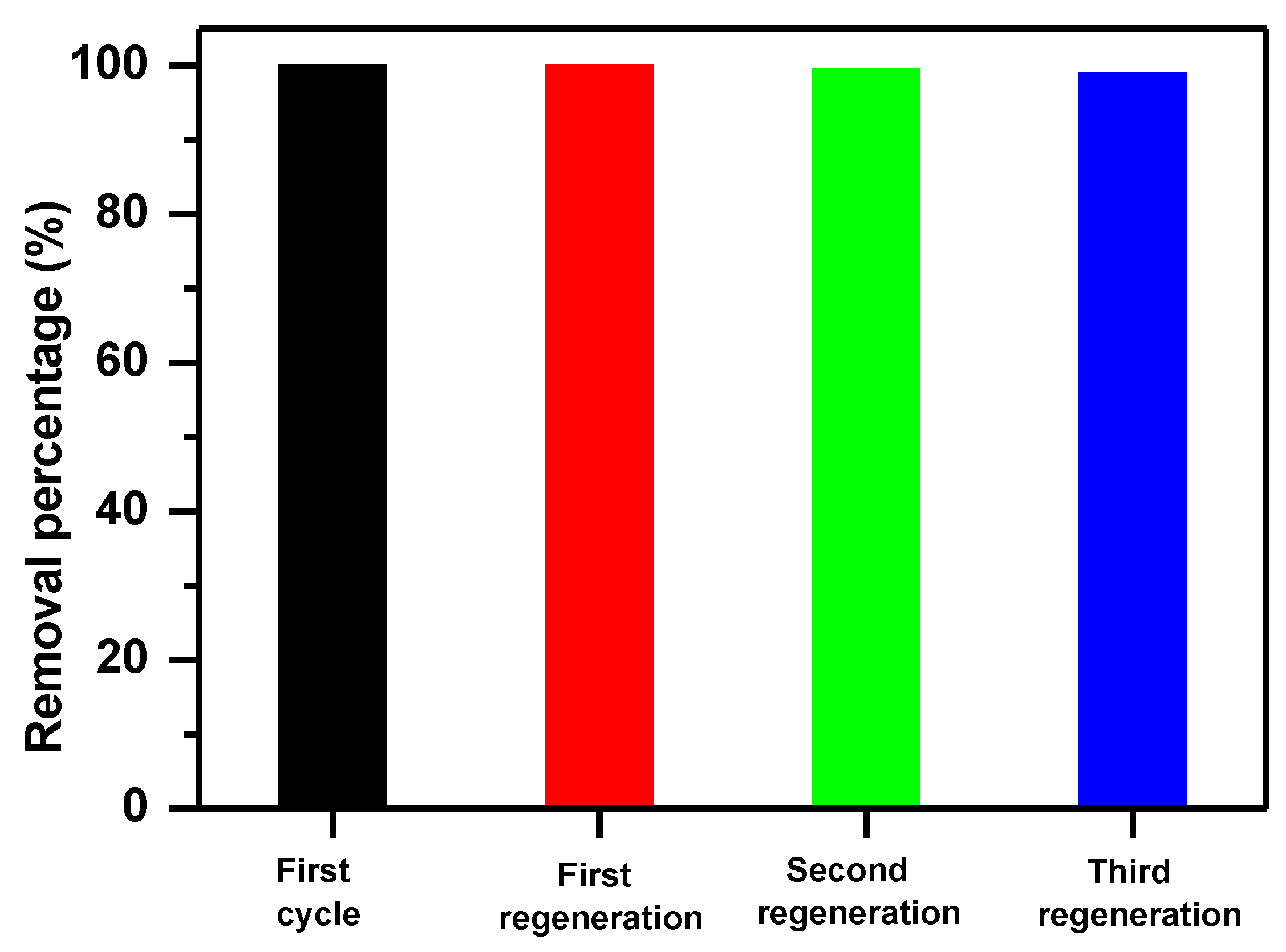
3.4.1. Regeneration Efficiency
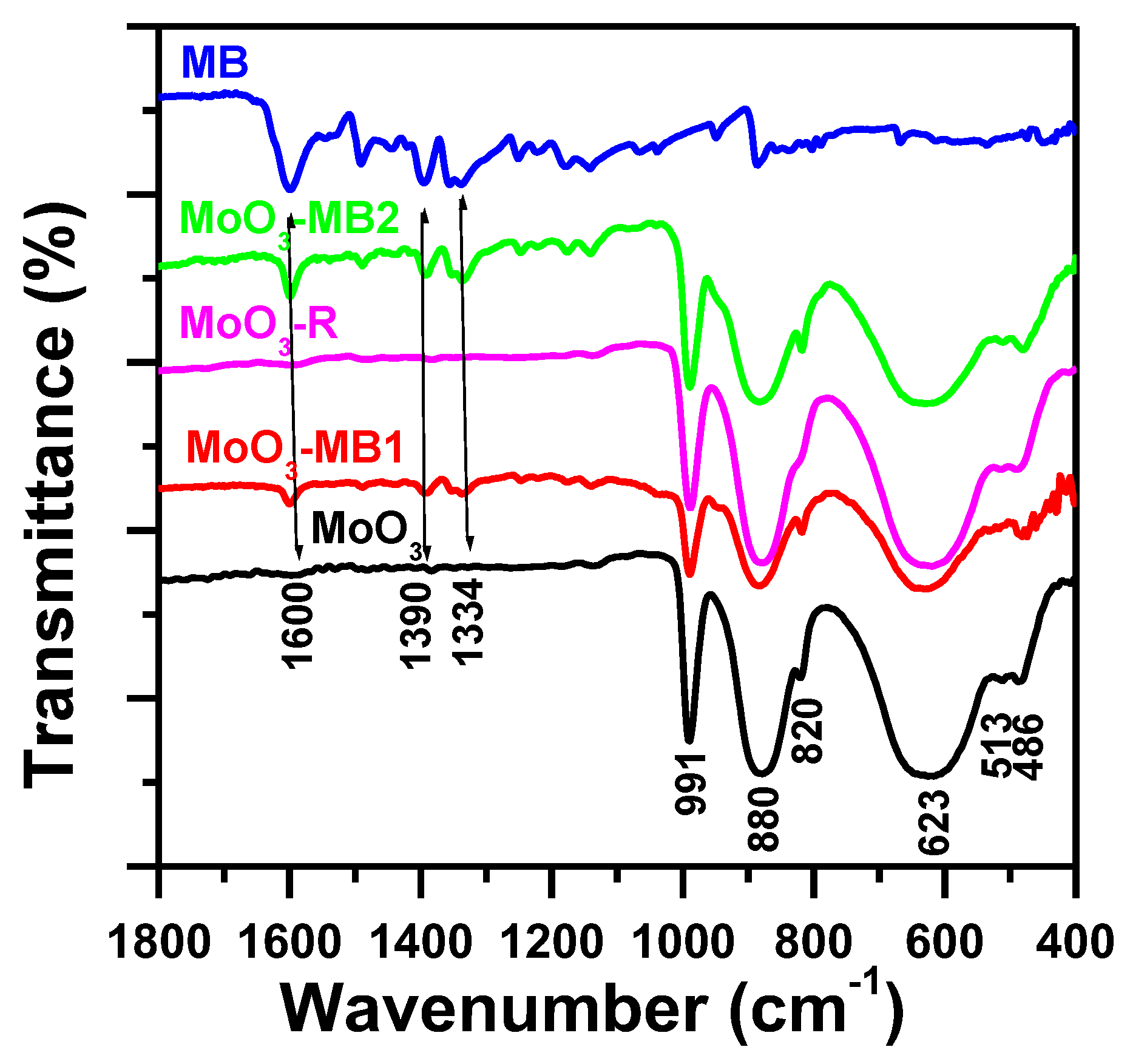
3.4.2. Fourier-Transform Infrared Spectroscopy
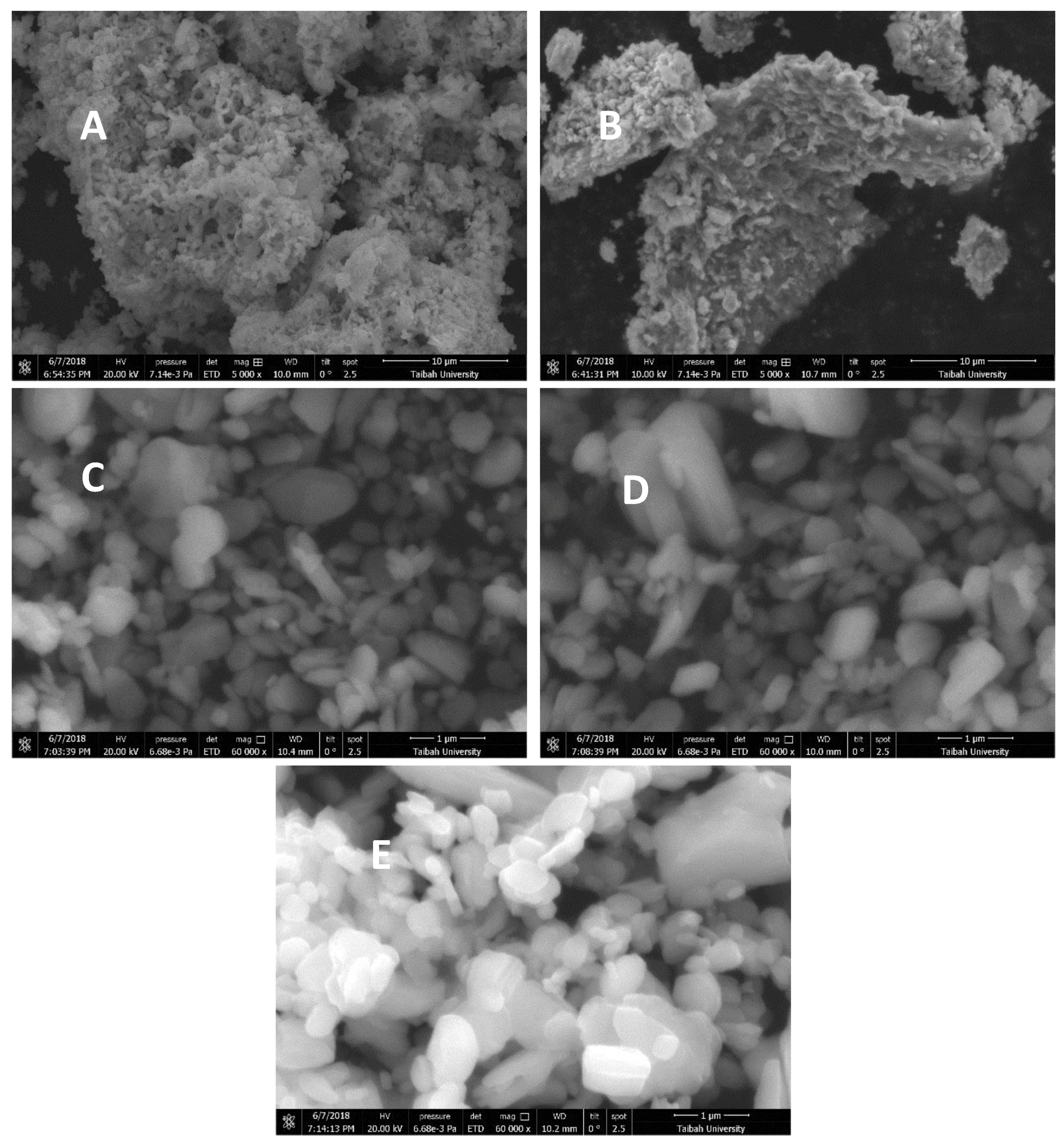
3.4.3. Scanning Electron Microscope (SEM) Analysis
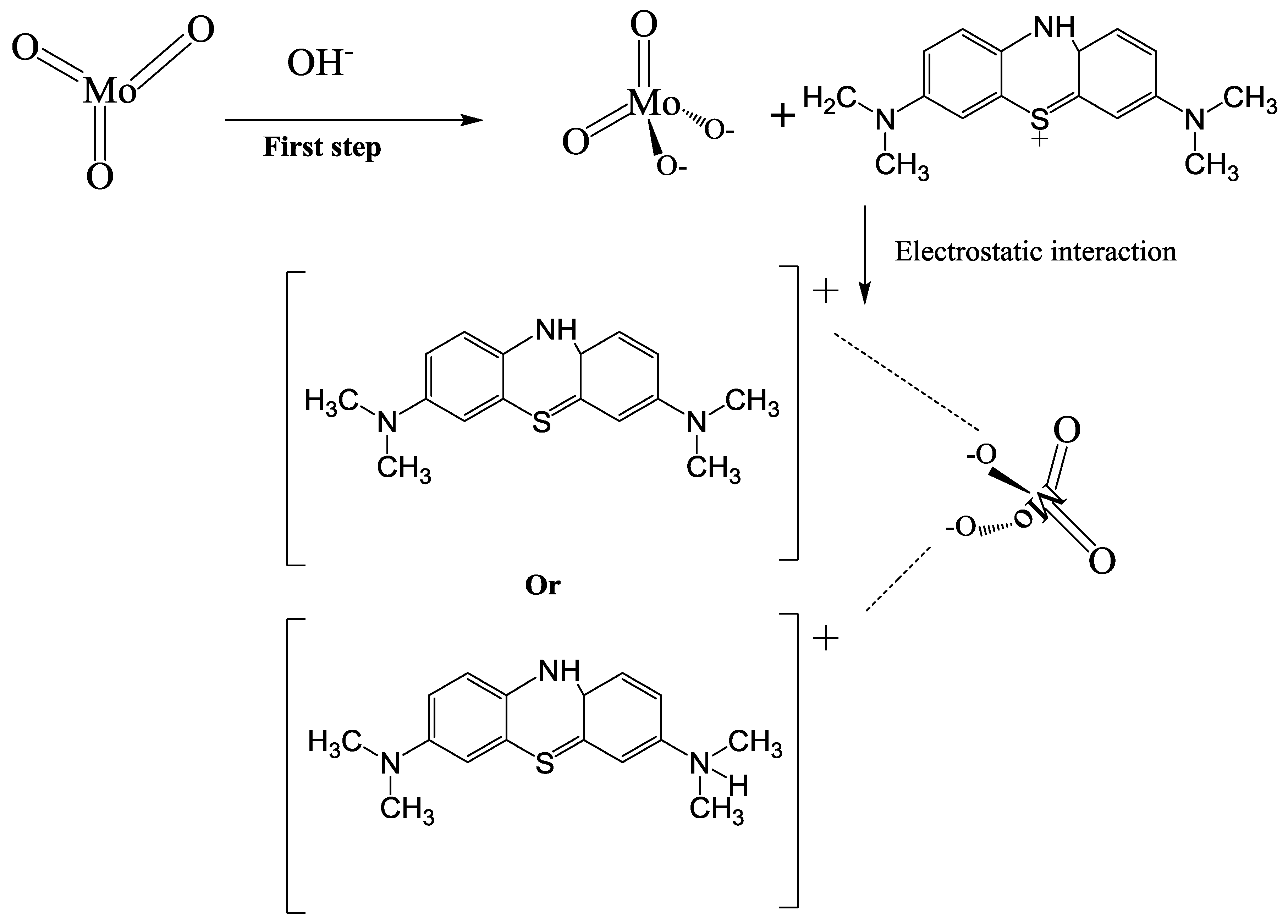
3.4.4. Removal Mechanism of MB
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Zhang, J.; Wang, A. Removal of methylene blue from aqueous solution using chitosan-g-poly (acrylic acid)/montmorillonite super adsorbent nanocomposite. Colloids Surf. A 2008, 32, 47–53. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Shitu, A.; Ibrahim, A. Removal of Methylene Blue Using Low Cost Adsorbent: A Review. Res. J. Chem. Sci. 2014, 4, 91–102. [Google Scholar]
- Ulson de Souza, S.M.A.G.; Forgiarini, E.; Ulson de Souza, A.A. Toxicity of textile dyes and their degradation by the enzyme horseradish peroxidase (HRP). J. Hazard Mater. 2007, 147, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Sucharita, A. Textile Dyes: Its Impact on Environment and its Treatment. J. Bioremed. Biodeg. 2014, 5, 1. [Google Scholar]
- Madrakian, T.; Afkhami, A.; Ahmadi, M.; Bagheri, H. Removal of some cationic dyes from aqueous solutions using magnetic modified multi-walled carbon nanotubes. J. Hazard Mater. 2011, 196, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhu, S.; Zhang, D.; Xu, S. Synthesis and properties of magnetic Fe3O4-activated carbon nanocomposite particles for dye removal. Mater. Lett. 2008, 62, 645–647. [Google Scholar] [CrossRef]
- Elemen, S.; Kumbasar, E.P.A.; Yapar, S. Modeling the adsorption of textile dye on organoclay using an artificial neural network. Dyes Pigment. 2012, 95, 102–111. [Google Scholar] [CrossRef]
- Solis, M.; Solis, A.; Perez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar] [CrossRef]
- Turgay, O.; Ersoz, G.; Atalay, S.; Forss, J.; Welander, U. The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation. Sep. Purif. Techol. 2011, 79, 26–33. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Greluk, M.; Hubicki, Z. Effect of basicity of anion exchangers and number and positions of sulfonic groups of acid dyes on dyes adsorption on macroporous anion exchangers with styrenic polymer matrix. Chem. Eng. J. 2013, 215–216, 731–739. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.C. Degradation of azo dyes by laccase: Biological method to reduce pollution load in dye wastewater. Clean Technol. Environ. Policy 2015, 17, 1443–1456. [Google Scholar] [CrossRef]
- Vanhulle, S.; Trovaslet, M.; Enaud, E.; Lucas, M.; Taghavi, S.; van der Lelie, D.; van Aken, B.; Foret, M.; Onderwater, R.C.A.; Wesenberg, D.; et al. Decolorization, cytotoxicity and genotoxicity reduction during a combined ozonation/fungal treatment of dye-contaminated wastewater. Environ. Sci. Technol. 2008, 42, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Cornelia, P.; Oana, P.; Robert, I.; Simona, G.M. Effective removal of methylene blue from aqueous solution using a new magnetic iron oxide nanosorbent prepared by combustion synthesis. Clean Technol. Environ. Policy 2016, 18, 705–715. [Google Scholar]
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Khalil, A.; Zerrouq, F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interface 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of methylene blue from water with montmorillonite nanosheets/chitosan hydrogels as adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Chen, Y.H. Synthesis, characterization and dye adsorption of ilmenite nanoparticles. J. Non-Cryst. Solids 2011, 357, 136–139. [Google Scholar] [CrossRef]
- Ozdemir, U.; Ozbay, I.; Ozbay, B.; Veli, S. Application of economical models for dye removal from aqueous solutions: Cash flow, cost–benefit and alternative selection methods. Clean Technol. Environ. Policy 2014, 16, 423–429. [Google Scholar] [CrossRef]
- Sadhukhan, B.; Mondal, N.K.; Chattoraj, S. Biosorptive removal of cationic dye from aqueous system: A response surface methodological approach. Clean Technol. Environ. Policy 2014, 16, 1015–1025. [Google Scholar] [CrossRef]
- George, Z.; Kyzas, J.F.; Kostas, A.M. The Change from Past to Future for Adsorbent Materials in Treatment of Dyeing Wastewaters. Materials 2013, 6, 5131–5158. [Google Scholar] [Green Version]
- Oudghiri-Hassani, H.; Rakass, S.; Abboudi, M.; Mohmoud, A.; Al Wadaani, F. Preparation and Characterization of α-Zinc Molybdate Catalyst: Efficient Sorbent for Methylene Blue and Reduction of 3-Nitrophenol. Molecules 2018, 23, 1462. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.C.; Luo, X.P.; Wang, X.; Guo, M.; Li, B. Removal of methylene blue from aqueous solution by modified bamboo hydrochar. Ecotoxicol. Environ. Saf. 2018, 157, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Low, S.K.; Tan, M.C. Dye adsorption characteristic of ultrasound pre-treated pomelo peel. J. Environ. Chem. Eng. 2018, 6, 3502–3509. [Google Scholar] [CrossRef]
- Mounia, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Bentahar, S.; Dbik, A.; El Khomri, M.; El Messaoudi, N.; Lacherai, A. Removal of a cationic dye from aqueous solution by natural clay. Groundw. Sustain. Dev. 2018, 6, 255–262. [Google Scholar] [CrossRef]
- Sadeghzadeh-Attar, A. Efficient photocatalytic degradation of methylene blue dye by SnO2 nanotubes synthesized at different calcination temperatures. Sol. Energy Mater. Sol. Cells 2018, 183, 16–24. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Liu, J.; Wang, T.; Wang, X.; Liu, B.; Liu, Y.; Huo, Q.; Chu, Z. Synthesis of hierarchical hollow sodium titanate microspheres and their application for selective removal of organic dyes. J. Colloid Interface Sci. 2018, 528, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Martinez, A.I.; Oliva, A.I.; Garcia, C.R.; Martinez-Luevanos, C.; Garcia-Lobato, M.; Ochoa-Valiente, M.; Berlanga, A. Flexible graphene composites for removal of methylene blue dye-contaminant from water. Appl. Surf. Sci. 2018, 436, 739–746. [Google Scholar] [CrossRef]
- Bayat, M.; Javanbakht, V.; Esmaili, J. Synthesis of zeolite/nickel ferrite/sodium alginate bionanocomposite via a co-precipitation technique for efficient removal of water-soluble methylene blue dye. Int. J. Biol. Macromol. 2018, 116, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kanakaraju, D.; Shahdad, N.R.M.; Lim, Y.C.; Pace, A. Magnetic hybrid TiO2/Alg/FeNPs triads for the efficient removal of methylene blue from water. Sustain. Chem. Pharm. 2018, 8, 50–62. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Tavallali, H.; Sharifi, M.; Nasiri Kokhdan, S.; Asghari, A. Preparation of low cost activated carbon from Myrtus communis and pomegranate and their efficient application for removal of Congo red from aqueous solution. Spectrochim. Acta Part A 2012, 86, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, F.; Ghaedi, M.; Kamali, K.; Sharifpour, E.; Sahraie, R.; Purkait, M.K. Comparison of nickel and/or zinc selenide nanoparticle loaded on activated carbon as efficient adsorbents for kinetic and equilibrium study of removal of Arsenazo (ΙΙΙ) dye. Powder Technol. 2013, 245, 217–226. [Google Scholar] [CrossRef]
- Sweet, M.J.; Chessher, A.; Singleton, I. Review: Metal-based nanoparticles; size, function and areas for advancement in applied microbiology. Adv. Appl. Microbiol. 2012, 80, 113–142. [Google Scholar] [PubMed]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.R.; Shrivastava, V.S. Adsorption removal of carcinogenic acid violet 19 dye from aqueous solution by polyaniline-Fe2O3 magnetic nano-composite. J. Mater. Environ. Sci. 2015, 6, 11–21. [Google Scholar]
- Vîrlan, C.; Ciocârlan, R.G.; Roman, T.; Gherca, D.; Cornei, N.; Pui, A. Studies on adsorption capacity of cationic dyes on several magnetic nanoparticles. Acta Chem. Iasi. 2013, 21, 19–30. [Google Scholar] [CrossRef]
- Chang, P.R.; Zheng, P.; Liu, B.; Anderson, D.P.; Yu, J.; Ma, X. Characterization of magnetic soluble starch-functionalized carbon nanotubes and its application for the adsorption of the dyes. J. Hazard. Mater. 2011, 186, 2144–2150. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Liu, S.S.; Juang, L.C.; Wang, C.C.; Lyu, M.D.; Hung, S.H. Application of titanate nanotubes for dyes adsorptive removal from aqueous solution. J. Hazard. Mater. 2007, 148, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (Chitosan–zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B 2010, 80, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, W.; He, G.; Zheng, W.; Yu, N.; Tan, M. Pore size and surface area control of MgO nanostructures using a surfactant-templated hydrothermal process: High adsorption capability to azo dyes. Colloids Surf. A 2012, 408, 79–86. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [Green Version]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable Salt-Templated Synthesis of Two-Dimensional Transition Metal Oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Ren, X.; Zheng, X.; Liu, Y.; Pang, L.; Jiang, J.; Liu, S. 2D MoO3 Nanosheets for Superior Gas Sensors. Nanoscale 2016, 8, 8696–8703. [Google Scholar] [CrossRef] [PubMed]
- Oudghiri-Hassani, H. Synthesis, characterization and catalytic performance of iron molybdate Fe2(MoO4)3 nanoparticles. Catal. Commun. 2015, 60, 19–22. [Google Scholar] [CrossRef]
- de Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-zadeh, K. Molybdenum Oxides—From Fundamentals to Functionality. Adv. Mater. 2017, 29, 1701619–1701650. [Google Scholar] [CrossRef] [PubMed]
- Chithambararaj, A.; Sanjini, N.S.; Bose, A.C.; Velmathi, S. Flower-like Hierarchical h-MoO3: New Findings of Efficient Visible Light Driven Nano Photocatalyst for Methylene Blue Degradation. Catal. Sci. Technol. 2013, 3, 1405–1414. [Google Scholar] [CrossRef]
- Negishi, H.; Negishi, S.; Kuroiwa, Y.; Sato, N.; Aoyagi, S. Anisotropic Thermal Expansion of Layered MoO3 Crystals. Phys. Rev. B 2004, 69, 064111. [Google Scholar] [CrossRef]
- McCarron, E.M.; Calabrese, J.C. The Growth and Single Crystal Structure of a High Pressure Phase of Molybdenum Trioxide: MoO3-II. J. Solid State Chem. 1991, 91, 121–125. [Google Scholar] [CrossRef]
- Parise, J.B.; McCarron, E.M.; Von Dreele, R.; Goldstone, J.A. Beta-MoO3 Produced From a Novel Freeze Drying Route. J. Solid State Chem. 1991, 93, 193–201. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen Vacancies Enhance Pseudocapacitive Charge Storage Properties of MoO3−x. Nat. Mater. 2016, 16, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kuwahara, Y.; Mori, K.; Cheng, H.; Wen, M.; Yamashita, H. High-Surface-Area Plasmonic MoO3−x: Rational Synthesis and Enhanced Ammonia Borane Dehydrogenation Activity. J. Mater. Chem. A 2017, 5, 8946–8953. [Google Scholar] [CrossRef]
- Alsaif, M.M.Y.A.; Chrimes, A.F.; Daeneke, T.; Balendhran, S.; Bellisario, D.O.; Son, Y.; Field, M.R.; Zhang, W.; Nili, H.; Nguyen, E.P.; et al. High-Performance Field Effect Transistors Using Electronic Inks of 2D Molybdenum Oxide Nanoflakes. Adv. Funct. Mater. 2016, 26, 91–100. [Google Scholar] [CrossRef]
- Truong, T.G.; Meriadec, C.; Fabre, B.; Bergamini, J.F.; de Sagazan, O.; Ababou-Girard, S.; Loget, G. Spontaneous Decoration of Silicon Surfaces with MoOx Nanoparticles for the Sunlight-Assisted Hydrogen Evolution Reaction. Nanoscale 2017, 9, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.I.; Capelli, S.C.; Vaz, P.D.; Nunes, C.D. Highly Selective and Recyclable MoO3 Nanoparticles in Epoxidation Catalysis. Appl. Catal. A 2015, 504, 344–350. [Google Scholar] [CrossRef]
- Alsaif, M.M.Y.A.; Field, M.R.; Daeneke, T.; Chrimes, A.F.; Zhang, W.; Carey, B.J.; Berean, K.J.; Walia, S.; van Embden, J.; Zhang, B.; et al. Exfoliation Solvent Dependent Plasmon Resonances in Two-Dimensional Sub-Stoichiometric Molybdenum Oxide Nanoflakes. ACS Appl. Mater. Interfaces 2016, 8, 3482–3493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, L.; Gong, Y. Exfoliated MoO3 Nanosheets for High-Capacity Lithium Storage. Electrochem. Commun. 2015, 52, 67–70. [Google Scholar] [CrossRef]
- Song, G.; Hao, J.; Liang, C.; Liu, T.; Gao, M.; Cheng, L.; Hu, J.; Liu, Z. Degradable Molybdenum Oxide Nanosheets with Rapid Clearance and Efficient Tumor Homing Capabilities as a Therapeutic Nanoplatform. Angew. Chem. Int. Ed. 2016, 55, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Abboudi, M.; Oudghiri-Hassani, H.; Wadaani, F.; Rakass, S.; Al Ghamdi, A.; Messali, M. Enhanced catalytic reduction of para-nitrophenol using α-MoO3 molybdenum oxide nanorods and stacked nanoplates as catalysts prepared from different precursors. J. Taibah Univ. Sci. 2018, 12, 133–137. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Luo, Z.; Huang, X.; Tan, C.; Li, H.; Zheng, B.; Li, B.; Huang, Y.; Yang, J.; et al. Liquid Phase Growth of Platinum Nanoparticles on Molybdenum Trioxide Nanosheets: An Enhanced Catalyst with Intrinsic Peroxidase-like Catalytic Activity. Nanoscale 2014, 6, 12340–12344. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yi, W.; Li, J.; Xi, G.; Li, Y.; Yang, H.; Liu, J. Direct Growth of Defect-Rich MoO3−X Ultrathin Nanobelts for Efficiently Catalyzed Conversion of Isopropyl Alcohol to Propylene under Visible Light. J. Mater. Chem. A 2016, 4, 1566–1571. [Google Scholar] [CrossRef]
- Santos-Beltran, M.; Paraguay-Delgado, F.; Garcıa, R.; Antunez-Flores, W.; Ornelas-Gutierrez, C.; Santos-Beltran, A. Fast methylene blue removal by MoO3 nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 28, 2935–2948. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of Nanoparticles in Waste Water Treatment. World Appl. Sci. J. 2008, 3, 417–433. [Google Scholar]
- Abboudi, M.; Oudghiri-Hassani, H.; Wadaani, F.; Messali, M.; Rakass, S. Synthesis Method of Precursors to produce Molybdenum Oxide MoO3 and related Materials. U.S. Patent 9,611,152B2, 4 April 2007. [Google Scholar]
- Benkhaya, S.; El Harfi, S.; El Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Envir. Eng. Sci. 2017, 3, 311–320. [Google Scholar]
- Rakass, S.; Mohmoud, A.; Oudghiri-Hassani, H.; Abboudi, M.; Kooli, F.; Wadaani, F. Modified Nigella Sativa Seeds as a Novel Efficient Natural Adsorbent for Removal of Methylene Blue Dye. Molecules 2018, 23, 1950. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181–182, 449–457. [Google Scholar] [CrossRef]
- Kannan, N.; Karuppasamy, K. Low cost adsorbents for the removal of phenyl aceticacid from aqueous solution. Indian J. Environ. Protec. 1998, 18, 683–690. [Google Scholar]
- Wawrzkiewicz, M.; Hubicki, Z. Removal of Tartrazine from aqueous solutions by strongly basic polystyrene anion exchange resins. J. Hazard. Mater. 2009, 164, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Anirudhan, T.S. A Preliminary examination of the adsorption characteristics of Pb(II) ions using sulphurised activated carbon prepared from bagasse pith. Indian J. Chem. Technol. 2002, 9, 32–40. [Google Scholar]
- Karaer, H.; Kaya, I. Synthesis, characterization of magnetic chitosan/active charcoal composite and using at the adsorption of methylene blue and reactive blue4. Micropor. Mesopor. Mat. 2016, 232, 26–38. [Google Scholar] [CrossRef]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 90–97. [Google Scholar] [CrossRef]
- Patil, S.; Renukdas, S.; Patel, N. Removal of methylene blue, a basic dye from aqueous solutions by adsorption using teak tree (Tectona grandis) bark powder. Inter. J. Environ. Sci. 2011, 1, 711–726. [Google Scholar]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Jihyun, R.K.; Santiano, B.; Kim, H.; Kan, E. Heterogeneous Oxidation of Methylene Blue with Surface-Modified Iron-Amended Activated Carbon. Am. J. Anal. Chem. 2013, 4, 115–122. [Google Scholar]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Furusawa, T.; Smith, J.M. Intraparticle mass transport in slurries by dynamic adsorption studies. AIChE J. 1974, 20, 88–93. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Shahwan, T.; Erten, H.N. Temperature effects in barium sorption on natural kaolinite and chlorite-illite clays. J. Radioanal. Nucl. Chem. 2004, 260, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Ma, J.; Yu, F.; Zhou, L.; Jin, L.; Yang, M.X.; Luan, J.S.; Tang, Y.H.; Fan, H.B.; Yuan, Z.W.; Chen, J.H. Enhanced adsorptive removal of methyl orange and methylene blue from aqueous solution by alkali-activated multiwalled carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 5749–5760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Wang, S.Q.; Shen, S.L.; Zhao, B.X. A novel water treatment magnetic nanomaterial for removal of anionic and cationic dyes under severe condition. Chem. Eng. J. 2013, 233, 258–264. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, Y.; Mai, J.X.; Sun, W.L.; Zhang, C.Y.; Wei, D.P.; Chen, Q.; Ni, J.R. Adsorption behavior of methylene blue onto titanate nanotubes. Chem. Eng. J. 2010, 156, 313–320. [Google Scholar] [CrossRef]
- Roosta, M.; Ghaedi, M.; Daneshfar, A.; Sahraei, R.; Asghari, A. Optimization of the ultrasonic assisted removal of methylene blue by gold nanoparticles loaded on activated carbon using experimental design methodology. Ultrason. Sonochem. 2014, 21, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Heidarpour, S.; Nasiri Kokhdan, S.; Sahraie, R.; Daneshfar, A.; Brazesh, B. Comparison of silver and palladium nanoparticles loaded on activated carbon for efficient removal of Methylene blue: Kinetic and isotherm study of removal process. Powder Technol. 2012, 228, 18–25. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, D.; Wu, D.; Ma, X.F. Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chem. Eng. J. 2011, 168, 959–963. [Google Scholar] [CrossRef]
- Chen, A.S.C.; Sorg, T.J.; Wang, L. Regeneration of iron-based adsorptive media used for removing arsenic from groundwater. Water Res. 2015, 77, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3·xH2O molybdenum trioxide hydrates. Spectrochim. Acta A Mol. Biomol. Spect. 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Ahmed, F.; Dewani, R.; Pervez, M.K.; Mahboob, S.J.; Soomro, S.A. Non-destructive FT-IR analysis of mono azo dyes. Bulg. Chem. Commun. 2016, 48, 71–77. [Google Scholar]
- Etman, A.S.; Abdelhamid, H.N.; Yuan, Y.; Wang, L.; Zou, X.; Sun, J. Facile Water-Based Strategy for Synthesizing MoO3−x Nanosheets: Efficient Visible Light Photocatalysts for Dye Degradation. ACS Omega 2018, 3, 2201–2209. [Google Scholar] [CrossRef]
- Aracena, A.; Sannino, A.; Jerez, O. Dissolution kinetics of molybdite in KOH media at different temperatures. Trans. Nonferrous Met. Soc. China 2018, 28, 177–185. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.; Ma, S.; Xu, Z.; Liu, W.; Liu, F. Adsorption and Desorption Mechanisms of Methylene Blue Removal with Iron-Oxide Coated Porous Ceramic Filter. J. Water Resour. Prot. 2009, 1, 35–40. [Google Scholar] [CrossRef]
- Santamarina, J.C.; Klein, K.A.; Wang, Y.H.; Prencke, E. Specific surface: Determination and relevance. Can. Geotech. J. 2002, 39, 233–241. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds molybdenum trioxide (α-MoO3) are available from the authors. |






| Adsorbent | Adsorbate | ∆H° (KJ·mol−1) | ∆S° (KJ·mol−1·K) | ∆G° (KJ·mol−1) | |||
|---|---|---|---|---|---|---|---|
| α-MoO3 | MB | 90 | 0.316 | 298 K | 323 K | 343 K | |
| −3.741 | −11.643 | −12.305 | |||||
| Model | Equation | Parameters |
|---|---|---|
| Pseudo first-order (PFD) [79] | qt: the removal capacity at time t (mg/g); qe: the removal capacity at equilibrium (mg/g); K1: the rate constant of pseudo first-order adsorption (1/min) | |
| Pseudo second-order (PSD) [79] | qt: the removal capacity at time t (mg/g); qe: the removal capacity at equilibrium (mg/g); K2: the pseudo second-order rate constant (g·mg−1·min−1) | |
| Intraparticle diffusion (IPD) [80]. | I (mg/g) and KI (mg/(g·min0.5)) are the intraparticle diffusion constants, qt: the removal capacity (mg/g) at time t; t: the contact time (min) |
| Dye Ci mg/L | Pseudo First-Order | Pseudo Second-Order | Intra-Particle-Diffusion Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qexp (mg/g) | qe (mg/g) | k1 (1/min) | R12 | qe (mg/g) | k2 (g/mg min) | R22 | I (mg/g) | ki (mg/g min0.5) | R32 | |
| 100 | 99.8 | 281 | 0.097 | 0.997 | 111 | 0.00097 | 0.998 | 60.20 | 4.42 | 0.832 |
| 130 | 129.5 | 321 | 0.097 | 0.996 | 136 | 0.00147 | 0.999 | 93.15 | 4.03 | 0.834 |
| 150 | 149.6 | 225 | 0.045 | 0.995 | 200 | 0.00017 | 1 | 34.35 | 11.75 | 0.910 |
| Model | Equation | Parameters |
|---|---|---|
| Freundlich [81] | n: the heterogeneity factor (g/L); qF: the Freundlich constant (mg(1−1/n)·L1/n·g−1); Ce: concentration of MB at equilibrium (ppm); qe: the MB dye amount adsorbed by α-MoO3 at equilibrium (mg/g) | |
| Langmuir [82] | Ce: concentration of MB at equilibrium (ppm); qe: the MB dye amount adsorbed by α-MoO3 at equilibrium (mg/g); KL: Langmuir constant of adsorption (L/mg); qm: the maximum amount of MB dye removed by α-MoO3 (mg/g) | |
| KL: the Langmuir constant; Ci: the initial concentration of MB; RL: values indicate that the removal of MB could be linear (RL = 1), irreversible (RL = 0), favorable (0 < RL < 1), or unfavorable (RL > 1). | ||
| Dubinin–Radushkevich (D-R) [83] | ε: the Polanyi potential; K: constant for the sorption energy (mol2/kJ2); R: the Universal gas constant (8.314 J.mol-1 K−1); T : the temperature (K); Ce: the equilibrium concentration of the MB dye left in the solution (ppm); qm: the theoretical saturation capacity. | |
| Temkin [84] | BT = RT/bT; bT: the Temkin constant related to heat of sorption (J/mol); AT: the Temkin isotherm constant (L/g); R: the gas constant (8.314 J/mol K); T: the absolute temperature (K) |
| Langmuir | Freundlich | Temkin | Dubinin–Radushkevich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | R2 | Range RL | qF (mg(1−1/n)·L1/n·g−1) | 1/n | R2 | AT (L/g) | BT (J/mol) | R2 | qm (mg/g) | R2 | E (KJ/mol) |
| 152 | 9.58 | 1 | 0.0007–0.0090 | 161 | 0.301 | 0.997 | 74.8 | 36.56 | 0.989 | 152 | 0.939 | 16 |
| Nanosorbent | Qmax (mg/g) | Reference |
|---|---|---|
| Magnetic iron oxide nanosorbent | 25.54 | [14] |
| Alkali-activated multiwalled carbon nanotubes | 399.00 | [85] |
| Fe3O4 magnetic nanoparticles modified with 3-glycidoxypropyltrimethoxysilane and glycine | 158.00 | [86] |
| Calcined titanate nanotubes | 133.33 | [87] |
| Gold nanoparticles loaded on activated carbon | 104.00–185.00 | [88] |
| Silver nanoparticles loaded on activated carbon | 71.43 | [89] |
| Palladium nanoparticles loaded on activated carbon | 75.40 | [89] |
| Magnetic halloysite nanotubes/iron oxide composites | 18.44 | [90] |
| Zinc molybdate nanoparticles | 217.86 | [22] |
| Molybdenum trioxide nanoparticles (hexagonal and orthorhombic phases) | 122.50 | [64] |
| Molybdenum trioxide nanorods and stacked nanoplates | 152.00 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakass, S.; Oudghiri Hassani, H.; Abboudi, M.; Kooli, F.; Mohmoud, A.; Aljuhani, A.; Al Wadaani, F. Molybdenum Trioxide: Efficient Nanosorbent for Removal of Methylene Blue Dye from Aqueous Solutions. Molecules 2018, 23, 2295. https://doi.org/10.3390/molecules23092295
Rakass S, Oudghiri Hassani H, Abboudi M, Kooli F, Mohmoud A, Aljuhani A, Al Wadaani F. Molybdenum Trioxide: Efficient Nanosorbent for Removal of Methylene Blue Dye from Aqueous Solutions. Molecules. 2018; 23(9):2295. https://doi.org/10.3390/molecules23092295
Chicago/Turabian StyleRakass, Souad, Hicham Oudghiri Hassani, Mostafa Abboudi, Fethi Kooli, Ahmed Mohmoud, Ateyatallah Aljuhani, and Fahd Al Wadaani. 2018. "Molybdenum Trioxide: Efficient Nanosorbent for Removal of Methylene Blue Dye from Aqueous Solutions" Molecules 23, no. 9: 2295. https://doi.org/10.3390/molecules23092295
APA StyleRakass, S., Oudghiri Hassani, H., Abboudi, M., Kooli, F., Mohmoud, A., Aljuhani, A., & Al Wadaani, F. (2018). Molybdenum Trioxide: Efficient Nanosorbent for Removal of Methylene Blue Dye from Aqueous Solutions. Molecules, 23(9), 2295. https://doi.org/10.3390/molecules23092295






