Ferrocene-Containing Impiridone (ONC201) Hybrids: Synthesis, DFT Modelling, In Vitro Evaluation, and Structure–Activity Relationships
Abstract
:1. Introduction
2. Results and Discussion
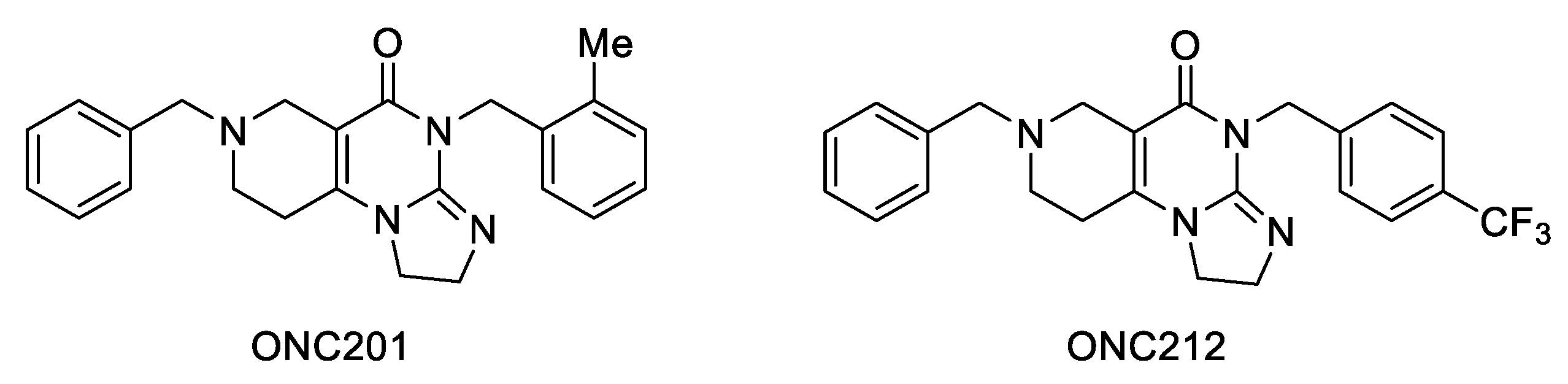
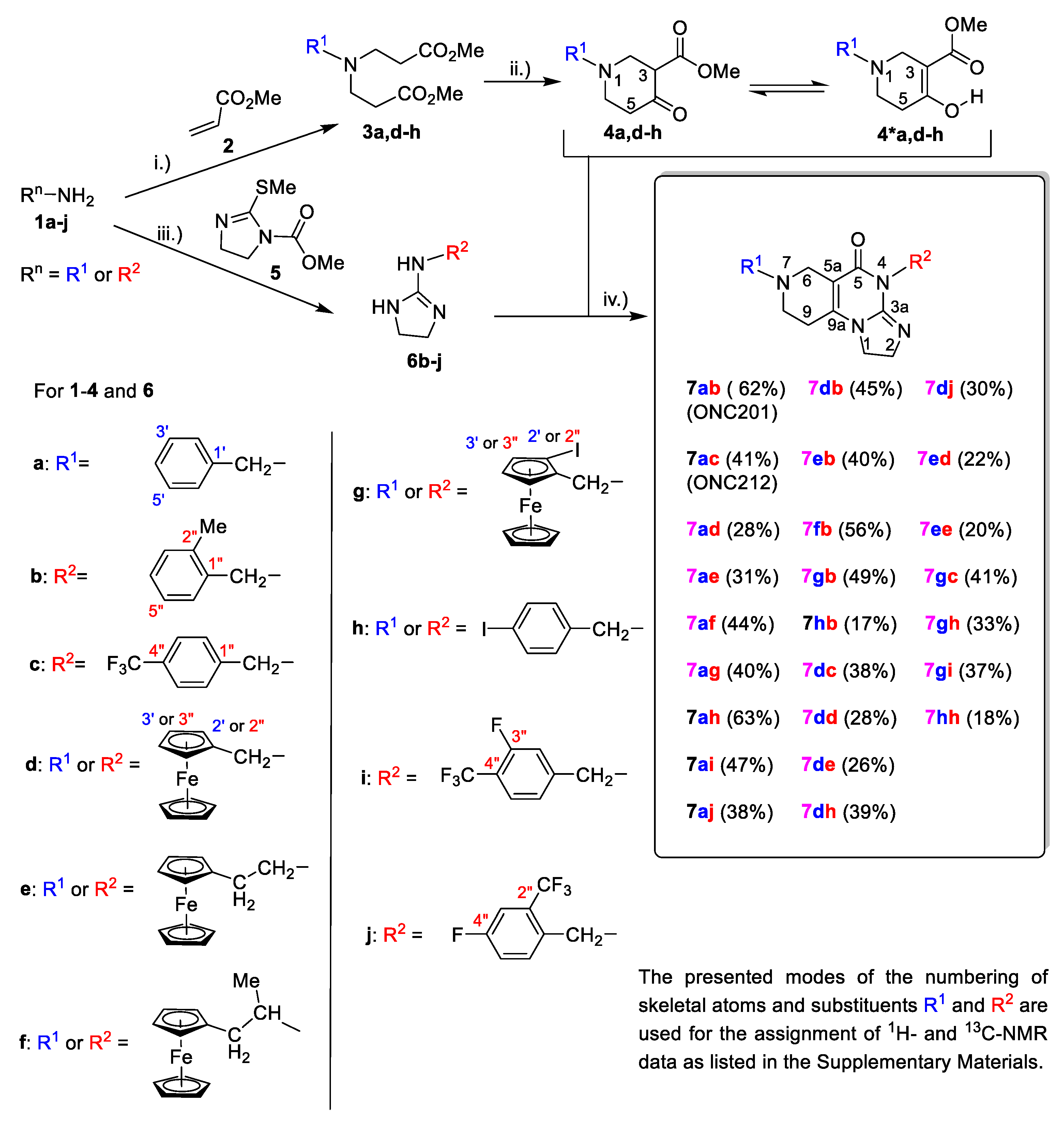
2.1. Synthesis of the Reference and Hybrid Impiridones
2.2. NMR Analysis of the Skeletal Structure of Novel Impiridones
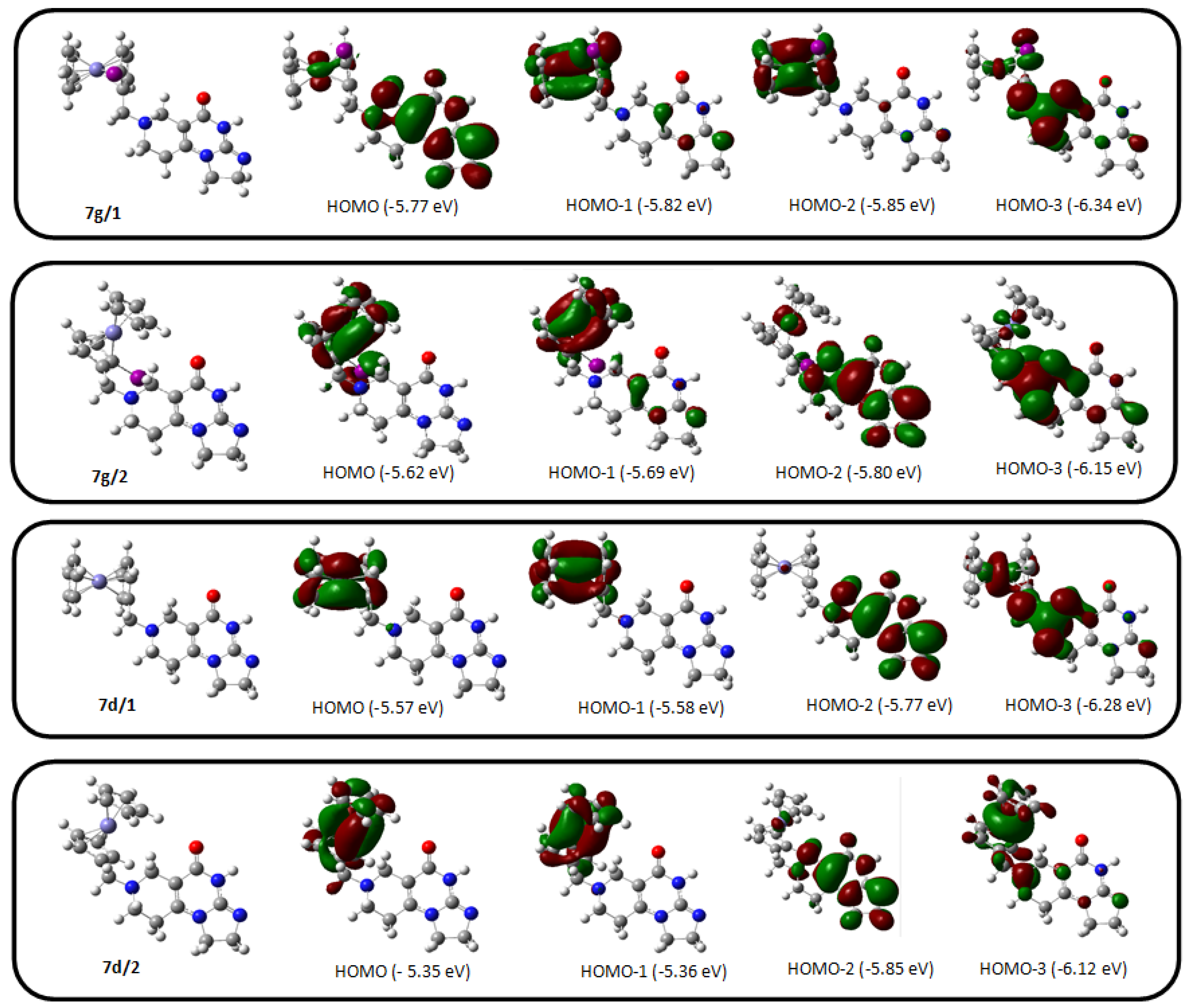
2.3. DFT Analysis of the Simplified Model Impiridones Carrying 2-Iodoferrocenylmethyl or Ferrocenylmethyl Group as R1-Substituent
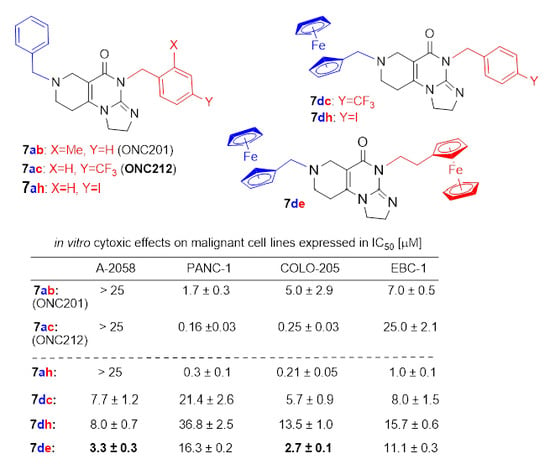
2.4. In Vitro Cytotoxicity Study of the Reference and Novel Impiridones
3. Materials and Methods
3.1. General Procedure for the Synthesis of Impiridones Type 7
3.1.1. Synthesis of 2-(Methylthio)-4,5-Dihydro-1H-imidazole-1-carboxylate (5)
3.1.2. Synthesis of N-Aralkyl-4,5-dihydro-1H-imidazol-2-amine as Representative Example for the Preparation of the Cyclic Guanidines 6b–j
3.1.3. General Procedure for the Synthesis of Impiridones Type 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosenberg, B.; Van Camp, L. The Successful Regression of Large Solid Sarcoma 180 Tumors by Platinum Compounds. Cancer Res. 1970, 30, 1799–1802. [Google Scholar] [PubMed]
- Dyson, P.J.; Sava, G. Metal-based antitumour drugs in the post genomic era. Dalton Trans. 2006, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.C.; Lippard, S.J. Inhibition of transcription by platinum antitumor compounds. Metallomics 2009, 1, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. Broadening the clinical use of platinum drug–based chemotherapy with new analogues. Expert Opin. Investig. Drugs 2007, 16, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.; Lippard, S. Direct Cellular Responses to Platinum-Induced DNA Damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, P.J.; Einhorn, L.H. Drugs five years later. Cisplatin. Ann. Intern. Med. 1984, 100, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, N.; Duranton, C.; Djerbi, N.; Puech, P.H.; Gounon, P.; Lagadic-Gossmann, D.; Dimanche-Boitrel, M.T.; Rauch, C.; Tauc, M.; Counillon, L.; et al. Nongenomic effects of cisplatin: Acute inhibition of mechanosensitive transporters and channels without actin remodeling. Cancer Res. 2010, 70, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. Abrogation by Curcumin on Testicular Toxicity Induced by Cisplatin in Rats. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Laginha, K.M.; Verwoert, S.; Charrois, G.J.R.; Allen, T.M. Determination of Doxorubicin Levels in Whole Tumor and Tumor Nuclei in Murine Breast Cancer Tumors. Clin. Cancer Res. 2005, 11, 6944–6949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, P.J.; Phillips, J.R.; Seiner, M.; Cass, C.E. Differential Activity of Vincristine and Vinblastine against Cultured Cells. Cancer Res. 1984, 44, 3307–3312. [Google Scholar] [PubMed]
- Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985. [Google Scholar] [CrossRef]
- Braga, S.S.; Silva, A.M.S. A New Age for Iron: Antitumoral Ferrocenes. Organometallics 2013, 32, 5626–5639. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti cancer drugs Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K. Recent developments in the chemistry of ferrocenyl secondary natural product conjugates. Coord. Chem. Rev. 2018, 366, 91–108. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives Nat. Rev. Chem. 2017, 66. [Google Scholar] [CrossRef]
- Jaouen, G.; Top, S.; Vessičres, A.; Alberto, R. New paradigms for synthetic pathways inspired by bioorganometallic chemistry. J. Organomet. Chem. 2000, 600, 23–36. [Google Scholar] [CrossRef]
- Tamura, H.; Miwa, M. DNA Cleaving Activity and Cytotoxic Activity of Ferricenium Cations. Chem. Lett. 1997, 26, 1177–1178. [Google Scholar] [CrossRef]
- Houlton, A.; Roberts, R.M.G.; Silver, J. Studies on the anti-tumour activity of some iron sandwich compounds. J. Organomet. Chem. 1991, 418, 107–112. [Google Scholar] [CrossRef]
- Osella, D.; Ferrali, M.; Zanello, P.; Laschi, F.; Fontani, M.; Nervi, C.; Cavigiolio, G. On the mechanism of the antitumor activity of ferrocenium derivatives. Inorg. Chim. Acta 2000, 306, 42–48. [Google Scholar] [CrossRef]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Pai, R.C.; Fong, S.; Leung, S.; Lawrence, D.A.; Marsters, S.A.; Blackie, C.; Chang, L.; McMurtrey, A.E.; Hebert, A.; et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Investig. 1999, 104, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voltan, R.; Secchiero, P.; Casciano, F.; Milani, D.; Zauli, G.; Tisato, V. Redox signalling and oxidative stress: Cross talk with TNF-related apoptosis inducing ligand activity. Int. J. Biochem. Cell Biol. 2016, 81, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Kline, C.L.; Van den Heuvel, A.P.; Allen, J.E.; Prabh, V.V.; Dicker, D.T.; El-Deiry, W.S. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci. Signal 2016, 9, ra18. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.V.; Allen, J.E.; Dicker, D.T.; El-Deiry, W.S. Small-Molecule ONC201/TIC10 Targets Chemotherapy-Resistant Colorectal Cancer Stem-like Cells in an Akt/Foxo3a/TRAIL-Dependent Manner. Cancer Res. 2015, 75, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Krigsfeld, G.; Mayes, P.A.; Patel, L.; Dicker, D.T.; Patel, A.S.; Dolloff, N.G.; Messaris, E.; Scata, K.A.; Wang, W.; et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci. Transl. Med. 2013, 5, 171ra17. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.E.; Crowder, R.; El-Deiry, W.S. First-In-Class Small Molecule ONC201 Induces DR5 and Cell Death in Tumor but Not Normal Cells to Provide a Wide Therapeutic Index as an Anti-Cancer Agent. PLoS ONE 2015, 10, e0143082. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Kline, C.L.; Pottorf, R.S.; Nallaganchu, B.R.; Olson, G.L.; Dicker, D.T.; Allen, J.E.; El-Deiry, W.S. The angular structure of ONC201, a TRAIL pathway-inducing compound, determines its potent anti-cancer activity. Oncotarget 2014, 5, 12728–12737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wang, H.; Ran, L.; Zhang, Z.; Jiang, R. The preclinical evaluation of TIC10/ONC201 as an anti-pancreatic cancer agent. Biochem. Biophys. Res. Commun. 2016, 476, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.Z.; Wang, W.; Fang, D.L.; Jin, Y.J. mTOR inhibition sensitizes ONC201-induced anti-colorectal cancer cell activity. Biochem. Biophys. Res. Commun. 2016, 478, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, J.; Li, Z.; Jiang, Y.; Zhou, Y. Small Molecular TRAIL Inducer ONC201 Induces Death in Lung Cancer Cells: A Preclinical Study. PLoS ONE 2016, 11, e0162133. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; Bertino, J.R.; Kaufman, H.L.; Mayer, T.; Moss, R.; Silk, A.; Chan, N.; Malhotra, J.; Rodriguez, L.; Aisner, J.; et al. First-in-human Clinical Trial of Oral ONC201 in Patients with Refractory Solid Tumors. Clin. Cancer Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Kline, C.L.; Ralff, M.D.; Lev, A.; Lulla, A.; Zhou, L.; Olson, G.L.; Nallaganchu, B.R.; Benes, C.H.; Allen, J.E.; et al. Preclinical evaluation of the imipridone family, analogs of clinical stage anti-cancer small molecule ONC201, reveals potent anti-cancer effects of ONC212. Cell Cycle 2017, 16, 1790–1799. [Google Scholar] [CrossRef] [Green Version]
- Lev, A.; Lulla, A.R.; Wagner, J.; Ralff, M.D.; Kiehl, J.B.; Zhou, Y.; Benes, C.H.; Prabhu, V.V.; Oster, W.; Astsaturov, I.; et al. Anti-pancreatic cancer activity of ONC212 involves the unfolded protein response (UPR) and is reduced by IGF1-R and GRP78/BIP. Oncotarget 2017, 8, 81776–81793. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.T.; Lockner, J.W.; Kravchenko, V.V.; Janda, K.D. Pharmacophore Reassignment for Induction of the Immunosurveillance Cytokine TRAIL. Angew. Chem. Int. Ed. 2014, 53, 6746–6749. [Google Scholar] [CrossRef]
- Baramee, A.; Coppin, A.; Mortuaire, M.; Pelinski, L.; Tomavoc, S.; Brocard, J. Synthesis and in vitro activities of ferrocenic aminohydroxynaphthoquinones against Toxoplasma gondii and Plasmodium falciparum. Bioorg. Med. Chem. 2006, 14, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, B.; Karst, U. Analysis of cysteine-containing proteins using precolumn derivatization with N-(2-ferroceneethyl)maleimide and liquid chromatography/electrochemistry/mass spectrometry. Anal. Bioanal. Chem. 2007, 388, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Love, B.; Flores, M. Notes-Ferrocene Derivatives. J. Org. Chem. 1961, 26, 3595. [Google Scholar] [CrossRef]
- Fodor, K.J.; Hegedüs, K.; Csomós, P.; Fodor, L.; Gubán, D.; Sohár, P.; Csámpai, A. Synthesis, Structural Elucidation, Cyclic Voltammetry, and Theoretical Modelling of 2-Ferrocenyl-4H-benzo[e][1,3]thiazines and 2-Aryl-4H-ferroceno[e][1,3]thiazines. Eur. J. Inorg. Chem. 2017, 511–520. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of Gaussian-type basis sets for local spin density functional calculations. Part I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. (Eds.) Gaussian 09; revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
Sample Availability: Samples of the compounds of type 7 are available from the authors. |
| Short-Term Treatment a | Long-Term Treatment b | |||||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | HT-29 | A-2058 | A-2058 | PANC-1 | COLO-205 | EBC-1 | |
| 7ab |  |  | 16.9 ± 8.6 | 27.6 ± 2.5 | >25 | 1.7 ± 0.3 | 5.0 ± 2.9 | 7.0 ± 0.5 |
| 7ac |  |  | 43.3 ± 38.6 | 35.1 ± 30.6 | >25 | 0.16 ± 0.03 | 0.25 ± 0.03 | 25.0 ± 2.1 |
| 7ad |  |  | - | - | >10 | >10 | >10 | >10 |
| 7ae |  |  | 58.6 ± 18.6 | - | >10 | >25 | >10 | >10 |
| 7af |  |  | 55.7 ± 3.8 | 45.4 ± 2.6 | 37.9 ± 15.3 | 36.6 ± 3.4 | 33.1 ± 6.8 | 28.9 ± 6.8 |
| 7ag |  |  | - | - | 12.9 ± 1.6 | >25 | 12.8 ± 1.7 | ~25 ± 5.9 |
| 7ah |  |  | 2.7 ± 1.1 | 8.8 ± 7.1 | >25 | 0.3 ± 0.1 | 0.21 ± 0.05 | 1.0 ± 0.1 |
| 7ai |  |  | 14.3 ± 7.8 | 14.2 ± 8.1 | >25 | 0.28 ± 0.06 | ~0.25 ± 0.03 | ~25.0 ± 1.9 |
| 7aj |  |  | 69.4 ± 7.8 | >100 | > 50 | ~50 ± 13.9 | 17.8 ± 1.0 | >50 |
| 7db |  |  | >100 | >100 | >10 | >25 | >10 | >10 |
| 7eb |  |  | >100 | >100 | >25 | >25 | >25 | >25 |
| 7fb |  |  | 72.4 ± 13.0 | 52.0 ± 1.1 | 15.9 ± 1.8 | 38.0 ± 3.1 | 16.7 ± 3.0 | 24.3 ± 2.3 |
| 7gb |  |  | 26.5 ± 15.2 | 36.3 ± 13.4 | 11.8 ± 0.7 | >25 | 11.4 ± 0.1 | 15.7 ± 3.4 |
| 7hb |  |  | >100 | 12.8 ± 0.2 | >50 | 21.6 ± 2.2 | 32.0 ± 1.4 | 18.0 ± 2.5 |
| 7dc |  |  | 40.9 ± 2.4 | 28.1 ± 13.3 | 7.7 ± 1.2 | 21.4 ± 2.6 | 5.7 ± 0.9 | 8.0 ± 1.5 |
| 7dd |  |  | 35.6 ± 0.5 | 31.2 ± 8.9 | 5.8 ± 0.7 | >25.0 | 5.0 ± 3.1 | >10 |
| 7de |  |  | 33.0 ± 23.3 | 20.9 ± 12.5 | 3.3 ± 0.3 | 16.3 ± 0.2 | 2.7 ± 0.1 | 11.1 ± 0.3 |
| 7dh |  |  | >100 | 57.2 ± 20.2 | 8.0 ± 0.7 | 36.8 ± 2.5 | 13.5 ± 1.0 | 15.7 ± 0.6 |
| 7dj |  |  | >100 | 42.0 ± 26.8 | 11.8 ± 0.4 | >50 | 13.0 ± 3.2 | 17.1 ± 1.1 |
| 7ed |  |  | 11.6 ± 3.5 | 13.5 ± 8.5 | 11.2 ± 5.6 | >25 | 9.4 ± 1.6 | 13.1 ± 2.4 |
| 7ee |  |  | 34.0 ± 9.9 | 17.6 ± 6.7 | 12.1 ± 4.9 | >25 | 8.5 ± 0.9 | >25 |
| 7gc |  |  | 40.2 ± 5.3 | 28.7 ± 11.9 | 13.1 ± 0.9 | 22.2 ± 2.9 | 7.7 ± 2.2 | 12.8 ± 1.8 |
| 7gh |  |  | >100 | 49.8 ± 13.6 | 22.9 ± 2.5 | >50 | 29.2 ± 2.3 | 28.2 ± 1.4 |
| 7gi |  |  | 39.9 ± 9.8 | 32.3 ± 12.5 | 17.0 ± 1.3 | 19.9 ± 0.6 | 20.6 ± 0.6 | 18.3 ± 0.3 |
| 7hh |  |  | 8.3 ± 0.8 | - | 31.6 ± 4.6 | 4.1 ± 0.2 | 2.3 ± 0.5 | 9.4 ± 3.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárány, P.; Oláh, R.S.; Kovács, I.; Czuczi, T.; Szabó, C.L.; Takács, A.; Lajkó, E.; Láng, O.; Kőhidai, L.; Schlosser, G.; et al. Ferrocene-Containing Impiridone (ONC201) Hybrids: Synthesis, DFT Modelling, In Vitro Evaluation, and Structure–Activity Relationships. Molecules 2018, 23, 2248. https://doi.org/10.3390/molecules23092248
Bárány P, Oláh RS, Kovács I, Czuczi T, Szabó CL, Takács A, Lajkó E, Láng O, Kőhidai L, Schlosser G, et al. Ferrocene-Containing Impiridone (ONC201) Hybrids: Synthesis, DFT Modelling, In Vitro Evaluation, and Structure–Activity Relationships. Molecules. 2018; 23(9):2248. https://doi.org/10.3390/molecules23092248
Chicago/Turabian StyleBárány, Péter, Rita Szabó Oláh, Imre Kovács, Tamás Czuczi, Csenge Lilla Szabó, Angéla Takács, Eszter Lajkó, Orsolya Láng, László Kőhidai, Gitta Schlosser, and et al. 2018. "Ferrocene-Containing Impiridone (ONC201) Hybrids: Synthesis, DFT Modelling, In Vitro Evaluation, and Structure–Activity Relationships" Molecules 23, no. 9: 2248. https://doi.org/10.3390/molecules23092248