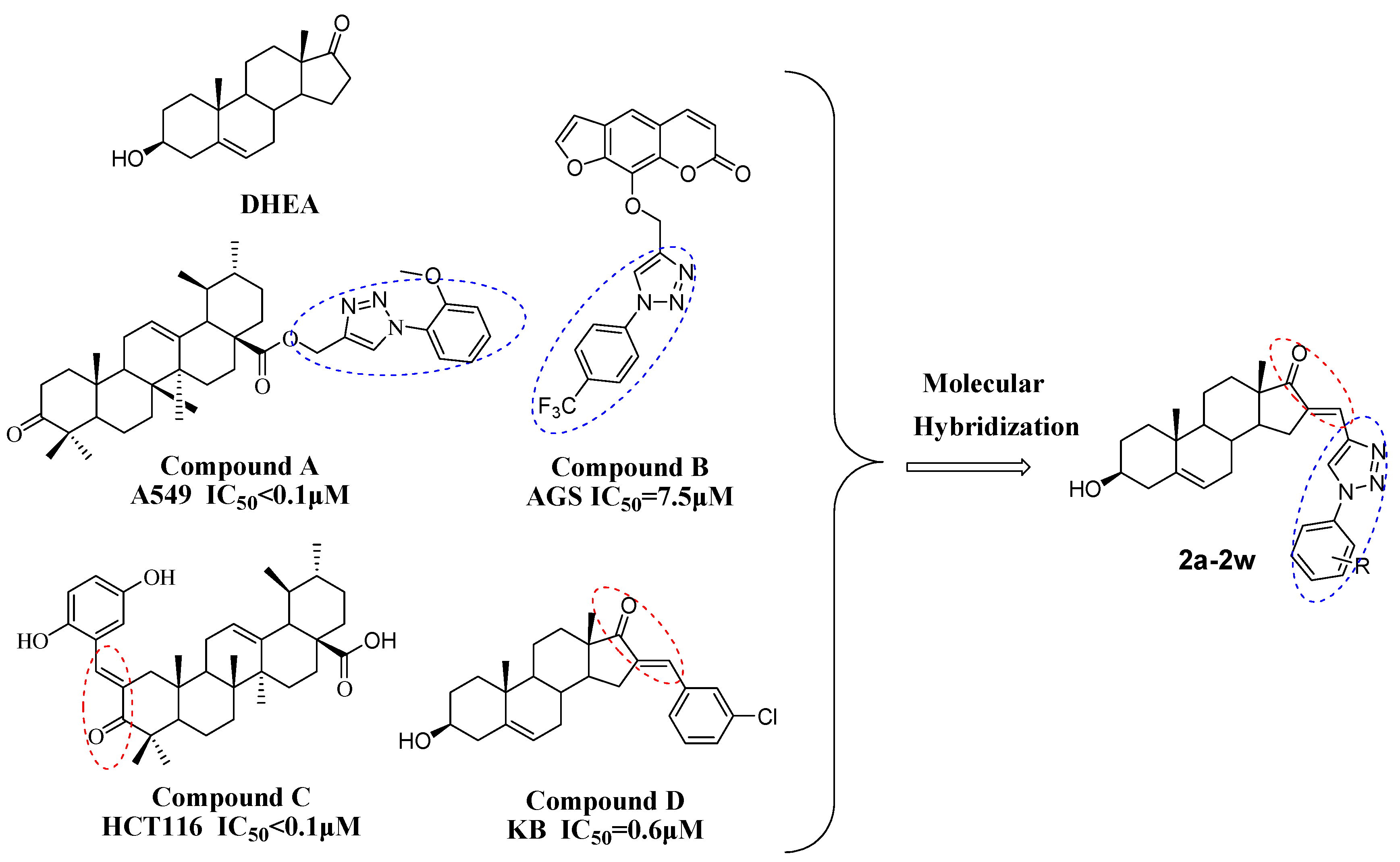
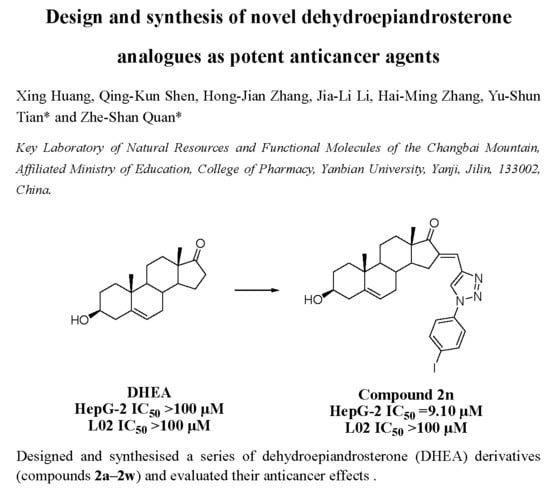
3.2.1. General Procedure for Preparation of 2a–2w
A mixture of dehydroepiandrosterone (DHEA) (2.0 mmol), aromatic aldehydes (2.1 mmol), and KOH (2.0 mmol) in EtOH (20 mL) was heated under reflux for about 1 h. After completion of the reaction as evident from TLC, the reaction solution was condensed under reduced pressure and the solid residue was transferred to 15 mL of water with 15 mL EA. The EA layer was washed with water (5 mL × 3) and saline (5 mL × 3) and dried using anhydrous MgSO
4. The mixture was then purified by normal-phase column chromatography (PE:EA = 5:2) to obtain the target compounds
2a–
2w (
Scheme 1).
(E)-3-hydroxy-10,13-dimethyl-16-((1-phenyl-1H-1,2,3-triazol-4-yl)methylene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2a) Yield 90%. 1H-NMR (300 MHz, CDCl3) δ 8.11 (s, 1H, triazole-H), 7.80 (s, 1H,-CO-C=CH), 7.77 (s, 1H, Ar-H), 7.58 (t, J = 6 Hz, 2H, Ar-H), 7.50 (t, J = 6 Hz, 2H, Ar-H), 5.44 (s, 1H, C6-H), 3.57 (s, 1H, -OH), 3.22 (dd, J1 = 15 MHz, J2 = 6 Hz, 1H, C3-H), 2.57–2.47 (m, 4H), 2.06–1.41 (m, 12H), 1.18–1.10 (m, 4H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 213.80, 149.37, 146.34, 142.43, 141.38, 134.59, 133.81, 127.47, 125.33, 125.04, 124.98, 75.49, 55.05, 54.07, 52.36, 47.05, 41.95, 41.47, 36.27, 35.91, 35.67, 34.29, 25.09, 34.23, 19.05; ESI-HR MS (m/z): calcd. for C28H34N3O2+ [M + H]+: 444.2646; found: 444.2651. Purity: 98.066% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 15.873 min, λ: 292 nm.
(E)-16-((1-(2-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2b) Yield 98%. 1H-NMR (300 MHz, CDCl3) δ 8.71 (s, 1H, triazole-H), 8.08 (s, 1H,-CO-C=CH), 7.86 (t, J = 6 Hz, 2H, Ar-H), 7.61–7.39 (m, 3H, Ar-H), 7.50 (t, J = 6 Hz, 2H, Ar-H), 5.53 (s, 1H, C6-H), 4,53 (s, 1H, -OH), 3.07 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 2.42–2.17 (m, 4H), 1.90–1.36 (m, 12H), 1.04 (s, 4H), 0.94 (m, 3H); 13C-NMR (75 MHz, DMSO-d6) δ 208.50, 143.89, 141.87, 137.99, 128.10, 128.05, 126.79, 126.05, 126.01, 120.47, 120.03, 117.74, 117.49, 70.43, 50.25, 48.97, 47.54, 42.69, 37.31, 36.77, 31.88, 31.63, 31.13, 30.78, 29.53, 20.47, 19.66, 14.43; ESI-HR MS (m/z): calcd. for C28H33FN3O2+ [M + H]+: 462.2551; found: 462.2558. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 80–100%), tR 5.820 min, λ: 292 nm.
(E)-16-((1-(3-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2c) Yield 98%. 1H-NMR (300 MHz, DMSO-d6) δ 8.95 (s, 1H, triazole-H), 7.86 (t, J = 6 Hz, 1H, -CO-C=CH), 7.69–7.56 (m, 2H, Ar-H), 7.47 (t, J = 6 Hz, 2H, Ar-H), 7.34 (s, 1H, Ar-H), 5.33 (s, 1H, C6-H), 4.61 (d, J = 3 Hz, 1H, -OH), 4.08 (dd, J1 = 9 Hz, J2 = 6 Hz, 1H, C3-H), 3.17 (d, J = 3 Hz, 2H), 3.07–2.99 (m, 1H), 2.20–2.12 (m, 3H), 1.83–1.66 (m, 6H), 1.53–1.30 (m, 4H), 1.01 (s, 4H), 0.89 (s, 3H); 13C-NMR (75 MHz, DMSO-d6) δ 213.37, 169.30, 149.37, 146.45, 142.61, 136.36, 128.27, 124.97, 120.86, 120.58, 120.27, 112.99, 112.64, 75.29, 55.03, 53.97, 52.30, 47.22, 41.98, 41.50, 36.40, 36.31, 35.95, 35.72, 34.26, 25.11, 24.30, 19.09; ESI-HR MS (m/z): calcd. for C28H33FN3O2+ [M + H]+: 462.2551; found: 462.2560. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 17.187 min, λ: 292 nm.
(E)-16-((1-(4-fluorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2d) Yield 96%. 1H-NMR (300 MHz, CDCl3) δ 8.06 (s, 1H, triazole-H), 7.78–7.74 (m, 2H, Ar-H), 7.47 (s, 1H, Ar-H), 7.29 (d, J = 6 Hz, 2H, Ar-H), 5.44 (s, 1H, C6-H), 3.57 (s, 1H, -OH), 3.22 (dd, J1 = 18 Hz, J2 = 6 Hz, 1H, C3-H), 2.57–2.46 (m, 1H), 2.40–2.29 (m, 3H), 2.06–1.40 (m, 12H), 1.30–1.15 (m, 1H), 1.10 (m, 4H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.32, 144.98, 141.09, 138.21, 122.69, 122.66, 122.57, 120.93, 119.56, 117.05, 116.74, 71.59, 50.37, 49.41, 47.71, 42.24, 37.17, 26.73, 31.59, 31.51, 31.21, 30.98, 29.70, 20.43, 19.47, 14.29; ESI-HR MS (m/z): calcd. for C28H33FN3O2+ [M + H]+: 462.2551; found: 462.2547. Purity: 97.271% by HPLC (A: H2O; B: methanol, graded: 80–100%), tR 5.987 min, λ: 292 nm.
(E)-16-((1-(2-chlorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-di-methyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2e) Yield 92%. 1H-NMR (300 MHz, CDCl3) δ 8.14 (s, 1H, triazole-H), 7.68–1,62 (m, 2H, Ar-H), 7.54–7.47(m, 3H, Ar-H), 5.43 (s, 1H, C6-H), 3.55 (s, 1H, -OH), 3.22 (dd, J1 = 18 Hz, J2 = 6 Hz, 1H, C3-H), 2.57–2.46 (m, 1H), 2.39–2.24 (m, 3H), 2.06–1.99 (m, 1H), 1.92–1.74 (m, 4H), 1.64–1.56 (m, 5H), 1.51–1.41 (m, 3H), 1.14–1.10 (m, 3H), 1.01 (s, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.31, 144.08, 141.06, 138.04, 131.03, 130.88, 128.63, 128.06, 127.68, 126.55, 120.95, 119.75, 71.59, 50.38, 49.43, 47.72, 42.23, 37.17, 36.73, 31.58, 31.22, 30.97, 29.68, 20.43, 19.46, 14.29; ESI-HR MS (m/z): calcd. for C28H33ClN3O2+ [M + H]+: 478.2256; found: 478.2663. Purity: 98.081% by HPLC (A: H2O; B: methanol, graded: 80–100%), tR 4.933 min, λ: 292 nm.
(E)-16-((1-(3-chlorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2f) Yield 90%. 1H-NMR (300 MHz, CDCl3) δ 8.07 (s, 1H, triazole-H), 7.80 (s, 1H,-CO-C=CH), 7.68 (d, J = 3 Hz, 1H, Ar-H), 7.50–7.45 (m, 3H, Ar-H), 5.42 (s, 1H, C6-H), 3.55 (s, 1H, -OH), 3.20 (dd, J1 = 12 Hz, J2 = 3 Hz, 1H, C3-H), 2.53–2.47 (m, 1H), 2.37–2.25 (m, 3H), 2.04–1.98 (m, 2H), 1.90–1.82 (m, 4H), 1.75–1.70 (m, 2H), 1.52–1.44 (m, 4H), 1.15–1.08 (m, 4H), 0.99 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.37, 145.06, 141.04, 137.45, 135.70, 130.98, 129.15, 122.39, 120.90, 120.78, 119.53, 119.50, 118.98, 71.56, 50.34, 49.38, 47.73, 42.20, 37.15, 36.70, 31.53, 31.20, 31.17, 30.97, 29.70, 20.42, 19.46, 14.29; ESI-HR MS (m/z): calcd. for C28H33ClN3O2+ [M + H]+: 478.2256; found: 478.2680. Purity: 98.406% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 19.453 min, λ: 292 nm.
(E)-16-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2g) Yield 97%. 1H-NMR (300 MHz, CDCl3) δ 8.09 (s, 1H, triazole-H), 7.75 (s, 1H,-CO-C=CH), 7.72 (s, 1H, Ar-H), 7.55 (d, J = 6 Hz, 2H, Ar-H), 7.46 (s, 1H, Ar-H), 5.43 (s, 1H, C6-H), 3.21 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 2.56–2.46 (m, 1H), 2.35–2.24 (m, 3H), 2.02–1.40 (m, 12H), 1.09 (m, 5H), 1.00 (s, 3H); 13C-NMR (75 MHz, CDCl3) δ 213.39, 149.39, 146.44, 142.52, 140.09, 138.92, 134.66, 128.13, 126.65, 124.96, 75.31, 55.04, 53.99, 52.30, 47.20, 41.98, 41.50, 36.32, 35.94, 35.70, 34.25, 25.12, 24.29, 23.56, 19.09; ESI-HR MS (m/z): calcd. for C28H33ClN3O2+ [M + H]+: 478.2256; found: 478.2563. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 95–100%), tR 2.860 min, λ: 292 nm.
(E)-16-((1-(3,4-dichlorophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2h) Yield 98%. 1H-NMR (300 MHz, CDCl3) δ 8.08 (s, 1H, triazole-H), 7.94 (s, 1H,-CO-C=CH), 7.66 (s, 2H, Ar-H), 7.45 (s, 1H, Ar-H), 5.44 (s, 1H, C6-H), 3.57 (s, 1H, -OH), 3.21 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 2.57–2.46 (m, 1H), 2.38–2.25 (m, 3H), 2.06–2.02 (m, 1H), 1.92–1.69 (m, 5H), 1.64–1.55 (m, 4H), 1.50–1.40 (m, 2H), 1.18–1.10 (m, 4H), 1.00 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.19, 145.29, 141.08, 138.72, 135.65, 134.17, 133.30, 131.62, 122.29, 122.13, 120.91, 119.52, 119.17, 71.61, 50.38, 49.38, 47.72, 42.23, 37.17, 36.73, 31.59, 31.51, 31.22, 30.99, 29.71, 20.43, 19.46, 14.28; ESI-HR MS (m/z): calcd. for C28H32Cl2N3O2+ [M + H]+: 512.1866; found: 512.1859. Purity: 98.857% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 22.315 min, λ: 292 nm.
(E)-16-((1-(2-bromophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2i) Yield 98%. 1H-NMR (300 MHz, CDCl3) δ 8.27 (s, 1H, triazole-H), 7.80 (d, J = 6 Hz, 1H, -CO-C=CH), 7.61–7.45 (m, 4H, Ar-H), 5.43 (s, 1H, C6-H), 3.22 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 3.25–3.18 (m, 1H), 2.55–2,46 (m, 1H), 2.35–2.24 (m, 3H), 1.93–1.88 (m, 3H), 1.82–1.58 (m, 6H), 1.51–1.45 (m, 3H), 1.09 (m, 4H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.34, 143.94, 141.14, 137.96, 136.11, 134.01, 131.48, 128.66, 128.10, 126.71, 120.85, 119.87, 118.57, 71.47, 50.34, 49.38, 47.71, 42.19, 37.17, 36.72, 31.53, 31.18, 30.95, 29.66, 20.42, 19.48, 14.30; ESI-HR MS (m/z): calcd. for C28H33BrN3O2+ [M + H]+: 522.1751; found: 522.1743. Purity: 99.701% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 15.587 min, λ: 292 nm.
(E)-16-((1-(3-bromophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan- thren-17(14H)-one (2j) Yield 98%. 1H-NMR (300 MHz, CDCl3) δ 8.09 (s, 1H, triazole-H), 7.97 (d, J = 6 Hz, 1H, -CO-C=CH), 7.75 (dd, J1 = 6 Hz, J2 = 3 Hz, 1H, Ar-H), 7.63 (t, J = 3 Hz, 1H, Ar-H), 7.48–7.43 (m, 2H, Ar-H), 5.43 (s, 1H, C6-H), 3.57 (s, 1H, -OH), 3.22 (dd, J1 = 18 Hz, J2 = 6 Hz, 1H, C3-H), 2.57–2.46 (m, 1H), 2.38–2.25 (m, 3H), 2.06–1.86 (m, 9H), 1.52–1.40 (m, 3H), 1.15–1.06 (m, 4H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.31, 145.07, 141.06, 138.42, 137.55, 132.10, 131.22, 123.61, 123.42, 122.35, 120.91, 119.48, 119.10, 71.58, 50.35, 49.37, 47.72, 42.21, 37.16, 36.72, 31.56, 31.20, 30.98, 29.69, 20.42, 19.47, 14.29; ESI-HR MS (m/z): calcd. for C28H33BrN3O2+ [M + H]+: 522.1751; found: 522.1740. Purity: 98.860% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 20.073 min, λ: 292 nm.
(E)-16-((1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-di-methyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan- thren-17(14H)-one (2k) Yield 95%. 1H-NMR (300 MHz, CDCl3) δ 8.09 (s, 1H, triazole-H), 7.72–7.65 (m, 4H, Ar-H), 7.46–7.44 (dd, J1 = 6 Hz, J2 = 3 Hz, 1H, Ar-H), 5.44 (s, 1H, C6-H), 3.60–3.52 (m, 1H), 3.25–3.17 (m, 1H, C3-H), 2.56–2.45 (m, 1H), 2.38–2.24 (m, 3H), 2.02–1.39 (m, 12H), 1.14–1.1.09 (m, 4H), 1.00 (s, 3H); 13C-NMR (75 MHz, CDCl3) δ 208.48, 144.40, 141.96, 138.06, 135.98, 135.26, 124.45, 122.79, 122.18, 120.43, 120.12, 70.43, 50.24, 49.06, 48.93, 47.54, 42.70, 37.28, 36.77, 31.89, 31.60, 31.16, 30.87, 29.48, 19.67, 14.42; ESI-HR MS (m/z): calcd. for C28H33BrN3O2+ [M + H]+: 522.1751; found: 522.1743. Purity: 99.001% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 19.640 min, λ: 292 nm.
(E)-3-hydroxy-16-((1-(2-iodophenyl)-1H-1,2,3-triazol-4-yl)methylene)-10,13-dim-ethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2l) Yield 91%. 1H-NMR (300 MHz, CDCl3) δ 8.06 (s, 1H, triazole-H), 8.03 (d, J = 3 MHz, 1H, -CO-C=CH), 7.59 (m, 3H, Ar-H), 7.32 (s, 1H, Ar-H), 5.43 (s, 1H, C6-H), 3.56 (s, 1H, -OH), 3.23 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 2.56–2.43 (m, 1H), 2.35–2.24 (m, 3H), 2.03–1.73 (m, 5H), 1.56–1.42 (m, 4H), 1.18–1.10 (m, 4H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.28, 144.05, 141.11, 140.35, 139.65, 137.98, 133.47, 131.92, 129.38, 127.36, 126.54, 124.42, 120.88, 119.82, 117.47, 71.51, 50.37, 49.81, 49.41, 48.82, 47.71, 42.21, 37.17, 36.72, 31.55, 31.51, 31.20, 30.96, 30.89, 29.69, 20.41, 19.45, 14.29; ESI-HR MS (m/z): calcd. for C28H33IN3O2+ [M + H]+: 570.1612; found: 570.1595. Purity: 98.823% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 15.273 min, λ: 292 nm.
(E)-3-hydroxy-16-((1-(3-iodophenyl)-1H-1,2,3-triazol-4-yl)methylene)-10,13-dim-ethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2m) Yield 96%. 1H-NMR (300 MHz, CDCl3) δ 8.14 (s, 1H, triazole-H), 8.08 (s, 1H,-CO-C=CH), 7.87–7.77 (m, 2H, Ar-H), 7.33 (s, 1H, Ar-H), 5.44 (s, 1H, C6-H), 3.58 (s, 1H, -OH), 3.21 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 2.56–2.47 (m, 1H), 2.34–2.25 (m, 3H), 2.08–1.74 (m, 7H), 1.54–1.27 (m, 5H), 1.10 (m, 4H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.30, 145.06, 141.05, 138.40, 138.05, 137.41, 131.28, 129.31, 122.32, 120.94, 119.82, 119.49, 71.54, 50.44, 49.32, 47.67, 42.20, 37.23, 36.74, 31.58, 31.51, 31.28, 31.17, 31.06, 29.73, 20.45, 19.53, 14.36; ESI-HR MS (m/z): calcd. for C28H33IN3O2+ [M + H]+: 570.1612; found: 570.1604. Purity: 98.003% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 20.673 min, λ: 292 nm.
(E)-3-hydroxy-16-((1-(4-iodophenyl)-1H-1,2,3-triazol-4-yl)methylene)-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2n) Yield 92%. 1H-NMR (300 MHz, CDCl3) δ 8.08 (s, 1H, triazole-H), 7.91 (d, J = 6 Hz, 1H, Ar-H), 7.53 (m, 3H, Ar-H), 5.44 (s, 1H, C6-H), 3.56 (s, 1H, -OH), 3.21 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 2.59–2.46 (m, 1H), 2.39–2.25 (m, 3H), 2.12–1.99 (m, 2H), 1.92–1.71 (m, 6H), 1.62–1.46 (m, 5H), 1.10 (s, 4H), 1.00 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.32, 145.12, 141.05, 139.02, 138.39, 136.27, 122.18, 122.07, 120.96, 119.45, 94.04, 71.62, 50.37, 49.40, 47.72, 42.21, 37.17, 36.73, 31.57, 31.51, 31.21, 30.98, 29.71, 20.43, 19.41, 14.30; ESI-HR MS (m/z): calcd. for C28H33IN3O2+ [M + H]+: 570.1612; found: 570.1601. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 20.460 min, λ: 292 nm.
(E)-3-hydroxy-10,13-dimethyl-16-((1-m-tolyl-1H-1,2,3-triazol-4-yl)methylene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2o) Yield 90%. 1H-NMR (300 MHz, CDCl3) δ 8.08 (s, 1H, triazole-H), 7.60 (s, 1H,-CO-C=CH), 7.55 (d, J = 6 Hz, 1H, Ar-H), 7.50–1.42 (m, 2H, Ar-H), 7.31 (s, 1H, Ar-H), 5.43 (s, 1H, C6-H), 3.57 (s, 1H, -OH), 3.22 (dd, J1 = 18 Hz, J2 = 6 Hz, 1H, C3-H), 2.56–2.47 (m, 4H), 2.35–2.25 (m, 3H), 2.06–1.87 (m, 5H), 1.83–1.74 (m, 3H), 1.61–1.40 (m, 8H), 1.13–1.06 (m, 4H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.40, 144.72, 141.09, 140.16, 137.90, 136.59, 129.87, 129.65, 122.62, 121.24, 120.92, 119.89, 117.70, 71.56, 50.38, 49.42, 47.71, 42.23, 37.17, 36.72, 31.56, 31.53, 31.20, 30.98, 29.68, 21.43, 20.43, 19.46, 14.29; ESI-HR MS (m/z): calcd. for C29H36N3O2+ [M + H]+: 458.2802; found: 458.2804. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 680–100%), tR 6.500 min, λ: 292 nm.
(E)-3-hydroxy-10,13-dimethyl-16-((1-p-tolyl-1H-1,2,3-triazol-4-yl)methylene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren-17(14H)-one (2p) Yield 96%. 1H-NMR (300 MHz, CDCl3) δ 8.06 (s, 1H, triazole-H), 7.66 (s, 1H,-CO-C=CH), 7.63 (s, 1H, Ar-H), 7.48 (s, 1H, Ar-H), 7.35 (t, J = 6 Hz, 2H, Ar-H), 5.44 (s, 1H, C6-H), 3.57 (s, 1H, -OH), 3.22 (dd, J1 = 15 MHz, J2 = 6 Hz, 1H, C3-H), 2.56–2.46 (m, 4H), 2.41–2.25 (m, 3H), 2.06–1.44 (m, 12H), 1.14–1.10 (m, 4H), 1.00 (s, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.44, 144.69, 141.05, 139.33, 137.85, 134.34, 130.37, 122.57, 120.96, 120.52, 119.91, 71.60, 50.35, 49.40, 47.71, 42.20, 31.17, 36.72, 31.50, 31.19, 30.97, 29.68, 21.15, 20.43, 19.47, 14.30; ESI-HR MS (m/z): calcd. for C29H36N3O2+ [M + H]+: 458.2802; found: 458.2811. Purity: 97.937% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 18.167 min, λ: 292 nm.
(E)-3-hydroxy-16-((1-(2-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methylene)-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2q) Yield 96%. 1H-NMR (300 MHz, CDCl3) δ 8.27 (s, 1H, triazole-H), 7.82 (d, J = 6 Hz, 1H,-CO-C=CH), 7.47 (t, J = 9 Hz, 2H, Ar-H), 7.15 (t, J = 9 Hz, 2H, Ar-H), 5.44 (s, 1H, C6-H), 3.93 (s, 3H, -OCH3), 3.23 (dd, J1 = 18 Hz, J2 = 3 Hz, 1H, C3-H), 2.55–2.47 (m, 1H), 2.33–2.24 (m, 3H), 2.06–1.88 (m, 3H), 1.82–1.62 (m, 5H), 1.58–1.28 (m, 5H), 1.10–1.01 (m, 8H); 13C-NMR (75 MHz, DMSO-d6) δ 209.55, 151.06, 143.74, 141.08, 137.31, 130.39, 126.93, 125.90, 125.31, 121.35, 120.98, 120.28, 112.36, 71.57, 56.07, 50.38, 49.47, 47.70, 42.23, 37.17, 36.73, 31.57, 31.21, 30.96, 29.66, 20.44, 19.46, 14.31; ESI-HR MS (m/z): calcd. for C29H36N3O3+ [M + H]+: 474.2751; found: 474.2762. Purity: 98.888% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 15.440 min, λ: 292 nm.
(E)-3-hydroxy-16-((1-(3-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methylene)-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2r) Yield 95%. 1H-NMR (300 MHz, CDCl3) δ 8.09 (s, 1H, triazole-H), 7.49 (s, 1H,-CO-C=CH), 7.45 (d, J = 3 Hz, 1H, Ar-H), 7.38 (d, J = 3 Hz, 1H, Ar-H), 7.32 (s, 1H, Ar-H), 7.02 (dd, J1 = 9 Hz, J2 = 3 Hz, 1H, Ar-H), 5.45 (s, 1H, C6-H), 3.92 (s, 3H, -OCH3), 3.57 (s, 1H, -OH), 3.21 (dd, J1 = 15 MHz, J2 = 6 MHz, 1H, C3-H), 2.57–2.46 (m, 1H), 2.36–2.25 (m, 3H), 2.07–1.40 (m, 13H), 1.18–1.10 (m, 3H), 1.01 (m, 3H); 13C-NMR (75 MHz, CDCl3) δ 209.35, 160.67, 144.79, 141.07, 138.03, 137.64, 130.67, 122.59, 120.94, 119.76, 114.76, 112.42, 106.56, 71.57, 55.67, 50.37, 49.48, 47.71, 42.23, 37.17, 36.72, 31.58, 31.51, 31.20, 30.97, 29.68, 20.43, 19.47, 14.30; ESI-HR MS (m/z): calcd. for C29H36N3O3+ [M + H]+: 474.2751; found: 474.2754. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 16.440 min, λ: 292 nm.
(E)-3-hydroxy-16-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methylene)-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2s) Yield 97%. 1H-NMR (300 MHz, CDCl3) δ 8.01 (s, 1H, triazole-H), 7.67 (s, 1H, -CO-C=CH), 7.64 (s, 1H, Ar-H), 7.47 (s, 1H, Ar-H), 7.05 (d, J = 6 Hz, 2H, Ar-H), 5.42 (s, 1H, C6-H), 3.88 (s, 3H, -OCH3), 3.55 (s, 1H, -OH), 3.19 (dd, J1 = 18 Hz, J2 = 6 Hz, 1H, C3-H), 2.54–2.23 (m, 4H), 2.05–1.44 (m, 12H), 1.08 (s, 4H), 0.99 (s, 3H); 13C-NMR (75 MHz, CDCl3) δ 213.78, 164.71, 149.14, 146.34, 142.15, 134.76, 127.85, 126.97, 125.16, 125.03, 119.59, 75.46, 60.43, 55.05, 54.07, 52.35, 47.07, 41.96, 41.47, 36.29, 35.90, 35.67, 34.26, 25.06, 24.24, 19.06; ESI-HR MS (m/z): calcd. for C29H36N3O3+ [M + H]+: 474.2751; found: 474.2759. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 95–100%), tR 2.527 min, λ: 292 nm.
(E)-16-((1-(3,4-dimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methylene)-3-hydroxy-10,13-dimethyl-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenan-thren-17(14H)-one (2t) Yield 93%. 1H-NMR (300 MHz, CDCl3) δ 8.04 (s, 1H, triazole-H), 7.47 (s, 1H,-CO-C=CH), 7.39 (d, J = 3 Hz, 1H, Ar-H), 7.20 (dd, J1 = 9 Hz, J2 = 3 Hz, 1H, Ar-H), 7.00 (d, J = 3 Hz, 1H, Ar-H), 5.44 (s, 1H, C6-H), 3.99 (s, 3H, -OCH3), 3.97(s, 3H, -OCH3), 3.21 (dd, J1 = 15 Hz, J2 = 6 Hz, 1H, C3-H), 2.56–2.25 (m, 5H), 2.05–1.98 (m, 2H), 1.91–1.74 (m, 5H), 1.62–1.44 (m, 4H), 1.17–1.00 (m, 8H); 13C-NMR (75 MHz, CDCl3) δ 209.49, 149.89, 144.68, 141.08, 137.88, 130.19, 122.76, 120.94, 119.85, 112.55, 111.25, 105.06, 71.59, 56.27, 50.36, 49.41, 47.71, 42.21, 37.16, 36.72, 31.56, 31.20, 30.97, 29.67, 20.42, 19.46, 14.30; ESI-HR MS (m/z): calcd. for C30H38N3O4+ [M + H]+: 504.2857; found: 504.2863. Purity: 98.680% by HPLC (A: H2O; B: methanol, graded: 95–100%), tR 2.427 min, λ: 292 nm.
(E)-3-hydroxy-10,13-dimethyl-16-((1-(3,4,5-trimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methylene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[a]phenanthren17(14H)-one (2u) Yield, 90%. 1H-NMR (300 MHz, CDCl3) δ 8.05 (s, 1H, triazole-H), 7.45 (s, 1H,-CO-C=CH), 6.97 (s, 2H, Ar-H), 5.44 (s, 1H, C6-H), 3.96 (s, 6H, -OCH3), 3.91 (s, 3H, -OCH3), 3.24 (dd, J1 = 18 Hz, J2 = 3 Hz, 1H, C3-H), 2.57–2.47 (m, 1H), 2.35–2.23 (m, 3H), 2.05–1.99 (m, 1H), 1.90–1.83 (m, 1H), 1.78–1.63 (m, 5H), 1.49–1.39 (m, 3H), 1.09–1.00 (m, 8H); 13C-NMR (75 MHz, CDCl3) δ 209.57, 153.96, 144.72, 141.12, 138.09, 132.47, 123.32, 122.93, 121.88, 121.61, 98.62, 71.55, 61.06, 56.49, 51.33, 49.56, 47.70, 42.20, 37.15, 36.70, 31.55, 31.17, 30.95, 29.68, 20.39, 19.45, 14.28; ESI-HR MS (m/z): calcd. for C31H40N3O5+ [M + H]+: 534.2962; found: 534.2955. Purity: 100% by HPLC (A: H2O; B: methanol, graded: 80–100%), tR 15.187 min, λ: 292 nm.
(E)-3-hydroxy-10,13-dimethyl-16-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methylene)1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[α]phenan- thren-17(14H)-one (2v) Yield 95%. 1H-NMR (300 MHz, DMSO-d6) δ 9.19 (s, 1H, triazole-H), 8.46 (s, 1H,-CO-C=CH), 8.43 (s, 1H, Ar-H), 8.25 (d, J = 6 Hz, 2H, Ar-H), 7.24 (s, 1H, Ar-H), 5.31 (s, 1H, C6-H), 4.60 (s, 1H, -OH), 2.99 (dd, J1 = 12 Hz, J2 = 3 Hz, 1H, C3-H), 2.42–2.15 (m, 4H), 1.77–1.66 (m, 7H), 1.49–1.34 (m, 4H), 0.98 (m, 5H), 0.86 (s, 3H); 13C-NMR (75 MHz, DMSO-d6) δ 208.29, 147.28, 144.76, 141.96, 140.95, 138.44, 125.94, 124.55, 121.26, 120.32, 119.74, 70.40, 50.24, 48.90, 47.50, 42.68, 40.30, 40.02, 39.74, 39.47, 39.19, 37.29.36.75, 31.86, 31.58, 31.14, 29.48, 20.42, 19.63, 14.37; ESI-HR MS (m/z): calcd. for C28H33N4O4+ [M + H]+: 489.2496; found: 489.2503. Purity: 98.048% by HPLC (A: H2O; B: methanol, graded: 80–100%), tR 5.413 min, λ: 292 nm.
(E)-3-hydroxy-10,13-dimethyl-16-((1-(4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol4-y)methylene)-1,3,4,7,8,9,10,11,12,13,15,16-dodecahydro-2H-cyclopenta[α] phenanthren-17(14H)-one (2w) Yield 93%. 1H-NMR (300 MHz, CDCl3) δ 8.13 (s, 1H, triazole-H), 7.94 (s, 1H,-CO-C=CH), 7.92 (s, 1H, Ar-H), 7.84 (t, J = 3 Hz, 2H, Ar-H), 7.45 (s, 1H, Ar-H), 5.42 (s, 1H, C6-H), 3.55 (s, 1H, -OH), 3.22 (dd, J1 = 9 Hz, J2 = 3 Hz, 1H, C3-H), 2.51 (t, J = 9 Hz, 2H, Ar-H), 2.36–2.25 (m, 3H), 2.01–1.69 (m, 7H), 1.52–1.44 (m, 3H), 1.15–1.03 (m, 4H), 0.99 (m, 3H), 0.86 (s, 2H); 13C-NMR (75 MHz, CDCl3) δ 209.22, 145.32, 141.08, 139.04, 138.74, 131.29, 127.28, 127.23, 122.21, 120.92, 120.54, 119.16, 71.60, 50.36, 49.38, 47.73, 42.23, 37.17, 36.73, 31.59, 31.50, 31.22, 30.98, 29.73, 20.43, 19.47, 14.29; ESI-HR MS (m/z): calcd. for C29H33F3N3O2+ [M + H]+: 512.2519; found: 512.2509. Purity: 99.465% by HPLC (A: H2O; B: methanol, graded: 60–100%), tR 19.600 min, λ: 292 nm.