Salivary Hydrogen Sulfide Measured with a New Highly Sensitive Self-Immolative Coumarin-Based Fluorescent Probe
Abstract
:1. Introduction
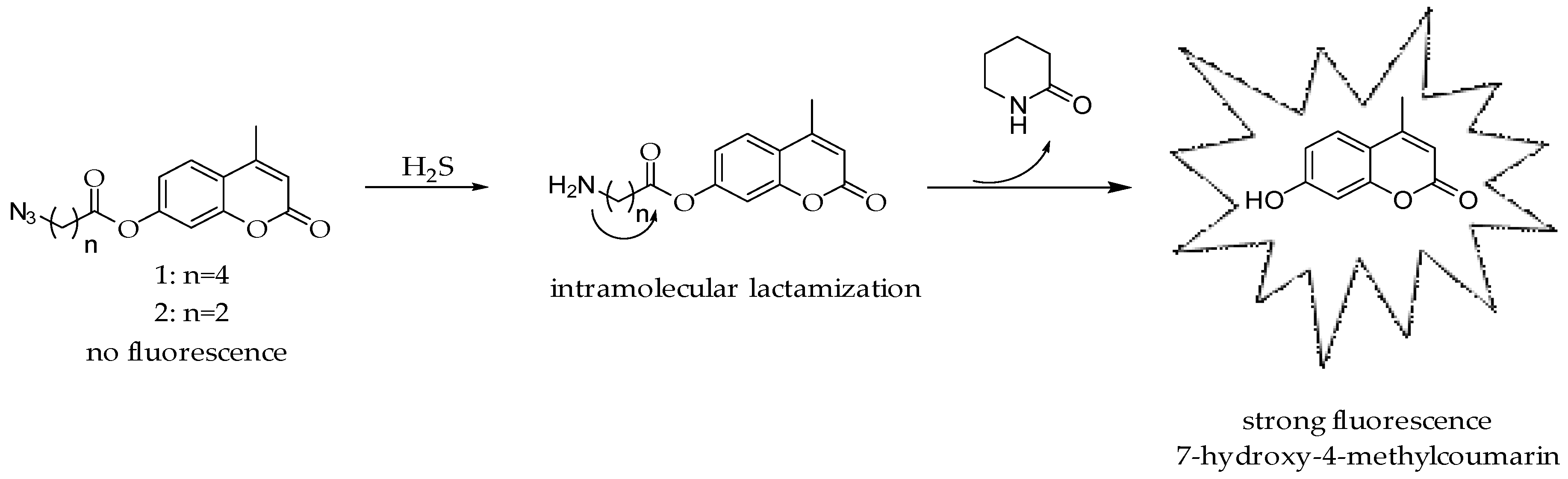
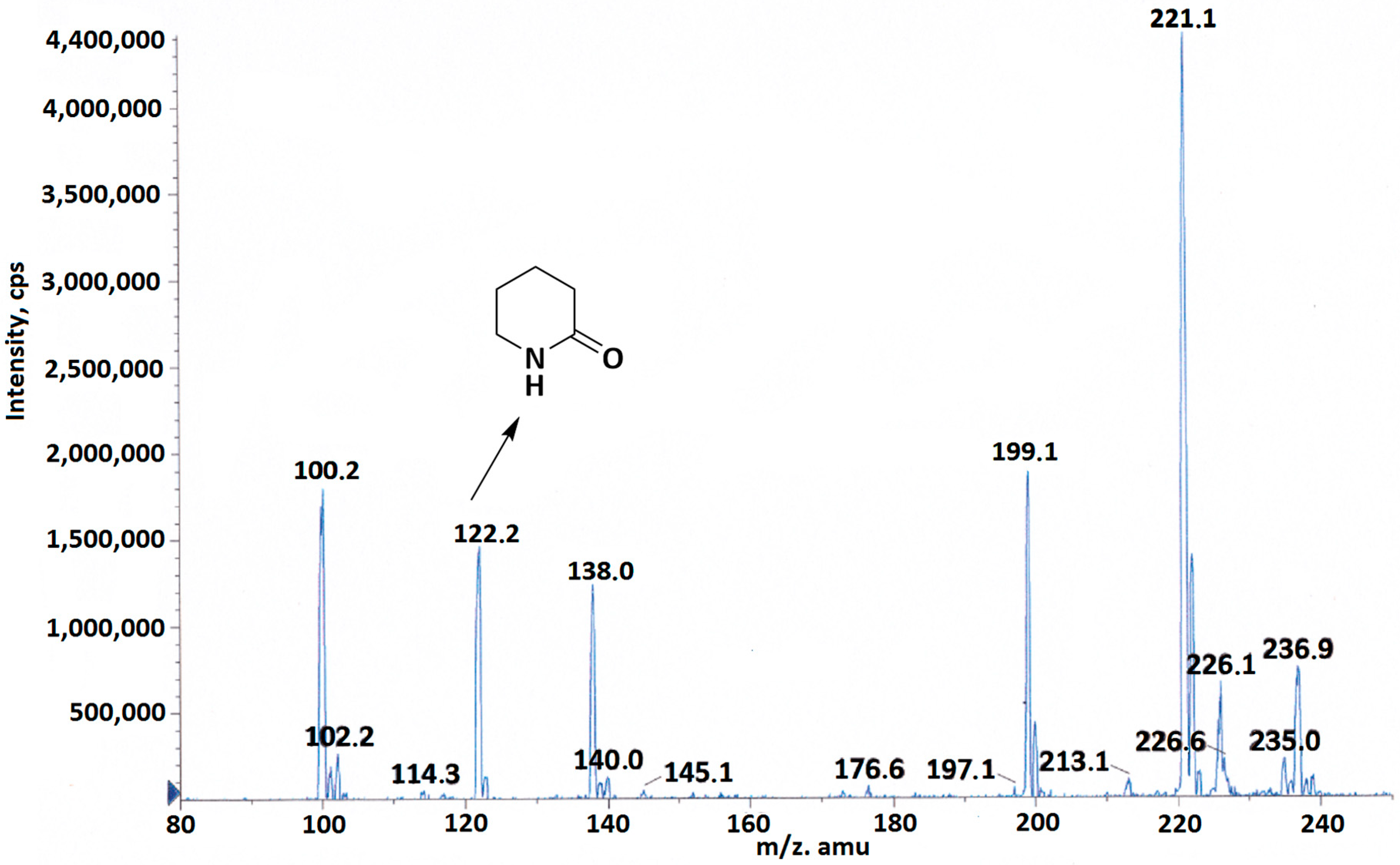
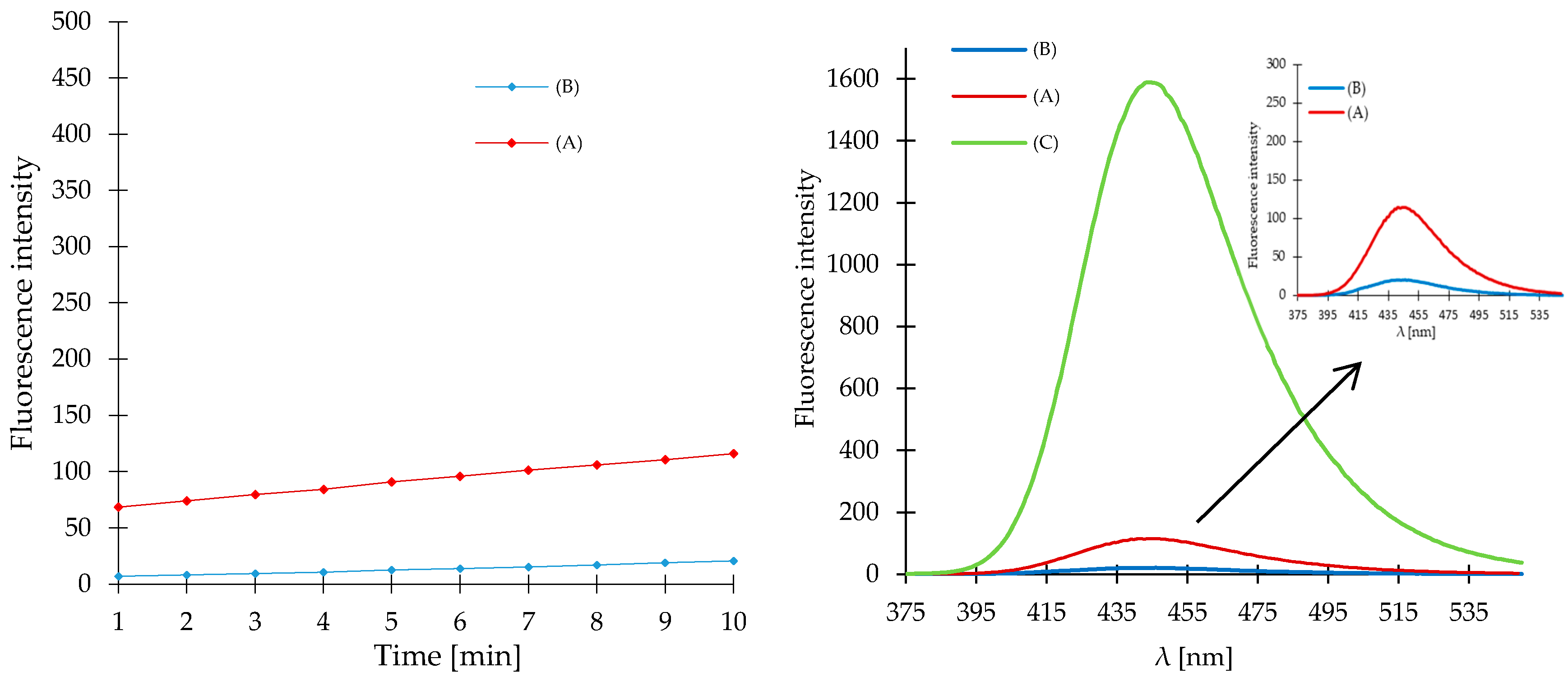
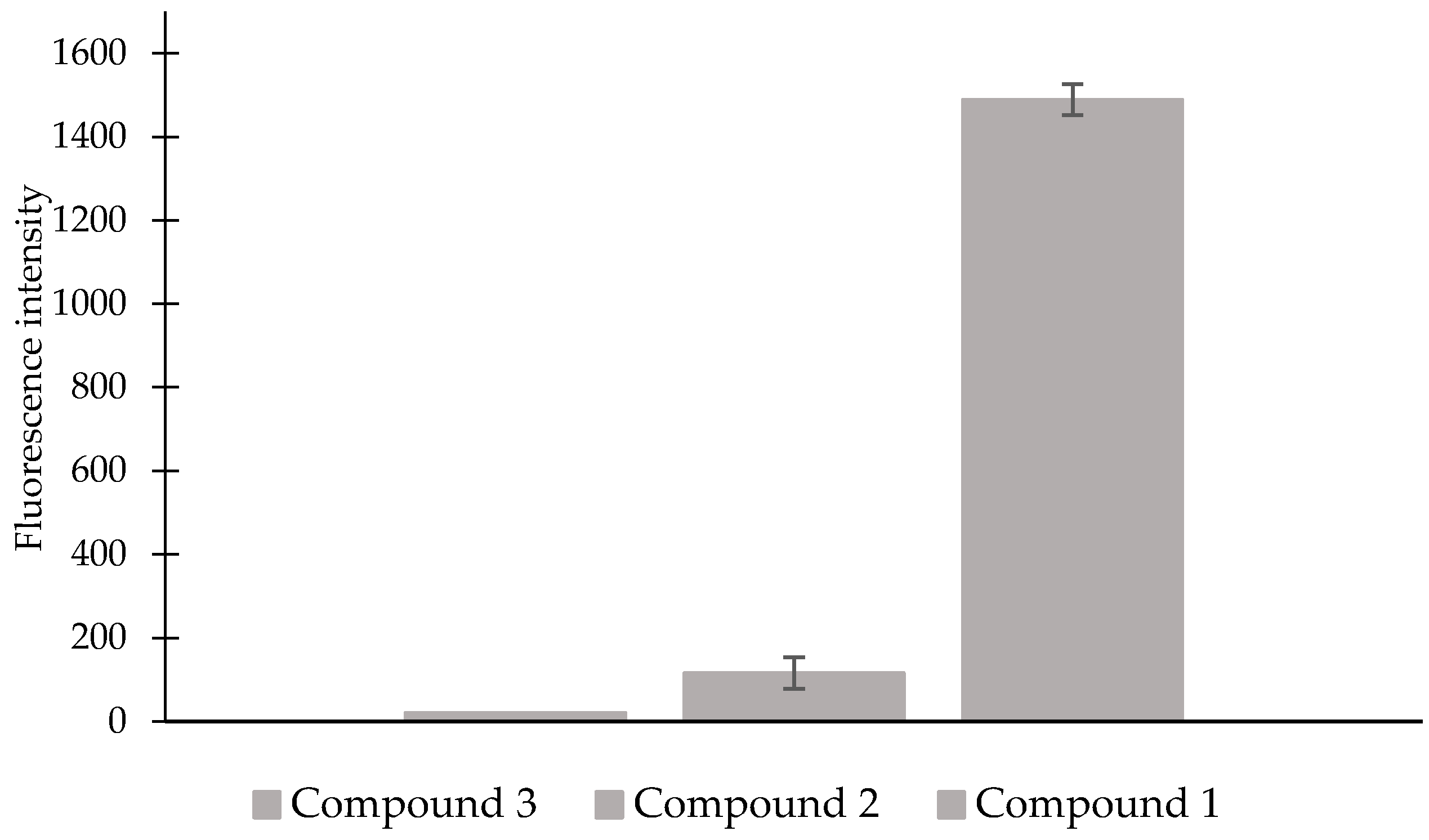
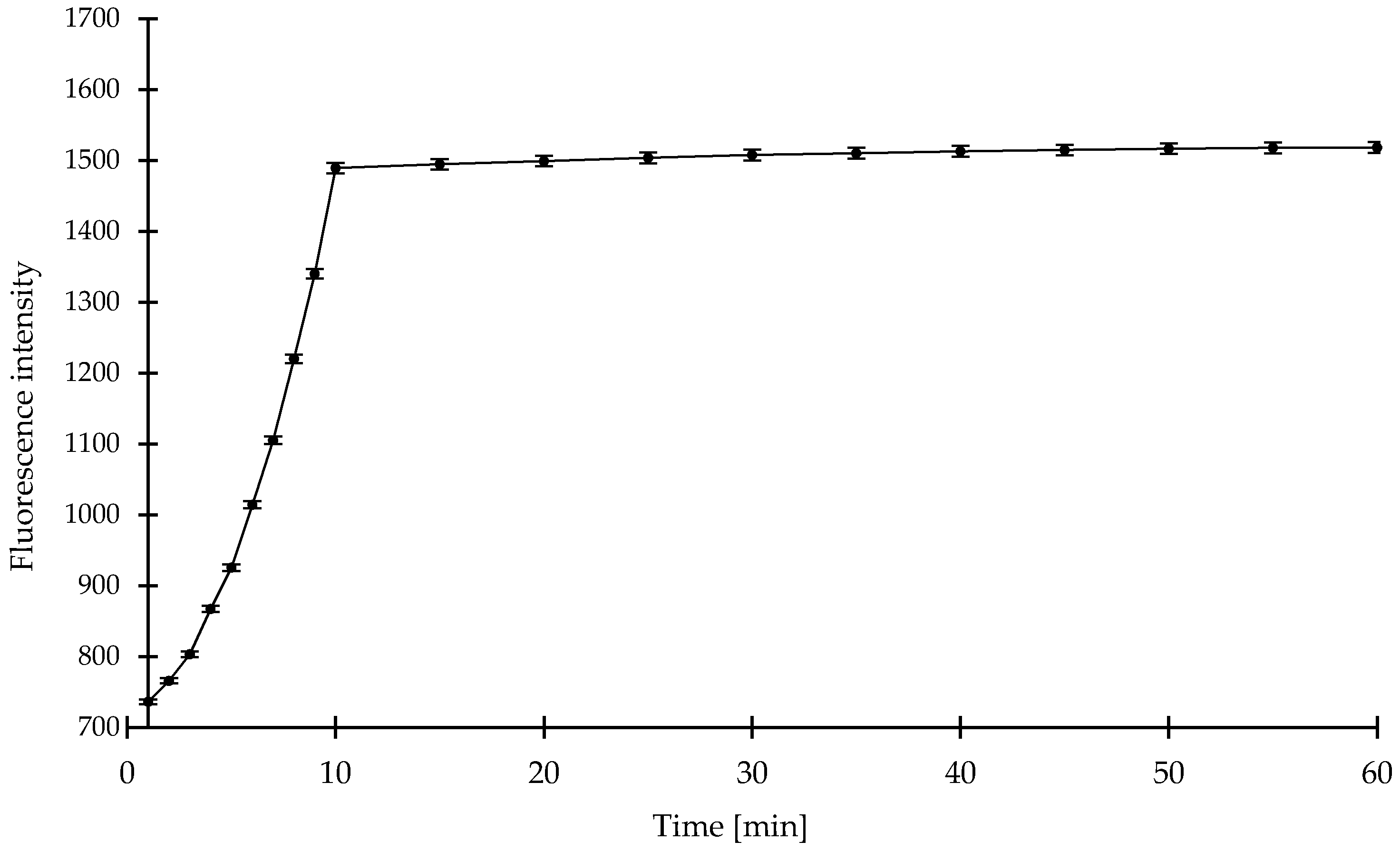
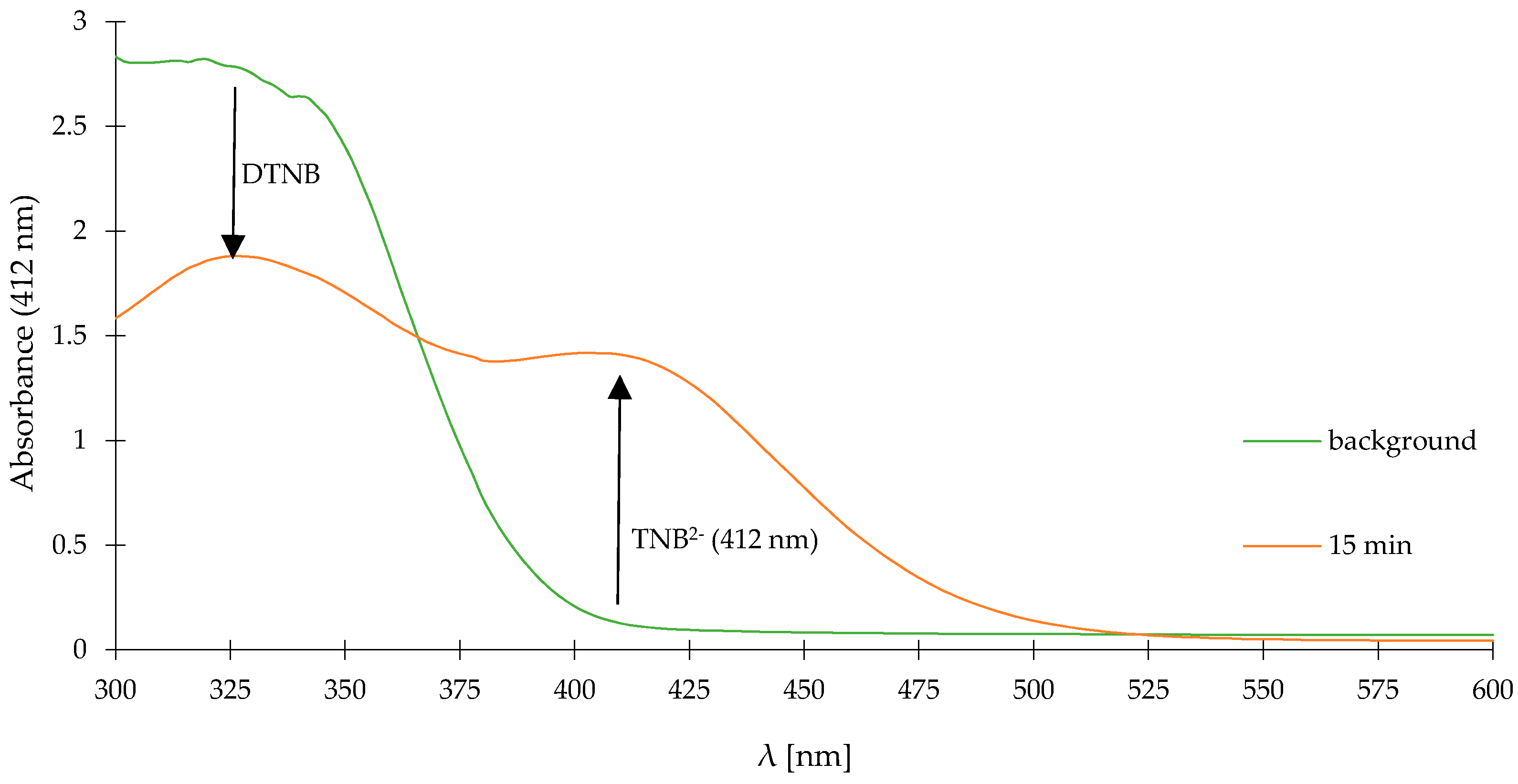
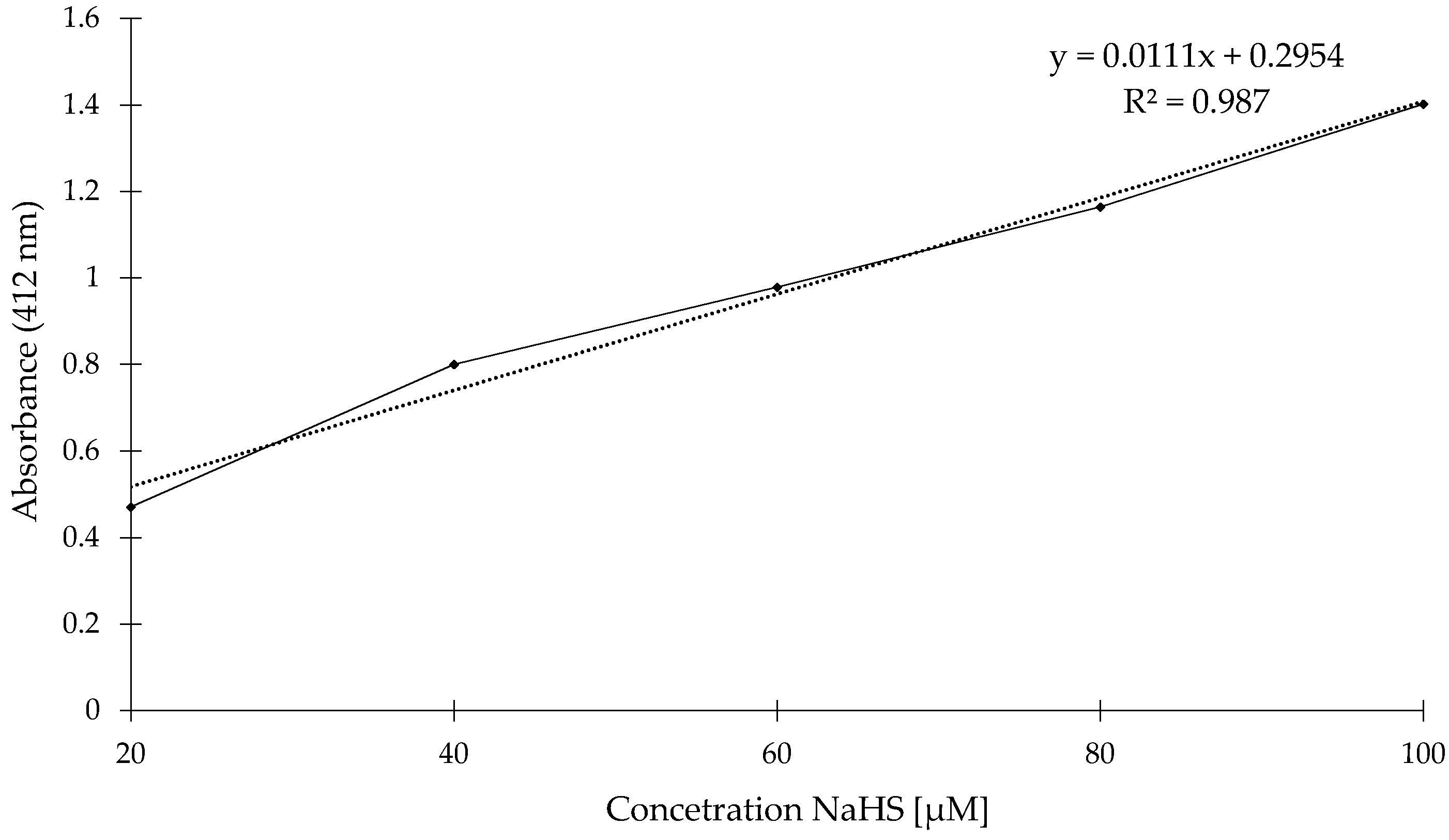
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis and Sensing Mechanisms
3.3. Synthesis of 3-Azidopropanoic Acid and 5-Azidopentanoic acid
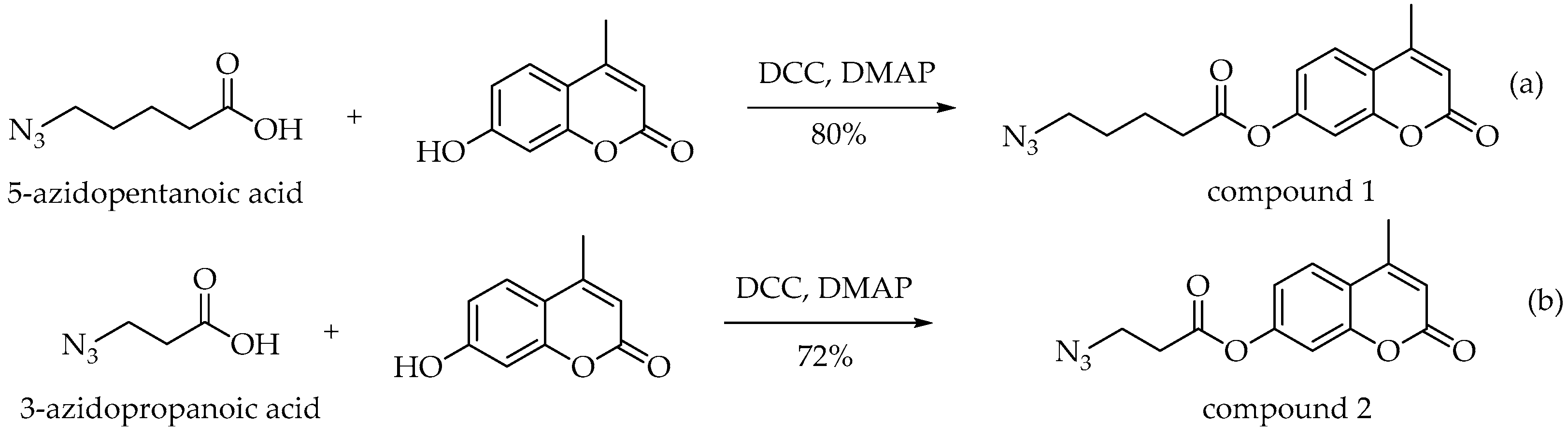
3.4. Synthesis of 4-Methyl-2-oxo-2H-chromen-7-yl 5-Azidopentanoate (1)
3.5. Synthesis of 4-Methyl-2-oxo-2H-chromen-7-yl 3-Azidopropanoate (2)
3.6. Synthesis of 4-Methyl-2-oxo-2H-chromen-7-yl Propionate (3)
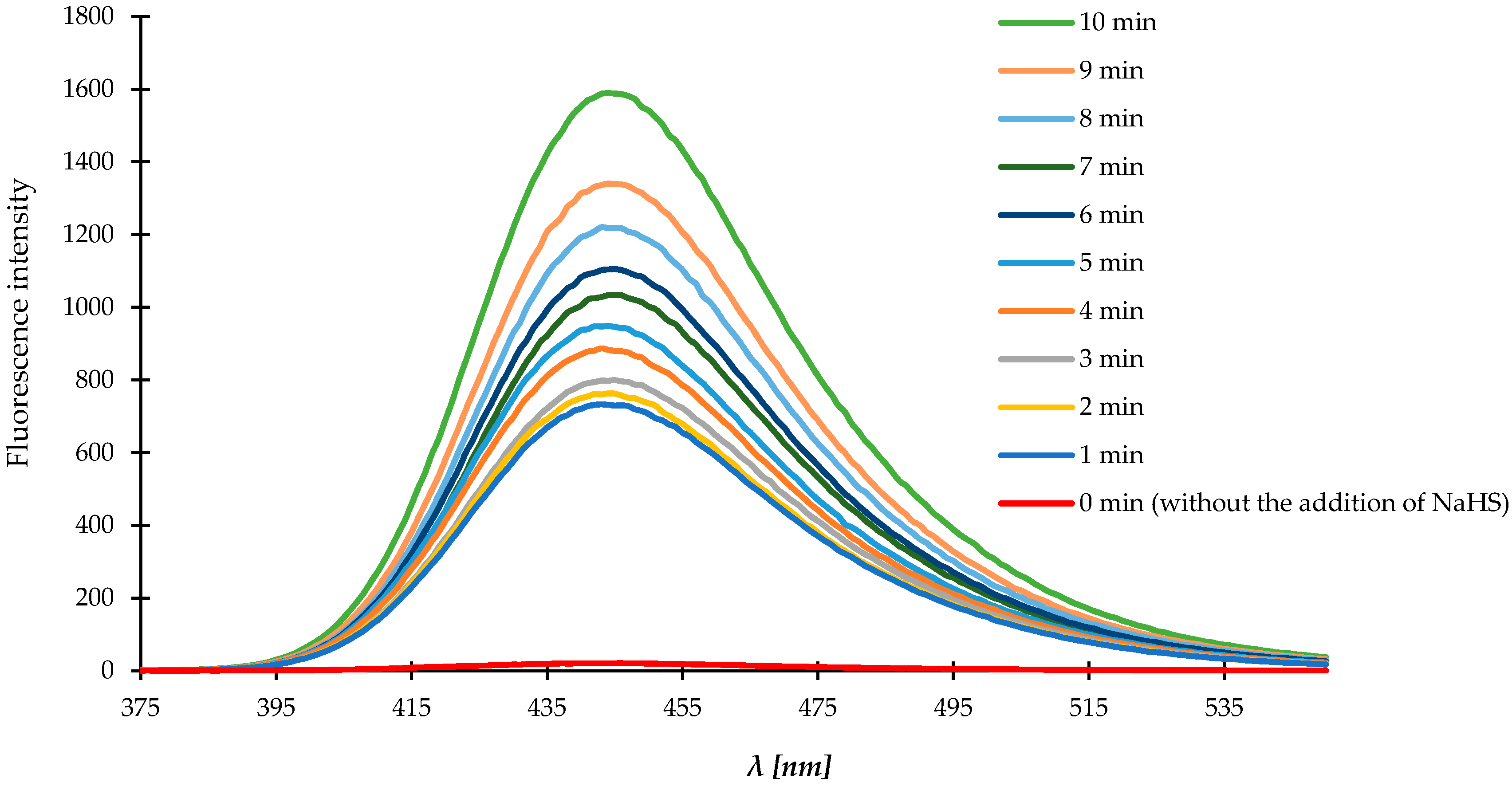
3.7. Characterization of the Fluorescence of Compound 1
3.8. Quantification of H2S Concentration Using Ellman’s Reagent (5,5-Dithiobis(2-Nitrobenzoic Acid) and the Developed Probe
3.9. H2S Detection in Saliva
3.10. General Procedure for H2S Detection by the Fluorescence Method
3.11. General Procedure for H2S Detection Using the Ellman’s Test
3.12. General Procedure for H2S Detection in Saliva Using Compound 1
3.13. Collection of Saliva Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Acid Labile Sulfide |
| CDCl3 | Deuterated chloroform |
| CH2Cl2 | Dichloromethane |
| CH3CN | Acetonitrile |
| CLSS | ChemiLuminescent Sulfide Sensors |
| 13C-NMR | Carbon-13 nuclear magnetic resonance |
| CuS | Copper sulfide |
| CTAB | Cetyltrimethylammonium bromide |
| DCC | N,N′-Dicyclohexylcarbodiimide |
| DMAP | N,N-Dimethylpyridin-4-amine |
| DTNB | 5,5′-Dithiobis(2-nitrobenzoic acid) |
| HCl | Hydrochloric acid |
| 1H-NMR | Proton nuclear magnetic resonance |
| HRMS | High Resolution Mass Spectrometry |
| H2S | Hydrogen sulfide |
| MgSO4 | Magnesium sulfate |
| mM | Millimolar concentration |
| MS | Mass Spectrometry |
| NaHS | Sodium hydrosulfide |
| R2 | Coefficient of determination |
| SE | Standard Error |
| SF | Sulfur fluride |
| TLC | Thin layer chromatography |
| TNB2− | 2-Nitro-5-thiobenzoate anion |
| UV-vis | Ultraviolet–visible spectroscopy |
| λex | Excitation wavelength |
| λem | Emission wavelength |
References
- Lavu, M.; Bhushan, S.; Lefer, D.J. Hydrogen sulfide-mediated cardioprotection: Mechanisms and therapeutic potential. Clin. Sci. 2011, 120, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Moore, P.K. Hydrogen sulfide and the vasculature: A novel vasculoprotective entity and regulator of nitric oxide bioavailability? J. Cell. Mol. Med. 2009, 13, 488–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, J.S. Hydrogen sulfide: A neuromodulator and neuroprotectant in the central nervous system. ACS Chem. Neurosci. 2014, 5, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.H.; Monjok, E.M.; Zeyssig, R.; Kouamou, G.; Bongmba, O.N.; Opere, C.A.; Njie, Y.F.; Ohia, S.E. Effect of hydrogen sulfide on sympathetic neurotransmission and catecholamine levels in isolated porcine iris-ciliary body. Neurochem. Res. 2009, 34, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Gu, H.; Ni, X. Hydrogen sulfide in the endocrine and reproductive systems. Expert Rev. Clin. Pharmacol. 2011, 4, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Srilatha, B.; Hu, L.; Adaikan, G.P.; Moore, P.K. Initial characterization of hydrogen sulfide effects in female sexual function. J. Sex. Med. 2009, 6, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Srilatha, B.; Adaikan, P.G.; Li, L.; Moore, P.K. Hydrogen sulphide: A novel endogenous gasotransmitter facilitates erectile function. J. Sex. Med. 2007, 4, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, W.; Wu, L.; Wang, R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J. Biol. Chem. 2007, 282, 16567–16576. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, G.; Jia, X.; Wu, L.; Wang, R. Activation of KATP channels by H2S in rat insulin-secreting cells and the underlying mechanisms. J. Physiol. 2005, 569, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef] [PubMed]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S biogenesis by human cystathionine gamma-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal. 2014, 20, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R.; Levitt, M.D.; Farrugia, G.; Szurszewski, J.H. Endogenous production of H2S in the gastrointestinal tract: Still in search of a physiologic function. Antioxid. Redox Signal. 2010, 12, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Sikora, N.; Dudek, M. Exogenous hydrogen sulfide produces hemodynamic effects by triggering central neuroregulatory mechanisms. Acta Neurobiol. Exp. 2008, 68, 382–388. [Google Scholar]
- Huc, T.; Jurkowska, H.; Wrobel, M.; Jaworska, K.; Onyszkiewicz, M.; Ufnal, M. Colonic hydrogen sulfide produces portal hypertension and systemic hypotension in rats. Exp. Biol. Med. 2018, 243, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Drapala, A.; Koszelewski, D.; Tomasova, L.; Ostaszewski, R.; Grman, M.; Ondrias, K.; Ufnal, M. Parenteral Na2S, a fast-releasing H2S donor, but not GYY4137, a slow-releasing H2S donor, lowers blood pressure in rats. Acta Biochim. Pol. 2017, 64, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Drapala, A.; Ufnal, M. Exogenous hydrogen sulfide causes different hemodynamic effects in normotensive and hypertensive rats via neurogenic mechanisms. Pharmacol. Rep. 2014, 66, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Tomasova, L.; Drapala, A.; Jurkowska, H.; Wróbel, M.; Ufnal, M. Na2S, a fast-releasing H2S donor, given as suppository lowers blood pressure in rats. Pharmacol. Rep. 2017, 69, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, P.A.; Johnson, P.W. The relationship between oral malodor, gingivitis, and periodontitis. A review. J. Periodontology 1999, 70, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Ayers, K.M.; Colquhoun, A. Halitosis: Causes, diagnosis, and treatment. N. Z. Dent. J. 1998, 94, 156–160. [Google Scholar] [PubMed]
- Searcy, D.G.; Peterson, M.A. Hydrogen sulfide consumption measured at low steady state concentrations using a sulfidostat. Anal. Biochem. 2004, 324, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Radford-Knoery, J.; Cutter, G.A. Determination of carbonyl sulfide and hydrogen sulfide species in natural waters using specialized collection procedures and gas chromatography with flame photometric detection. Anal. Chem. 1993, 65, 976–982. [Google Scholar] [CrossRef]
- Hou, F.P.; Huang, L.; Xi, P.X.; Cheng, J.; Zhao, X.F.; Xie, G.Q.; Shi, Y.J.; Cheng, F.J.; Yao, X.J.; Bai, D.C.; et al. A retrievable and highly selective fluorescent probe for monitoring sulfide and imaging in living cells. Inorg. Chem. 2012, 51, 2454–2460. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Sheng, C.; Cao, Y.; He, W.; Wang, W. Fluorescent probes for the detection of hydrogen sulfide in biological systems. Angew. Chem. Int. Ed. 2012, 51, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Tang, Y.Y.; Zhu, W.H.; Xie, Y.S. Fluorescent and colorimetric ion probes based on conjugated oligopyrroles. Chem. Soc. Rev. 2015, 44, 1101–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.B.; Zhu, W.H.; Xie, Y.S. Development of ion chemosensors based on porphyrin analogues. Chem. Rev. 2016, 117, 2203–2256. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, X.; Guo, W. A reaction-based and highly selective fluorescent probe for hydrogen sulfide. Dyes Pigment. 2017, 139, 482–486. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, G.; Zeng, G.; Li, Z.; Chen, A.; Wang, J.; Jiang, L. Fluorescence chemosensors for hydrogen sulfide detection in biological systems. Analyst 2015, 140, 1772–1786. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Feng, W.; Feng, G. Rapid and highly selective detection of H2S by nitrobenzofurazan (NBD) ether-based fluorescent probes with an aldehyde group. Sens. Actuators B Chem. 2017, 238, 619–625. [Google Scholar] [CrossRef]
- Han, Q.; Mou, Z.; Wang, H.; Tang, X.; Dong, Z.; Wang, L.; Dong, X.; Liu, W. Highly selective and sensitive one-and two-photon ratiometric fluorescent probe for intracellular hydrogen polysulfide sensing. Anal. Chem. 2016, 88, 7206–7212. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, S.; She, M.; Jia, Y.; Hao, L.; Yin, B.; Wang, L.; Obst, M.; Shen, Y.; Zhang, Y. Novel fluorescein-based fluorescent probe for detecting H2S and Its real applications in blood plasma and biological imaging. Anal. Chem. 2016, 88, 11253–11260. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Yang, S.; Li, Y.; Qing, Z.; Zheng, J.; Li, J.; Yang, R. Ratiometric visualization of NO/H2S cross-talk in living cells and tissues using a nitroxyl-responsive two-photon fluorescence probe. Anal. Chem. 2017, 89, 4587–4594. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Z.; Yang, L.; Han, J.; Han, S. Fluorogenic detection of hydrogen sulfide via reductive unmasking of o-azidomethylbenzoyl-coumarin conjugate. Chem. Commun. 2012, 48, 10120–10122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, S.; Hong, M.; Xu, Y.; Wang, S.; Liu, Y.; Qian, Y.; Zhao, J. A colorimetric and ratiometric fluorescent probe for the imaging of endogenous hydrogen sulphide in living cells and sulphide determination in mouse hippocampus. J. Org. Biomol. Chem. 2014, 12, 5115–5125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Du, X.J.; Dai, C.G.; Song, Q.H. Ratiometric two-photon fluorescent probes for mitochondrial hydrogen sulfide in living cells. J. Org. Chem. 2014, 79, 9481–9489. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, W.; Feng, W.; Wang, Y.; Chen, G.; Wang, S.; Li, S.; Li, H.; Wang, K.; Zhang, J. A novel dual-emission fluorescent probe for the simultaneous detection of H2S and GSH. Chem. Commun. 2016, 52, 4628–4631. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.L.; Liu, W.; Liu, X.J.; Ouyang, F.; Kuang, Y.Q.; Jiang, J.H. An azidocoumarin-based fluorescent probe for imaging lysosomal hydrogen sulfide in living cells. Anal. Methods 2017, 9, 2859–2864. [Google Scholar] [CrossRef]
- Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T. Development of a highly selective fluorescence probe for hydrogen sulfide. J. Am. Chem. Soc. 2011, 133, 18003–18005. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.G.; Cha, S.; Lee, H.; Jeon, H.L.; Chang, S.K. Sulfide-selective chemosignaling by a Cu2+ complex of dipicolylamine appended fluorescein. Chem. Commun. 2009, 47, 7390–7392. [Google Scholar] [CrossRef] [PubMed]
- Sathyadevi, P.; Lee, L.Y.; Wang, Y.L.; Chen, Y.J.; Chen, C.Y.; Wang, Y.M. A water soluble and fast response fluorescent turn-on copper complex probe for H2S detection in zebra fish. Talanta 2016, 147, 445–452. [Google Scholar]
- Chen, Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew. Chem. 2013, 125, 1732–1735. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Cao, D. A nitroolefin functionalized DPP fluorescent probe for the selective detection of hydrogen sulfide. New J. Chem. 2017, 41, 3367–3373. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, D.; Wang, Q.; Liu, S.; Lin, Q.; Sun, H. Molecular engineering of a mitochondrial-targeting two-photon in and near-infrared out fluorescent probe for gaseous signal molecules H2S in deep tissue bioimaging. Biosens. Bioelectron. 2017, 91, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Nashet, A.S.; Osuga, D.T.; Feeney, R.E. Hydrogen Sulfide in Redox Biology. Anal. Biochem. 1977, 79, 394–405. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Gura, T. Just spit it out. Nat. Med. 2008, 14, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.E.; Moore, L.V. The bacteria of periodontal diseases. Periodontolgy 2000 1994, 5, 66–77. [Google Scholar] [CrossRef]
- Matsuyama, T.; Kawai, T.; Izumi, Y.; Taubman, M.A. Expression of major histocompatibility complex class II and CD80 by gingival epithelial cells induces activation of CD4+ T cells in response to bacterial challenge. Infect. Immun. 2005, 73, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, I.; Westbay, G. Oral malodor. Crit. Rev. Oral Biol. Med. 1990, 1, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T. Assay methods and biological roles of labile sulfur in animal tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 781, 227–249. [Google Scholar] [CrossRef]
- Kanehira, T.; Hongo, H.; Takehara, J.; Asano, K.; Osada, K.; Izumi, H.; Fujii, Y.; Sakamoto, W. A novel visual test for hydrogen sulfide on the tongue dorsum. Open J. Stomatol. 2012, 2, 314–321. [Google Scholar] [CrossRef]
- Krolla, J.K.; Werchana, C.A.; Reevesb, A.G.; Bruemmerb, K.J.; Lippertb, A.R.; Thomas Ritza, T. Sensitivity of salivary hydrogen sulfide to psychological stress and its association with exhaled nitric oxide and affect. Physiol. Behav. 2017, 179, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Tarnai, Z.; Berkovits, C.; Novák, P.; Mohácsi, A.; Braunitzer, G.; Rakonczay, Z.; Turzó, K.; Nagy, K.; Szabó, G. Volatile sulphur compound measurement with OralChroma (TM): A methodological improvement. J. Breath Res. 2015, 9, 016001. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Bull, R.; Rains, J.L.; Bass, P.F.; Levine, S.N.; Reddy, S.; McVie, R.; Bocchini, J.A. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid. Redox Signal. 2010, 12, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; DeLeon, E.R.; Liu, F. Controversies and conundrums in hydrogen sulfide biology. Nitric Oxide 2014, 41, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cai, L.; Chen, Z.; Wang, W. Coumarin-Derived Fluorescent Chemosensors. IntechOpen 2012, 121–151. [Google Scholar] [CrossRef]
- Żądło-Dobrowolska, A.; Szczygieł, M.; Koszelewski, D.; Paprocki, D.; Ostaszewski, R. Self-immolative versatile fluorogenic probes for screening of hydrolytic enzyme activity. Org. Biomol. Chem. 2016, 14, 9146–9150. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3-azidopropanonic acid, 5-azidopentanonic acid, 4-Methyl-2-oxo-2H-chromen-7-yl 5-Azidopentanoate (1), 4-Methyl-2-oxo-2H-chromen-7-yl 3-Azidopropanoate (2) and 4-Methyl-2-oxo-2H-chromen-7-yl Propionate (3) are available from the authors. |
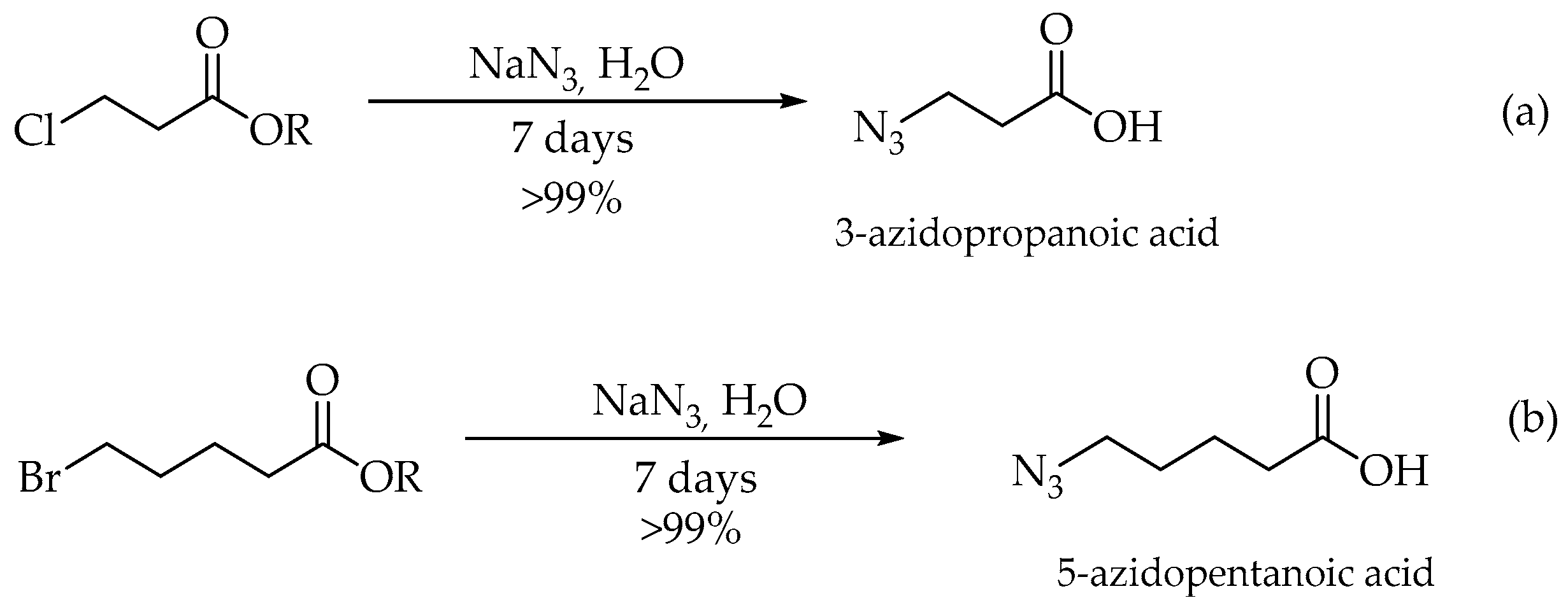
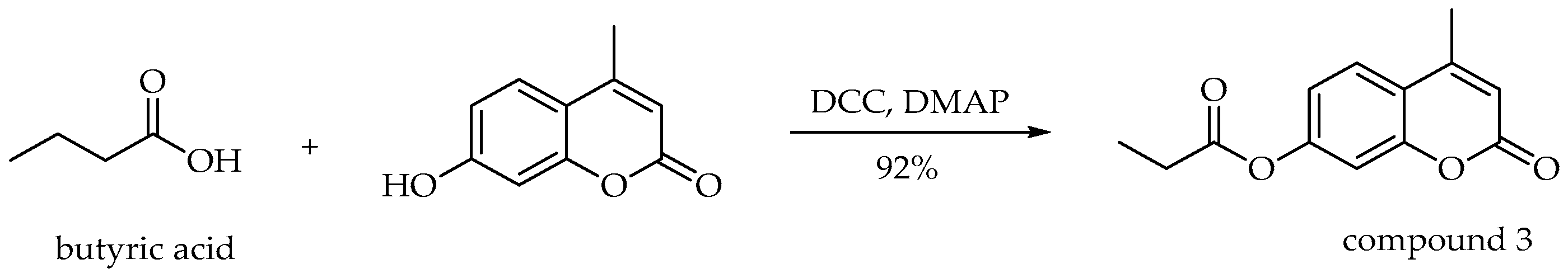
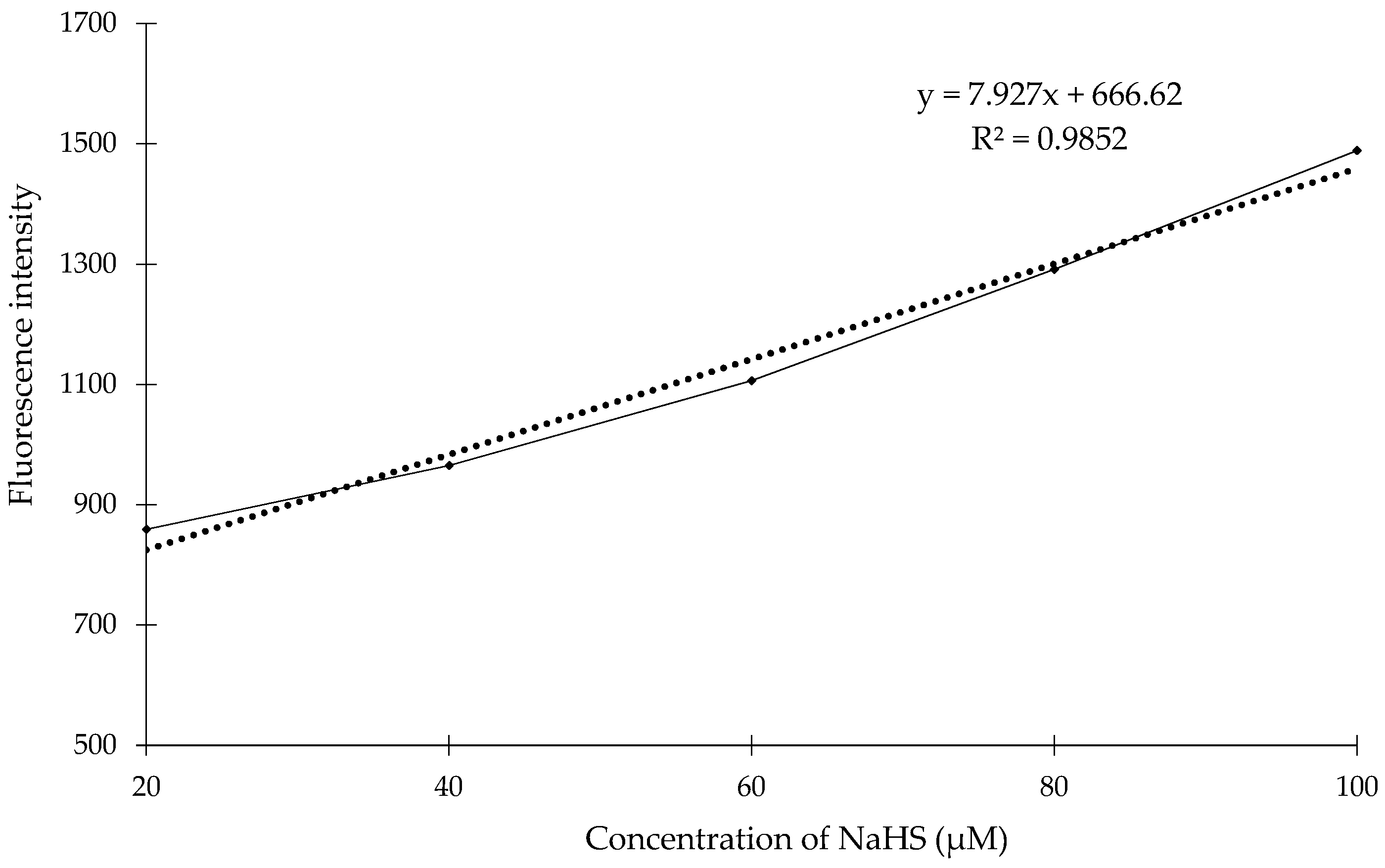
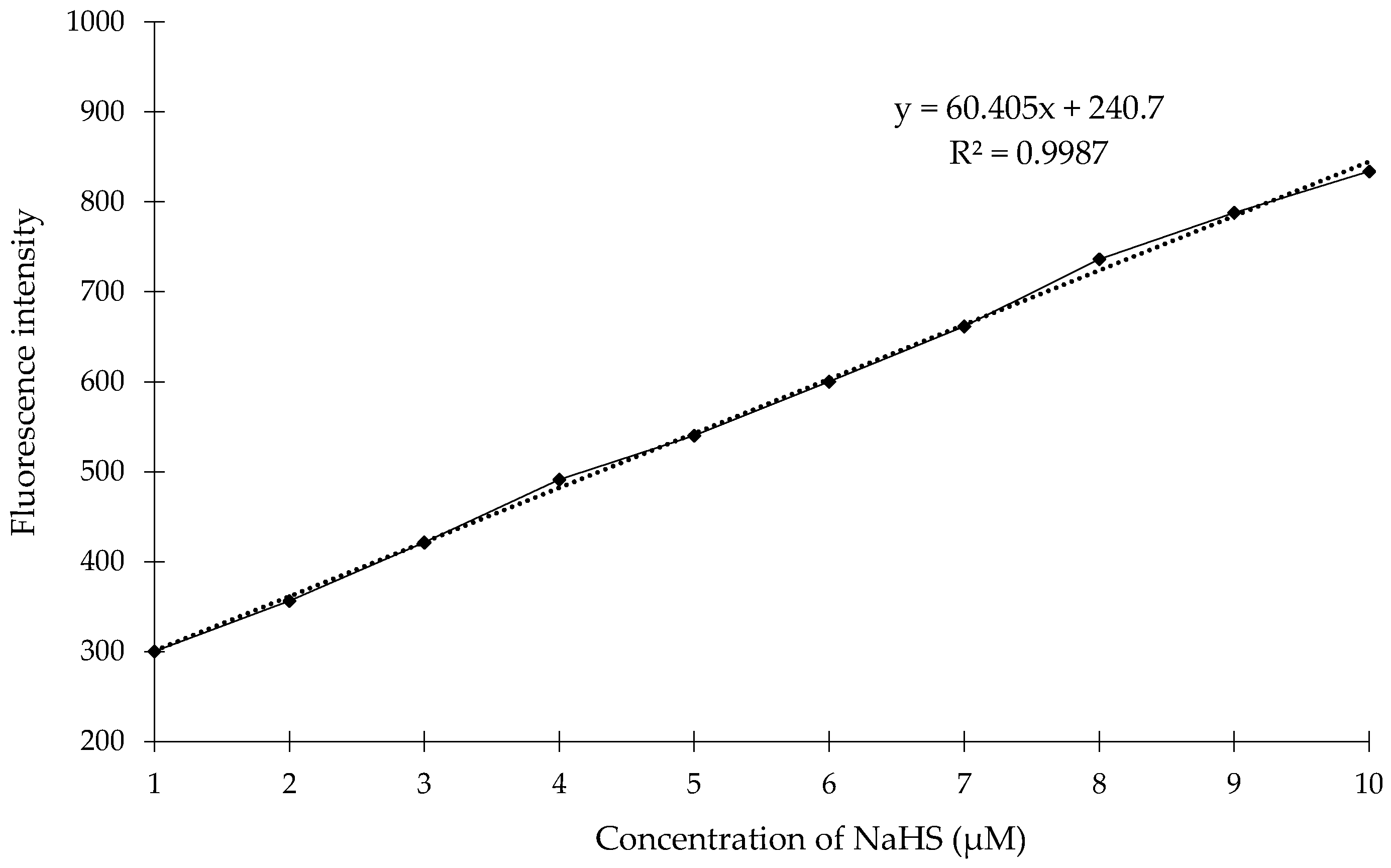
| Samples of Saliva (n = 15) | |||
|---|---|---|---|
| H2S concentration [μM] | Range | Mean | SE |
| 1.641–7.124 | 3.424 | ±0.547 | |
| Concentration of NaHS [μM] | Ellman’s Test (μM) a | Fluorescence Method Using the Compound 1 (μM) b | ||
|---|---|---|---|---|
| C H2S[μM] | V [μM s−1] | C H2S[μM] | V [μM s−1] | |
| 20 | 15.82 | 0.018 | 17.00 | 0.028 |
| 40 | 36.91 | 0.041 | 38.15 | 0.064 |
| 60 | 57.63 | 0.064 | 58.10 | 0.097 |
| 80 | 78.25 | 0.087 | 78.60 | 0.13 |
| 100 | 94.52 | 0.105 | 97.23 | 0.16 |
| Participant Demographics and Physical Characteristics. | |||
| Healthy volunteers (n) | 15 | ||
| Age (years) | Mean | SE (standard error) | Range |
| 28 | 1.6 | 18–40 | |
| Sex (m/f) | 7/8 | ||
| Ethnicity: | |||
| Caucasian | 100% | ||
| Other | - | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaorska, E.; Konop, M.; Ostaszewski, R.; Koszelewski, D.; Ufnal, M. Salivary Hydrogen Sulfide Measured with a New Highly Sensitive Self-Immolative Coumarin-Based Fluorescent Probe. Molecules 2018, 23, 2241. https://doi.org/10.3390/molecules23092241
Zaorska E, Konop M, Ostaszewski R, Koszelewski D, Ufnal M. Salivary Hydrogen Sulfide Measured with a New Highly Sensitive Self-Immolative Coumarin-Based Fluorescent Probe. Molecules. 2018; 23(9):2241. https://doi.org/10.3390/molecules23092241
Chicago/Turabian StyleZaorska, Ewelina, Marek Konop, Ryszard Ostaszewski, Dominik Koszelewski, and Marcin Ufnal. 2018. "Salivary Hydrogen Sulfide Measured with a New Highly Sensitive Self-Immolative Coumarin-Based Fluorescent Probe" Molecules 23, no. 9: 2241. https://doi.org/10.3390/molecules23092241






