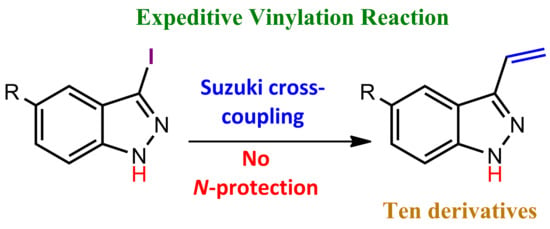
3.2. Syntheses and Characterization Procedures
![Molecules 23 02051 i003]()
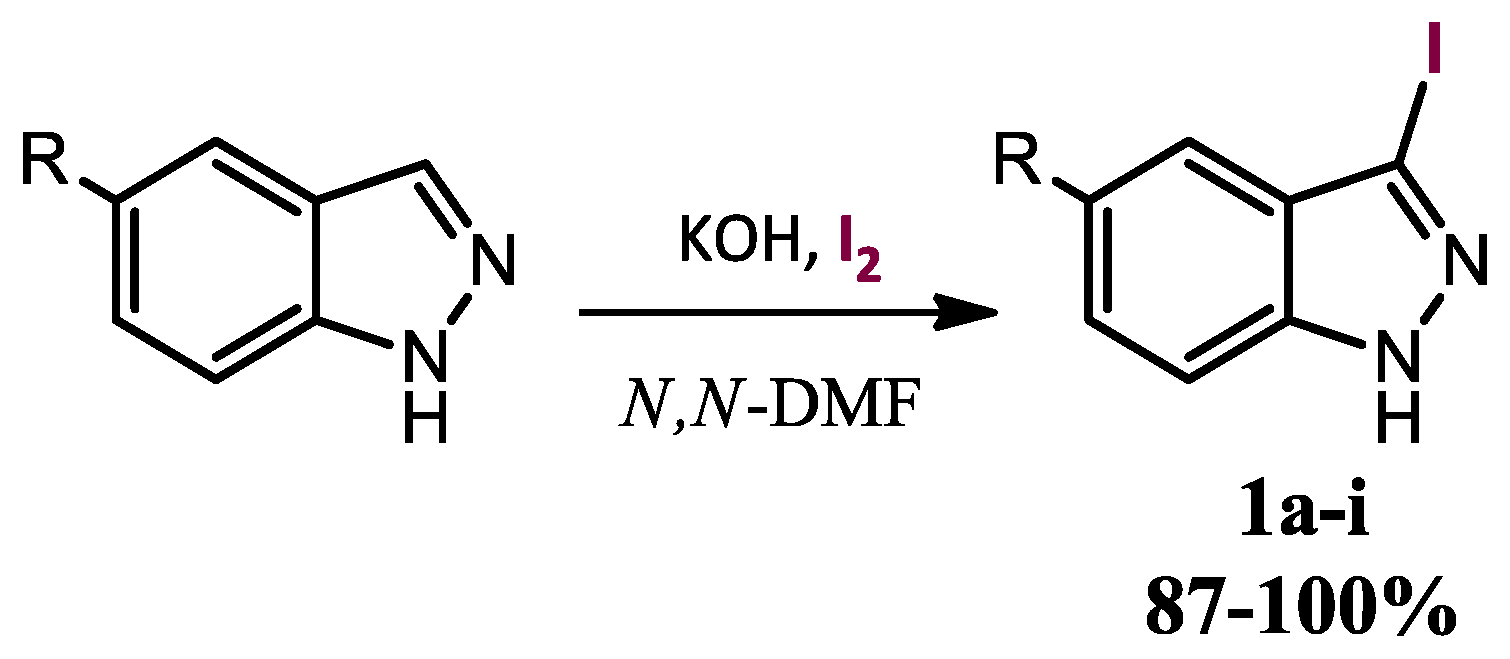
3-Iodo-1H-indazole (
1a). 3-Iodoindazoles were obtained by direct iodination of commercial indazoles by the method previously described by Bocchi [
28] with slight modifications. A solution of 1
H-indazole (3 g, 25.4 mmol), iodine (12.7 g, 50.03 mmol) and potassium hydroxide (5.34 g, 95.25 mmol) in DMF (7 mL) was stirred for 3 h at room temperature. The reaction was quenched by dilution with saturated solution of sodium bisulfite (150 mL) and a precipitated was formed. The precipitated was filtered over vacuum and washed with water (3 × 30 mL). The solid was left to dry at 30 °C in a vacuum oven overnight obtaining 6.17 g of a pale yellow solid. Yield: 100%; m.p.: 136–138 °C (lit.: [
36] 134–136 °C); IR (KBr) ν (cm
−1): 3086 (NH); 424 (C-I).
1H-NMR δ (ppm): 13.50 (1H, s, H-1); 7.55 (1H, d,
J = 8.6 Hz, H-7); 7.45–7.40 (2H, m, H-6 and H-4); 7.19 (1H, dd,
J = 7.5 Hz, H-5).
13C-NMR δ (ppm): 140.41; 127.22; 126.79; 121.23; 120.39; 110.51; 93.49; HRMS calculated for C
7H
5IN
2: 243.9497, Found: 243.9499.
![Molecules 23 02051 i004]()
3-Iodo-5-nitro-1H-indazole (
1b). Prepared from 5-nitro-1
H-indazole (3 g, 18.4 mmol), iodine (9.0 g, 35.46 mmol), potassium hydroxide (3.87 g, 69.00 mmol) and DMF (7 mL) to give 5.31 g of a pale yellow solid. Yield: 100%; m.p.: 216–217 °C (lit.: [
22] 214 °C); IR (KBr) ν (cm
−1): 3200 (NH); 1528 (NO
2 asymmetrical); 1335 (NO
2 symmetrical); 424 (C-I).
1H-NMR δ (ppm): 14.13 (1H, s, H-1); 8.30 (1H, d,
J = 1.8 Hz, H-4); 8.23 (1H, dd,
J = 9.2 and 2.1 Hz, H-6); 7.75 (1H, d,
J = 9.2, H-7).
13C-NMR δ (ppm): 142.99; 142.52; 126.77; 122.42; 118.57; 112.18; 97.67; HRMS calculated for C
7H
4IN
3O
2: 288.9348, Found: 288.9353.
![Molecules 23 02051 i005]()
5-Bromo-3-iodo-1H-indazole (
1c). Prepared from 5-bromo-1
H-indazole (1 g, 5.07 mmol), iodine (2.5 g, 9.85 mmol), potassium hydroxide (1.07 g, 19.06 mmol) and DMF (4 mL) to give 1.63 g of a pale yellow solid. Yield: 100%; m.p.: 209–210 °C (lit.: [
22] 196 °C); IR (KBr) ν (cm
−1): 3124 (NH); 424 (C-I and C-Br).
1H-NMR δ (ppm): 13.69 (1H, s, H-1); 7.60 (1H, s, H-4); 7.57–7.50 (2H, m, H-6 and H-7).
13C-NMR δ (ppm): 139.24; 129.98; 128.46; 122.59; 113.46; 112.74; 92.47; HRMS calculated for C
7H
4BrIN
2: 321.8603, Found: 321.8617.
![Molecules 23 02051 i006]()
3-Iodo-5-methoxy-1H-indazole (
1d). Prepared from 5-methoxy-1
H-indazole (0.2 g, 1.35 mmol), iodine (0.7 g, 2.76 mmol), potassium hydroxide (284 mg, 5.06 mmol) and DMF (3 mL) to give 0.34 g of a pale brown solid. Yield: 90%; m.p.: 162–163 °C (lit.: [
22] 178–179 °C); IR (KBr) ν (cm
−1): 3185 (NH); 1288 (C-O); 424 (C-I).
1H-NMR δ (ppm): 13.36 (1H, s, H-1); 7.46 (1H, d,
J = 8.9 Hz, H-7); 7.08 (1H, d,
J = 8.8 Hz, H-6); 6.76 (1H, s, H-4); 3.82 (3H, s, OCH
3).
13C-NMR δ (ppm): 154.71; 136.16; 127.13; 119.51; 111.74; 99.24; 92.25; 55.45; HRMS calculated for C
8H
7IN
2O: 273.9603, Found: 273.9599.
![Molecules 23 02051 i007]()
5-Fluoro-3-iodo-1H-indazole (
1e). Prepared from 5-fluoro-1
H-indazole (0.2 g, 1.47 mmol), iodine (0.75 g, 2.95 mmol), potassium hydroxide (0.31 g, 5.52 mmol) and DMF (3 mL) to give 0.33 g of a pale brown solid. Yield: 87%; m.p.: 158–159 °C (lit.: [
37] 166 °C); IR (KBr) ν (cm
−1): 3171 (NH); 1165 (C-F); 424 (C-I).
1H-NMR δ (ppm): 13.61 (1H, s, H-1); 7.61 (1H, dd,
J = 9.0 and 4.1 Hz, H-7); 7.32 (1H, td,
J = 9.1 and 2.1 Hz, H-6); 7.17 (1H, dd,
J = 8.7 and 1.7 Hz, H-4).
13C-NMR δ (ppm): 157.51 (d,
J = 237.3 Hz); 137.56; 126.96 (d,
J = 10.4 Hz); 116.84 (d,
J = 27.5 Hz); 112.41 (d,
J = 9.7 Hz); 104.44 (d,
J = 24.2 Hz); 92.69 (d,
J = 5.8 Hz); HRMS calculated for C
7H
4FIN
2: 261.9403, Found: 261.9408.
![Molecules 23 02051 i008]()
5-Chloro-3-iodo-1H-indazole (1f). Prepared from 5-chloro-1H-indazole (0.2 g, 1.31 mmol), iodine (0.65 g, 2.56 mmol), potassium hydroxide (275 mg, 4.90 mmol) and DMF (3 mL) to give 0.33 g of a pale brown solid. Yield: 90%; m.p.: 182–183 °C; IR (KBr) ν (cm−1): 3125 (NH); 710 (C-Cl); 424 (C-I). 1H-NMR δ (ppm): 13.69 (1H, s, H-1); 7.60 (1H, d, J = 8.7 Hz, H-7), 7.46–7.40 (2H, m, H-4 and H-6). 13C-NMR δ (ppm): 139.10; 127.82; 127.64; 125.77; 119.48; 112.51; 92.64; HRMS calculated for C7H4ClIN2: 277.9108, Found: 277.9116.
![Molecules 23 02051 i009]()
3-Iodo-5-methyl-1H-indazole (1g). Prepared from 5-methyl-1H-indazole (0.2 g, 1.51 mmol), iodine (0.75 g, 2.95 mmol), potassium hydroxide (318 mg, 5.67 mmol) and DMF (3 mL) to give 0.36 g of a pale brown solid. Yield: 93%; m.p.: 159–160 °C; IR (KBr) ν (cm−1): 3186 (NH); 2916 (C-H); 424 (C-I). 1H-NMR δ (ppm): 13.37 (1H, s, H-1); 7.44 (1H, d, J = 8.2 Hz, H-7); 7.25 (1H, d, J = 8.1 Hz, H-6); 7.18 (1H, s, H-4); 2.41 (3H, s, CH3). 13C-NMR δ (ppm): 139.12; 130.43; 129.33; 127.10; 119.24; 110.31; 92.59; 20.91; HRMS calculated for C8H7IN2: 257.9654, Found: 257.9659.
![Molecules 23 02051 i010]()
3-Iodo-1H-indazole-5-carbonitrile (1h). Prepared from 1H-indazole-5-carbonitrile (0.2 g, 1.4 mmol), iodine (0.7 g, 2.76 mmol), potassium hydroxide (295 mg, 5.26 mmol) and DMF (3 mL) to give 0.34 g of a white solid. Yield: 91%; m.p.: 206–207 °C; IR (KBr) ν (cm−1): 3340 (NH); 2283 (CN); 424 (C-I). 1H-NMR δ (ppm): 13.99 (1H, s, H-1); 8.04 (1H, s, H-7); 7.73 (2H, s, H-4 and H-6). 13C-NMR δ (ppm): 141.53; 129.27; 127.39; 126.73; 119.30; 112.25; 103.73; 95.36. HRMS calculated for C8H4IN3: 268.9450, Found: 268.9455.
![Molecules 23 02051 i011]()
tert-Butyl (3-iodo-1H-indazol-5-yl)carbamate (1i). Prepared from tert-butyl(1-H-indazol-5-yl)carbamate (0.35 g, 1.5 mmol), iodine (0.75 g, 2.95 mmol), potassium hydroxide (316 mg, 5.63 mmol) and DMF (3 mL). The brown solid obtained was purificated by column chromatography on silica gel using n-hexane/ethyl acetate 1:1 v/v to obtain 0.29 g of pure product as brown crystalline plates. Yield: 54%; m.p.: 181–182 °C; IR (KBr) ν (cm−1): 3348 (NH); 1750 (C=O); 1157 (C-O); 424 (C-I). 1H-NMR δ (ppm): 13.35 (1H, s, H-1); 9.40 (1H, s, H-5); 7.70 (1H, s, H-4); 7.44 (1H, d, J = 8.9 Hz, H-8); 7.38 (1H, d, J = 8.9 Hz, H-7); 1.50 (9H, s, H-6). 13C-NMR δ (ppm): 153.05; 136.82; 133.63; 126.91; 120.89; 110.71; 107.38; 92.78; 79.04; 28.19; HRMS calculated for C12H14IN3O2: 359.0131, Found: 359.0128.
![Molecules 23 02051 i012]()
3-Iodo-1H-indazol-5-amine (1j). To a solution of tert-butyl (3-iodo-1-H-indazol-5-yl)carbamate (0.2 g, 0.56 mmol) (1i) and trifluoroacetic acid (5 mL, 65.34 mmol) in CH2Cl2 (5 mL) was stirred vigorously for 2 h at room temperature. After stirring, the reaction was neutralized to pH = 7 using NaOH 1M and the organic layer was extracted with ethyl acetate (3 × 20 mL) The combined organic layers were dried over anhydrous sodium sulfate and removal of the solvent under vacuum afforded a 144 mg of pure product as a black solid. Yield: 100%; m.p.: 178–179 °C; IR (KBr) ν (cm−1): 3375 (NH); 424 (C-I). 1H-NMR δ (ppm): 13.07 (1H, s, H-1); 7.25 (1H, d, J = 8.8 Hz, H-7), 6.85 (1H, dd, J = 8.8 and 1.7 Hz, H-6); 6.44 (1H, s, H-4); 5.01 (2H, br s, H-5). 13C-NMR δ (ppm): 143.46; 134.79; 127.96; 119.39; 110.91; 99.83; 90.59; HRMS calculated for C7H6IN3: 258.9606, Found: 258.9602.
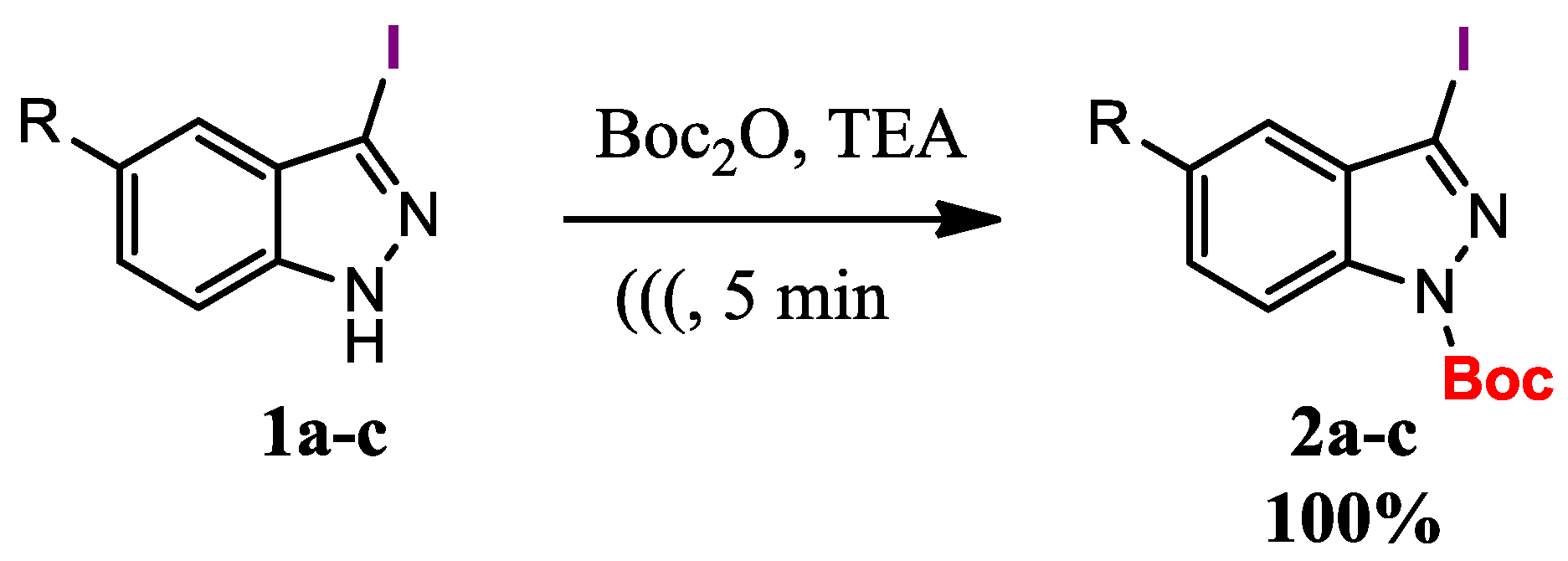
![Molecules 23 02051 i013]()
tert-Butyl 3-iodo-1H-indazole-1-carboxylate (2a). A mixture of 3-iodo-1H-indazole (0.2 g, 0.82 mmol), di-tert-butyldicarbonate (0.2 g, 0.92 mmol) and triethylamine (1 mL) were put under ultrasonic irradiation for 10 min. The resulted solution was neutralized using HCl 1M and then extracted with dichloromethane (3 × 30 mL). The combined organic layers were dried with anhydrous sodium sulfate and removal of the solvent under vacuum afforded a pure product as a pale yellow crystals. Yield: 100%; m.p.: 93–95 °C; IR (KBr) ν (cm−1): 1728 (C=O); 1150 (C-O); 424 (C-I). 1H-NMR (CDCl3) δ (ppm): 8.09 (1H, d, J = 8.5 Hz, H-7); 7.55 (1H, t, J = 7.8 Hz, H-4); 7.46 (1H, d, J = 7.9 Hz, H-6); 7.33 (1H, t, J = 7.6 Hz, H-5); 1.71 (9H, s, CH3). 13C-NMR δ (ppm): 148.35; 139.59; 130.17; 129.98; 124.21; 121.96; 114.56; 102.95; 85.48; 28.18; HRMS calculated for C12H13IN2O2: 344.0022, Found: 344.0016.
![Molecules 23 02051 i014]()
tert-Butyl 3-iodo-5-nitro-1H-indazole-1-carboxylate (2b). Prepared from 3-iodo-5-nitro-1H-indazole (0.2 g, 0.69 mmol), di-tert-butyldicarbonate (0.17 g, 0.78 mmol) and triethylamine (1 mL) to give 0.27 g of a pale yellow solid. Yield: 100%; m.p.: 144–145 °C; IR (KBr) ν (cm−1): 1744 (C=O); 1528 (NO2 asymmetrical); 1381 and 1342 (NO2 symmetrical); 1258 (C-O); 617 (C-I). 1H-NMR δ (ppm): 8.49 (1H, br s, H-4); 8.32 (1H, br s, H-7); 8.25 (1H, br s, H-6); 1.66 (9H, s, CH3). 13C-NMR δ (ppm): 147.11; 144.13; 141.57; 129.78; 124.83; 118.31; 115.39; 105.88; 86.23; 27.56; HRMS calculated for C12H12IN3O4: 388.9872, Found: 388.9871.
![Molecules 23 02051 i015]()
tert-Butyl 5-bromo-3-iodo-1H-indazole-1-carboxylate (2c). Prepared from 5-bromo-3-iodo-1H-indazole (0.2 g, 0.62 mmol), di-tert-butyldicarbonate (0.15 g, 0.69 mmol) and triethylamine (1 mL) to give 0.27 g of a pale yellow solid. Yield: 100%; m.p.: 152–153 °C; IR (KBr) ν (cm−1): 1750 (C=O); 1157 (C-O); 424 (C-I and C-Br). 1H-NMR (CDCl3) δ (ppm): 8.01 (1H, d, J = 8.8 Hz, H-7); 7.66 (1H, d, J = 9.2 Hz, H-6); 7.64 (1H, s, H-4); 1.64 (9H, s, CH3). 13C-NMR δ (ppm): 148.14; 138.67; 133.15; 131.89; 124.69; 117.56; 116.15; 101.11; 86.13; 28.23; HRMS calculated for C12H12BrIN2O2: 421.9127, Found: 421.9128.
![Molecules 23 02051 i016]()
tert-Butyl 3-iodo-5-methoxy-1H-indazole-1-carboxylate (2d). Prepared from 3-iodo-5-methoxy-1H-indazole (0.1 g, 0.365 mmol), di-tert-butyldicarbonate (0.08 g, 0.366 mmol) and triethylamine (1 mL) to give 119 mg of a pale yellow solid. Yield: 88%; m.p.: 130–131 °C; IR (KBr) ν (cm−1): 1740 (C=O); 1298 (H3C-O); 1157 (C-O); 424 (C-I). 1H-NMR (CDCl3) δ (ppm): 8.01 (1H, d, J = 9.1 Hz, H-7); 7.22 (1H, dd, J = 9.1 and 2.4 Hz, H-6); 6.82 (1H, d, J = 2.2 Hz, H-4); 3.93 (3H, s, OCH3); 1.74 (9H, s, CH3). 13C-NMR δ (ppm): 157.902; 148.32; 134.85; 130.95; 121.12; 115.58; 102.20; 101.71; 85.45; 55.86; 28.21; HRMS calculated for C13H15IN2O3: 374.0127, Found: 374.0118.
![Molecules 23 02051 i017]()
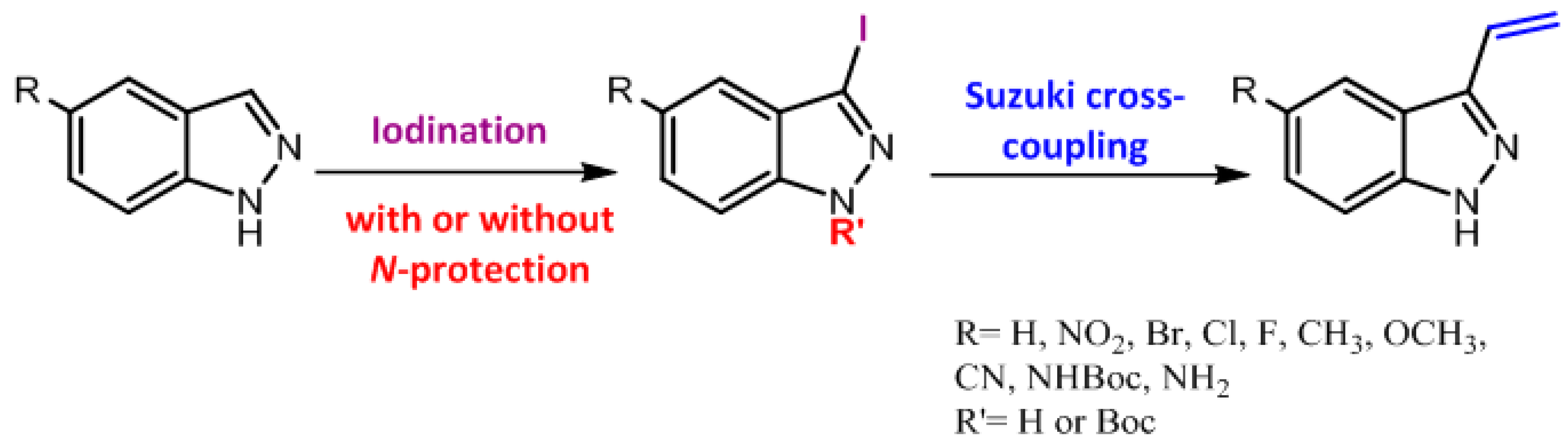
3-Vinyl-1H-indazole (3a). Method a: A mixture of 3-iodoindazole (0.2 g, 0.82 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.27 mL, 1.62 mmol), tetrakis triphenylphosphine palladium (52 mg, 0.045 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and 1,4-dioxane (7 mL), were placed in a microwave glass tube and purged with nitrogen. The closed tube was placed under microwave irradiation to 120 °C for 40 min. After irradiation was completed, the reaction was stopped by dilution using 50 mL of brine. The organic layer was extracted with ethylacetate (3 × 45 mL) and the combined organic layers were dried over anhydrous sodium sulfate. Removal of the solvent under vacuum afforded a brown oil crude residue. The oil was purified by column chromatography on silica gel (hexane/ethylacetate 7:3) to yield 89 mg of white crystalline plates. Yield: 75%.
Method b: Prepared from tert-butyl 3-iodo-1H-indazole-1-carboxylate (0.2 g, 0.58 mmol), 2 eq. of vinyl boronic acid pinacol ester (0.27 mL, 1.62 mmol), tetrakistriphenylphosphine palladium (52 mg, 0.045 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above to obtain 50 mg of a crystalline plates: Yield: 60%.
Method c: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 1 equivalent of vinyl boronic acid pinacol ester (0.14 mL, 0.823 mmol), tetrakistriphenylphosphine palladium (52 mg, 0.045 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above to obtain 58 mg of a crystalline plates. Yield: 48%.
Method d: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 3 equivalents of vinyl boronic acid pinacol ester (0.42 mL, 2.47 mmol), tetrakistriphenylphosphine palladium (52 mg, 0.045 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using the previously described microwave method to obtain 70 mg of a crystalline plates. Yield: 59%.
Method e: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.28 mL, 1.62 mmol), palladium (II) acetate (5 mol%, 9 mg, 0.041 mmol), Xantphos (5 mol%, 24 mg, 0.041 mmol) an aqueous solution of potassium phosphate 1M (2 mL) and dioxane (7 mL) using microwave method described above to obtain 23 mg of a crystalline plates. Yield: 20%.
Method f: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.28 mL, 1.62 mmol), palladium (II) acetate (5 mol%, 9 mg, 0.041 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above to obtain 40 mg of a crystalline plates. Yield: 34%.
Method g: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.28 mL, 1.62 mmol), bis(triphenylphosphine)palladium (II) dichloride (5 mol%, 29 mg, 0.041 mmol), triphenylphosphine (5 mol%, 11 mg, 0.041 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above to obtain 58 mg of a crystalline plates. Yield: 49%.
Method h: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.28 mL, 1.62 mmol), [1,2-bis(diphenylphosphino)ethane]dichloro nickel (II) (5 mol%, 22 mg, 0.041 mmol), ethylenebis(diphenylphosphine) (5 mol%, 16 mg, 0.041 mmol), an aqueous solution of potassium phosphate 1M (2 mL) and dioxane (7 mL) using microwave method described above to obtain 24 mg of a crystalline plates. Yield: 20%.
Method i: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.28 mL, 1.62 mmol), tetrakistriphenylphosphine palladium (52 mg, 0.045 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above, but at a reaction temperature of 180 °C, to obtain 80 mg of a crystalline plates. Yield: 68%.
Method j: Prepared from 3-iodoindazole (0.2 g, 0.82 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.28 mL, 1.62 mmol), tetrakistriphenylphosphine palladium (52 mg, 0.045 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above, but with a reaction time of 60 min, to obtain 40 mg of a crystalline plates. Yield: 34%.
Method k: Prepared from 3-iodoindazole (0.1 g, 0.41 mmol), 2 eq. of vinyl boronic acid pinacol ester (0.1 mL, 0.81 mmol), tetrakistriphenylphosphine palladium (26 mg, 0.023 mmol), an aqueous solution of sodium carbonate 2N (1 mL) and dioxane (3.5 mL) using method a described above, but employing conventional heating, to obtain 26 mg of a crystalline plates: Yield: 44%.
m.p.: 104–105 °C (lit.: [
11] 115.5–116.5 °C); IR (KBr) ν (cm
−1): 3047 (NH); 1620 (C=C).
1H-NMR (CDCl
3) δ (ppm): 11.32 (1H, s, H-1); 7.95 (1H, d,
J = 8.2 Hz, H-7); 7.48 (1H, d,
J = 8.4 Hz, H-4); 7.40 (1H, t,
J = 7.6 Hz, H-6); 7.21 (1H, t,
J = 7.5 Hz, H-5); 7.13 (1H, dd,
Jtrans = 18.0 Hz and
Jcis = 11.5 Hz, H-1′); 6.16 (1H, d,
Jtrans = 18.0 Hz, H-3′); 5.58 (1H, d,
Jcis = 11.4 Hz, H-2′).
13C-NMR (CDCl
3) δ (ppm): 144.18; 141.59; 128.90; 127.03; 121.48; 121.14; 121.00; 117.18; 110.30; HRMS calculated for C
9H
8N
2: 144.0687, Found: 144.0681.
![Molecules 23 02051 i018]()
5-Nitro-3-vinyl-1H-indazole (3b). Method a: Prepared from 3-iodo-5-nitro-1H-indazole (0.2 g, 0.69 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.23 mL, 1.35 mmol), tetrakis- triphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 0.113 g of a pale brown solid. Yield: 87%.
Method b: Prepared from tert-butyl 3-iodo-5-nitro-1H-indazole-1-carboxylate (0.2 g, 0.514 mmol), 2 eq. of vinyl boronic acid pinacol ester (0.17 mL, 1.03 mmol), tetrakistriphenylphosphine palladium (35 mg, 0.03 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above to obtain 13 mg of a pale brown solid: Yield: 13%.
Method c: Prepared from 3-iodo-5-nitro-1H-indazole (0.18 g, 0.62 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.21mL, 1.24 mmol), tetrakistriphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using method a described above, but employing conventional heating, to give 24 mg of pale brown solid. Yield: 21%.
m.p.: 166–168 °C; IR (KBr) ν (cm−1): 3179 (NH); 1620 (C=C); 1528 (NO2 asymmetrical); 1335 (NO2 symmetrical). 1H-NMR δ (ppm): 13.75 (1H, s, H-1); 8.93 (1H, s, H-4); 8.20 (1H, d, J = 8.6 Hz, H-7); 7.71 (1H, d, J = 9.2 Hz, H-6); 7.14 (1H, dd, Jtrans = 17.8 Hz and Jcis =11.4 Hz, H-1′); 6.19 (1H, d, Jtrans = 17.8 Hz, H-3′); 5.58 (1H, d, Jcis = 11.4 Hz, H-2′).13C-NMR δ (ppm): 145.12; 142.97; 141.89; 127.65; 121.14; 119.61; 118.21; 117.95; 111.31; HRMS calculated for C9H7N3O2: 189.0538, Found: 189.0535.
![Molecules 23 02051 i019]()
5-Bromo-3-vinyl-1H-indazole (3c). Method a: Prepared from 5-bromo-3-iodo-1H-indazole (0.2 g, 0.621 mmol), 2 equivalent of vinyl boronic acid pinacol ester (0.21 mL, 1.24 mmol), tetrakis- triphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 82 mg of a pale yellow solid. Yield: 60%.
Method b: Prepared from tert-butyl 5-bromo-3-iodo-1H-indazole-1-carboxylate (0.2 g, 0.47 mmol), 2 eq. of vinyl boronic acid pinacol ester (0.16 mL, 0.96 mmol), tetrakistriphenylphosphine palladium (28 mg, 0.024 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above to obtain 45 mg of a pale yellow solid: Yield: 43%.
m.p.: 250–251 °C; IR (KBr) ν (cm−1): 3112 (NH); 1689 (C=C); 424 (C-Br). 1H-NMR (CDCl3) δ (ppm): 11.68 (1H, s, H-1); 8.03 (1H, s, H-4); 7.44 (1H, d, J = 8.8 Hz, H-7); 7.32 (1H, d, J = 8.8 Hz, H-6); 7.01 (1H, dd, Jtrans = 18.0 Hz and Jcis = 11.5 Hz, H-1′); 6.07 (1H, d, Jtrans = 18.0 Hz, H-3′); 5.57 (1H, d, Jcis = 11.5 Hz, H-2′). 13C-NMR (CDCl3) δ (ppm): 143.45;140.15; 130.21; 128.12; 123.48; 122.54; 117.83; 114.66; 111.77; HRMS calculated for C9H7BrN2: 221.9793, Found: 221.9797.
![Molecules 23 02051 i020]()
5-Methoxy-3-vinyl-1H-indazole (3d). Method a: Prepared from 3-iodo-5-methoxy-1H-indazole (0.2 g, 0.73 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.24 mL, 1.43 mmol), tetrakis- triphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 74 mg of a pale yellow solid. Yield: 58%.
Method b: Prepared from tert-butyl 3-iodo-5-methoxy-1H-indazole-1-carboxylate (0.135 g, 0.361 mmol), 2 eq. of vinyl boronic acid pinacol ester (0.12 mL, 0.72 mmol), tetrakistriphenylphosphine palladium (21 mg, 0.018 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL) using microwave method described above to obtain 29 mg of a pale yellow solid. Yield: 46%.
Method c: Prepared from 3-iodo-5-methoxy-1H-indazole (0.2 g, 0.73 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.24 mL, 1.43 mmol), tetrakistriphenylphosphine palladium (52 mg, 0.045 mmol), an aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7.0 mL) using method described above, but employing conventional heating, to obtain 96 mg of a pale yellow solid. Yield: 75%.
m.p.: 88–89 °C; IR (KBr) ν (cm−1): 3155 (NH); 1635 (C=C); 1219 (C-O). 1H-NMR δ (ppm): 12.95 (1H, s, H-1); 7.44 (1H, d, J = 8.9 Hz, H-7); 7.33 (1H, s, H-4); 7.06–6.96 (2H, m, H-6 and H-1’); 6.02 (1H, d, Jtrans = 18.0 Hz, H-3′); 5.41 (1H, d, Jcis = 11.5 Hz, H-2′); 3.82 (1H, s, OCH3). 13C-NMR δ (ppm): 154.50; 141.71; 137.02; 129.38; 120.70; 118.05; 114.90; 111.47; 100.02; 55.50; HRMS calculated for C10H10N2O: 174.0793, Found: 174.0788.
![Molecules 23 02051 i021]()
5-Fluoro-3-vinyl-1H-indazole (3e). Method a: Prepared from 5-fluoro-3-iodo-1H-indazole (0.2 g, 0.763 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.26 mL, 1.54 mmol), tetrakis- triphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 58 mg of a pale yellow solid. Yield: 47%.
Method b: Prepared from 5-fluoro-3-iodo-1H-indazole (0.08 g, 0.305 mmol), 2 eq. of vinyl boronic acid pinacol ester (0.075 mL, 0.61 mmol), tetrakistriphenylphosphine palladium (17 mg, 0.015 mmol), an aqueous solution of sodium carbonate 2N (1 mL) and dioxane (3.5 mL) using method a described above, but employing conventional heating, to obtain 50 mg of the precursor 5-fluoro-3-iodo-1H-indazole: Yield of recovery: 63%.
m.p.: 104–105 °C; IR (KBr) ν (cm−1): 3163 (NH); 1636 (C=C); 1118 (C-F). 1H-NMR (CDCl3) δ (ppm): 11.73 (1H, s, H-1); 7.52 (1H, dd, J = 8.9 and 1.7 Hz, H-4); 7.41 (1H, dd, J = 9.0 and 4.2 Hz, H-7); 7.17 (1H, td, J = 8.9 and 2.0 Hz, H-6); 7.05 (1H, dd, Jtrans = 18.0 Hz and Jcis = 11.5 Hz, H-1′); 6.05 (1H, d, Jtrans = 18.0 Hz, H-3′); 5.56 (1H, d, Jcis = 11.5 Hz, H-2′). 13C-NMR (CDCl3) δ (ppm): 158.39 (d, J = 238.8 Hz), 144.04 (d, J = 5.5 Hz), 138.52, 128.38, 121.01 (d, J = 10.0 Hz), 117.25, 111.49 (d, J = 9.7 Hz), 105.21 (d, J = 24.1 Hz); HRMS calculated for C9H7FN2: 162.0593, Found: 162.0590.
![Molecules 23 02051 i022]()
5-Chloro-3-vinyl-1H-indazole (3f). Prepared from 5-chloro-3-iodo-1H-indazole (0.2 g, 0.722 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.24 mL, 1.42 mmol), tetrakistriphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 77 mg of a yellow solid. Yield: 60%; m.p.: 131–132 °C; IR (KBr) ν (cm−1): 3181 (NH); 1628 (C=C); 649 (C-Cl). 1H-NMR δ (ppm): 13.28 (1H, s, H-1); 8.05 (1H, s, H-4); 7.56 (1H, d, J = 8.8 Hz, H-7); 7.37 (1H, d, J = 8.7 Hz, H-6); 7.01 (1H, dd, Jtrans = 18.0 Hz and Jcis = 11.5 Hz, H-1′); 6.08 (1H, d, Jtrans = 18.0 Hz, H-3′); 5.46 (1H, d, Jcis = 11.5 Hz, H-2′). 13C-NMR δ (ppm): 142.00; 139.72; 128.57; 126.56; 125.45, 121.23, 119.60, 116.29, 112.17; HRMS calculated for C9H7ClN2: 178.0298, Found: 178.0296.
![Molecules 23 02051 i023]()
5-Methyl-3-vinyl-1H-indazole (3g). Method a: Prepared from 3-iodo-5-methyl-1H-indazole (0.2 g, 0.775 mmol), 2 equivalent of vinyl boronic acid pinacol ester (0.26 mL, 1.54 mmol), tetrakis- triphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 60 mg of a pale brown solid. Yield: 49%.
Method b: Prepared from 3-iodo-5-methyl-1H-indazole (0.095 g, 0.368 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.090 mL, 0.736 mmol), tetrakistriphenylphosphine palladium (21 mg, 0.018 mmol), an aqueous solution of sodium carbonate 2N (1 mL) and dioxane (3.5 mL) using method a described above, but employing conventional heating, to obtain 10 mg of a pale brown solid. Yield: 17%.
m.p.: 88–89 °C; IR (KBr) ν (cm−1): 3179 (NH); 2916 (C-H); 1628 (C=C). 1H-NMR (CDCl3) δ (ppm): 11.63 (1H, s, H-1); 7.72 (1H, s, H-4); 7.37 (1H, d, J = 8.5 Hz, H-7); 7.24–7.11 (2H, m, H-1′ and H-6); 6.16 (1H, d, Jtrans = 18.0 Hz, H-3′); 5.57 (1H, d, Jcis = 11.4 Hz, H-2′); 2.50 (3H, s, CH3). 13C-NMR (CDCl3) δ (ppm):143.28; 140.28; 130.84; 129.02; 128.96; 121.38; 119.96; 116.69; 110.12; 21.58; HRMS calculated for C10H10N2: 158.0844, Found: 158.0840.
![Molecules 23 02051 i024]()
5-Cyano-3-vinyl-1H-indazole (3h). Prepared from 5-cyano-3-iodo-1H-indazole (0.2 g, 0.743 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.25 mL, 1.48 mmol), tetrakistriphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 77 mg of a white solid. Yield: 61%; m.p.: 156–157 °C; IR (KBr) ν (cm−1): 3209 (NH); 2222 (CN); 1612 (C=C). 1H-NMR δ (ppm): 13.59 (1H, s, H-1); 8.63 (1H, s, H-4), 7.68 (2H, s, H-7 and H-6); 7.04 (1H, dd, Jtrans = 18.0 Hz and Jcis = 11.5 Hz, H-1′), 6.21 (1H, d, J = 18.0 Hz, H-3′), 5.53 (1H, d, J = 11.5 Hz, H-2′). 13C-NMR δ (ppm): 143.56; 142.01; 128.38; 128.01; 127.43; 119.99; 119.82; 117.82; 111.98; 103.29; HRMS calculated for C10H7N3: 169.0640, Found: 169.0637.
![Molecules 23 02051 i025]()
tert-Butyl (3-vinyl-1H-indazol-5-yl)carbamate (3i). Prepared from tert-butyl (3-iodo-1-H-indazol-5-yl) carbamate (0.2 g, 0.56 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.19 mL, 1.12 mmol), tetrakistriphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 80 mg of a White solid. Yield: 55%; m.p.: 189–190 °C; IR (KBr) ν (cm−1): 3356 (NH); 1700 (C=O); 1636 (C=C); 1165 (C-O). 1H-NMR δ (ppm): 12.96 (1H, s, H-1); 9.30 (1H, s, H-5); 8.13 (1H, s, H-4); 7.45–7.37 (2H, m, H-8 and H-7); 6.98 (1H, dd, Jtrans = 18.0 Hz and Jcis = 11.4 Hz, H-1′); 5.92 (1H, d, Jtrans = 18.0 Hz, H-3′); 5.44 (1H, d, Jcis = 11.4 Hz, H-2′); 1.49 (9H, s, H-6). 13C-NMR δ (ppm): 153.18; 141.83; 137.80; 133.36; 129.70; 120.33; 119.92; 114.88; 110.54; 107.98; 78.91; 28.22; HRMS calculated for C14H17N3O2: 259.1321, Found: 259.1330.
![Molecules 23 02051 i026]()
5-Amino-3-vinyl-1H-indazole (3j). Method a: Prepared from 5-amino-3-iodo-1H-indazole (1j, 0.2 g, 0.77 mmol), 2 equivalents of vinyl boronic acid pinacol ester (0.26 mL, 1.54 mmol), tetrakis- triphenylphospine palladium (52 mg, 0.045 mmol), aqueous solution of sodium carbonate 2N (2 mL) and dioxane (7 mL), to give 44 mg of a brown solid. Yield: 36%.
Method b: A solution of tert-butyl (3-vinyl-1H-indazol-5-yl) carbamate (3i, 145 mg, 0.4 mmol) and trifluoroacetic acid (2 mL, 26.13 mmol) in CH2Cl2 (2 mL) was stirred vigorously for 2 h at room temperature. After stirring, the reaction was neutralized to pH = 7 using NaOH 1M and the organic layer was extracted with ethyl acetate (3 × 20 mL) The combined organic layers were dried with anhydrous sodium sulfate and removal of the solvent under vacuum afforded a 80 mg of pure product as a brown solid. Yield: 100%; m.p.: 157–158 °C; IR (KBr) ν (cm−1): 3461 (NH); 1682 (C=O). 1H-NMR δ (ppm):12.64 (1H, s, H-1); 7.24 (1H, d, J = 8.7 Hz, H-7); 7.00 (1H, s, H-4); 6.91 (1H, dd, Jtrans = 18.0 and Jcis = 11.5 Hz, H-1′); 6.80 (1H, d, J = 8.8 Hz, H-6); 5.84 (1H, d, Jtrans = 18.0 Hz, H-3′); 5.34 (1H, d, Jcis = 11.5 Hz, H-2′); 4.87 (2H, s, H5). 13C-NMR δ (ppm): 143.20; 140.39; 135.83; 130.33; 121.46; 118.02; 113.61; 110.81; 100.76; HRMS calculated for C9H9N3: 159.0796, Found: 159.0792.