Computational-Based Study of QuEChERS Extraction of Cyclohexanedione Herbicide Residues in Soil by Chemometric Modeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Chromatographic Method
2.2. Modeling of the QuEChERS Extraction Method
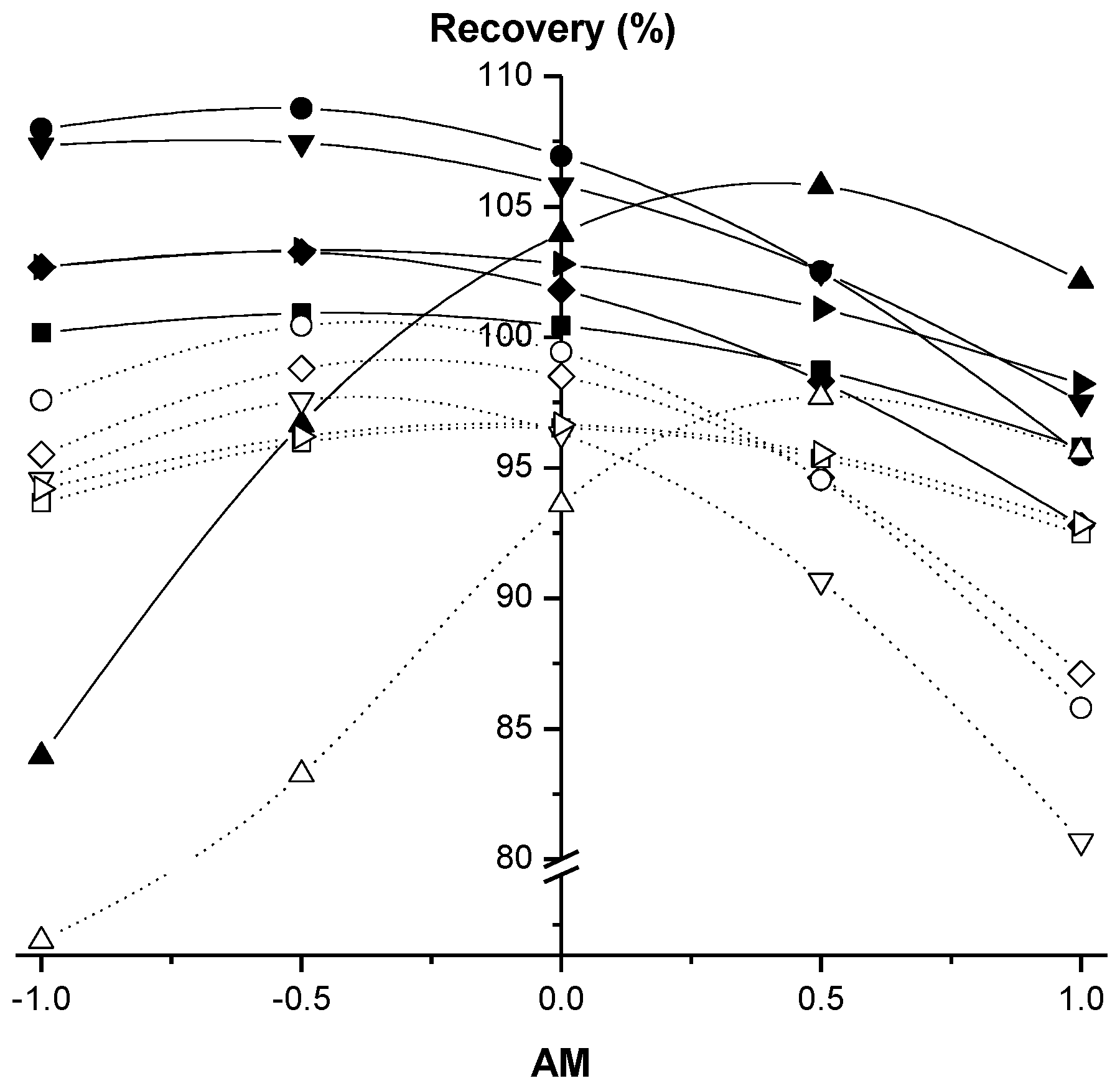
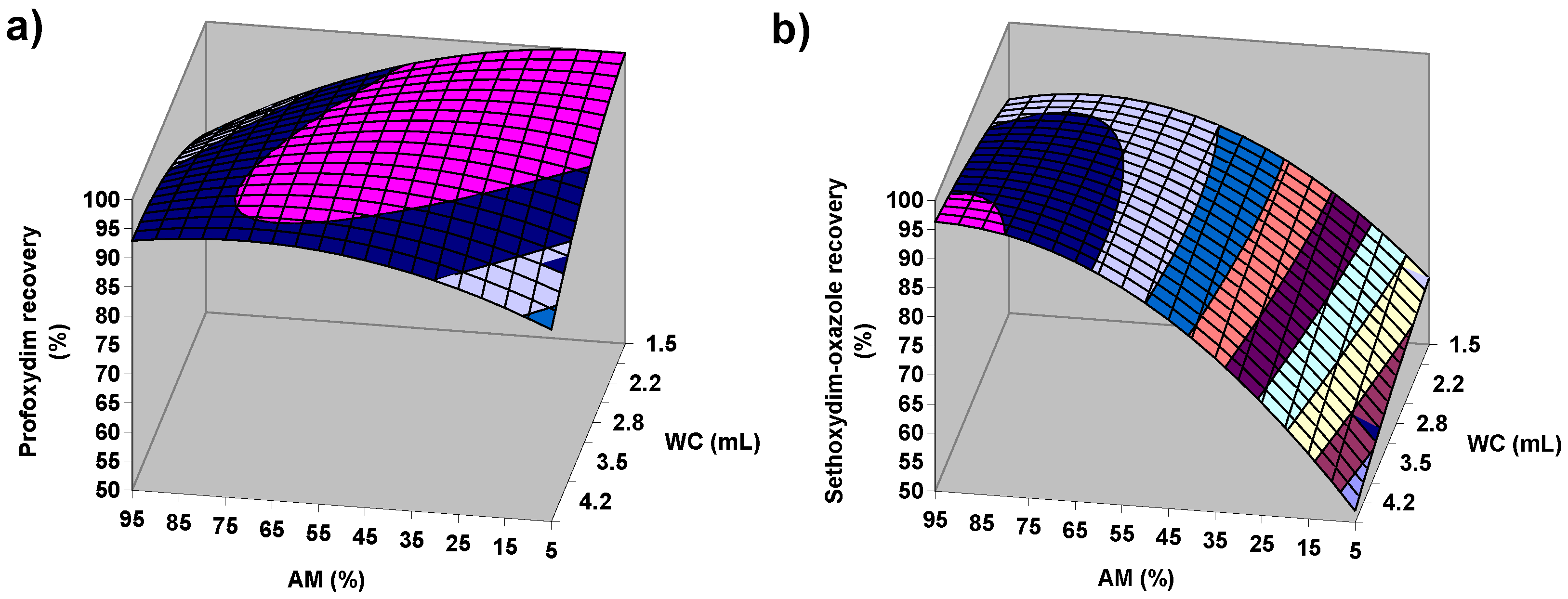
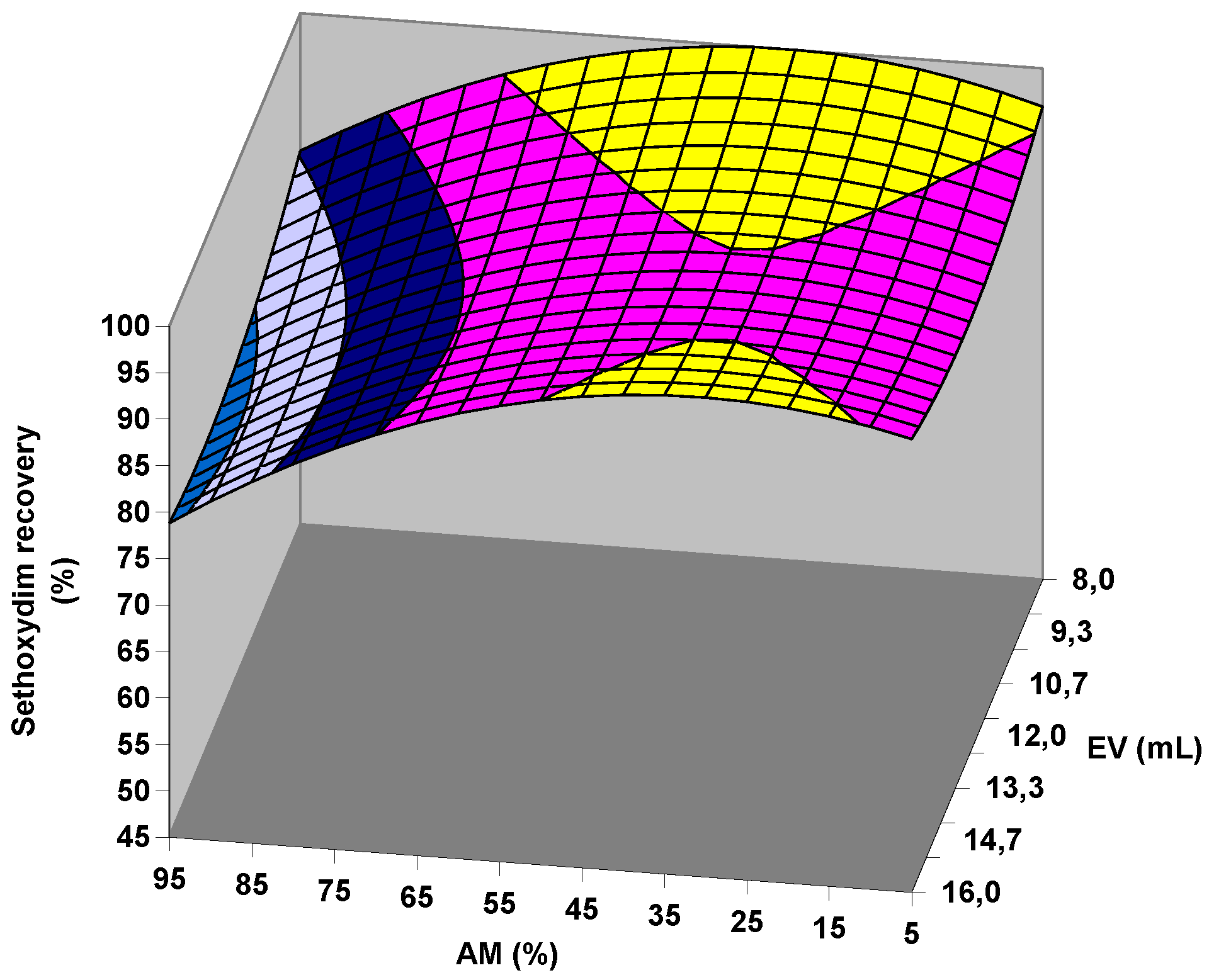
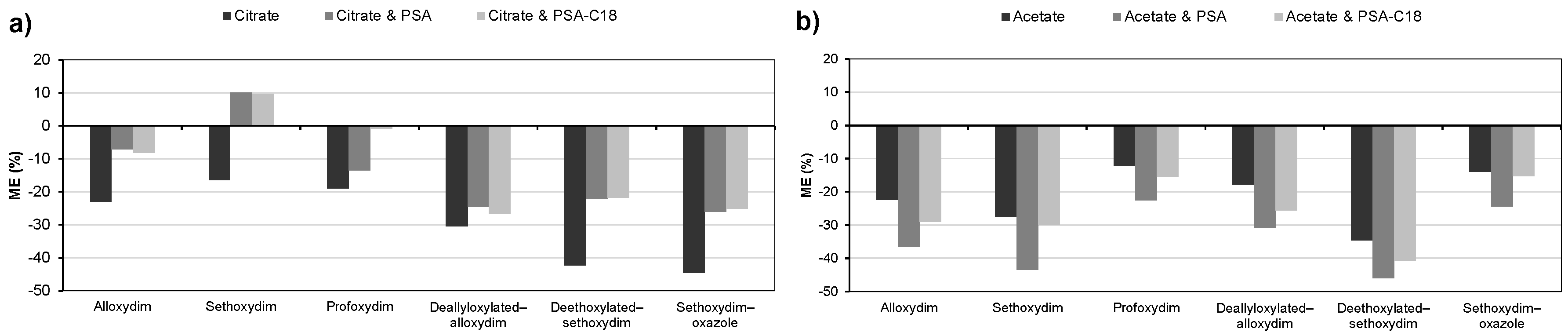
2.2.1. Factorial Design of Citrate QuEChERS
2.2.2. Factorial Design of Acetate QuEChERS
2.3. Handling of the Polynomial Models
2.4. Confirmation of Extraction Conditions and Clean-Up Assessment
3. Materials and Methods
3.1. Chemicals
3.2. Soil Samples
3.3. QuEChERS Procedure
3.4. Factorial Design of QuEChERS Extraction
3.5. Chromatographic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tong, Z.; Wu, Y.-C.; Liu, Q.-Q.; Shi, Y.-H.; Zhou, L.-J.; Liu, Z.-Y.; Yu, L.-S.; Cao, H.-Q. Multi-residue analysis of pesticide residues in crude pollens by UPLC-MS/MS. Molecules 2016, 21, 1652. [Google Scholar] [CrossRef] [PubMed]
- Ccanccapa, A.; Masiá, A.; Navarro-Ortega, A.; Picó, Y.; Barceló, D. Pesticides in the Ebro river basin: Occurrence and risk assessment. Environ. Pollut. 2016, 211, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Trends in analysis of pesticide residues to fulfil the European Regulation (EC) No. 1107/2009. TrAC Trends Anal. Chem. 2016, 80, 568–580. [Google Scholar] [CrossRef]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Tot. Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Mantzos, N.; Karakitsou, A.; Hela, D.; Patakioutas, G.; Leneti, E.; Konstantinou, I. Persistence of oxyfluorfen in soil, runoff water, sediment and plants of a sunflower cultivation. Sci. Tot. Environ. 2014, 472, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Bargańska, Ż.; Ślebioda, M.; Namieśnik, J. Determination of pesticide residues in honeybees using modified QUEChERS sample work-up and liquid chromatography-tandem mass spectrometry. Molecules 2014, 19, 2911. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Computational methodologies for the risk assessment of pesticides in the European Union. J. Agric. Food Chem. 2017, 65, 2017–2018. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Calvo, L.; Alonso-Prados, J.L.; Sandín-España, P. Photolysis of clethodim herbicide and a formulation in aquatic environments: Fate and ecotoxicity assessment of photoproducts by QSAR models. Sci. Tot. Environ. 2018, 615, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Sevilla-Morán, B.; Calvo, L.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Photodegradation behaviour of sethoxydim and its comercial formulation Poast® under environmentally-relevant conditions in aqueous media. Study of photoproducts and their toxicity. Chemosphere 2017, 168, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.R. Metabolic Pathways of Agrochemicals; Royal Society of Chemistry: Cambridge, UK, 1998; p. 849. [Google Scholar]
- Villaverde, J.J.; Santín-Montanyá, I.; Sevilla-Morán, B.; Alonso-Prados, J.; Sandín-España, P. Assessing the effects of alloxydim phototransformation products by QSAR models and a phytotoxicity study. Molecules 2018, 23, 993. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Liang, L.; Liu, F. Dissipation and residues of clethodim and its oxidation metabolites in a rape-field ecosystem using QuEChERS and liquid chromatography/tandem mass spectrometry. Food Chem. 2014, 143, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Marek, L.J.; Koskinen, W.C.; Bresnahan, G.A. LC/MS analysis of cyclohexanedione oxime herbicides in water. J. Agric. Food Chem. 2000, 48, 2797–2801. [Google Scholar] [CrossRef] [PubMed]
- Sandín-España, P.; González-Blázquez, J.J.; Magrans, J.O.; García-Baudín, J.M. Determination of herbicide tepraloxydim and main metabolites in drinking water by solid-phase extraction and liquid chromatography with UV detection. Chromatographia 2002, 55, 681–686. [Google Scholar] [CrossRef]
- Tsochatzis, E.D.; Tzimou-Tsitouridou, R.; Menkissoglu-Spiroudi, U.; Karpouzas, D.G.; Maria Papageorgiou, G. Development and validation of an HPLC-DAD method for the simultaneous determination of most common rice pesticides in paddy water systems. Intern. J. Environ. Anal. Chem. 2012, 92, 548–560. [Google Scholar] [CrossRef]
- Saha, M.; Harrison, B.; Collins, L. GC-MSD method for the determination of tepraloxydim and its major metabolites residues in soil using conversion to the common analyte approach. In Proceedings of the 220th ACS National Meeting, Washington DC, USA, 20–24 August 2000; p. U50. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive soildphase extraction for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [PubMed]
- Romero-González, R.; Arrebola Liébanas, F.J.; López-Ruiz, R.; Garrido Frenich, A. Sample treatment in pesticide residue determination in food by high-resolution mass spectrometry: Are generic extraction methods the end of the road? J. AOAC Int. 2016, 99, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Rutkowska, E.; Jankowska, M. Influence of QuEChERS modifications on recovery and matrix effect during the multi-residue pesticide analysis in soil by GC/MS/MS and GC/ECD/NPD. Environ. Sci. Pollut. Res. 2017, 24, 7124–7138. [Google Scholar] [CrossRef] [PubMed]
- Berlioz-Barbier, A.; Vauchez, A.; Wiest, L.; Baudot, R.; Vulliet, E.; Cren-Olivé, C. Multi-residue analysis of emerging pollutants in sediment using QuEChERS-based extraction followed by LC-MS/MS analysis. Anal. Bioanal. Chem. 2014, 406, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Azevedo de Brito, W.; Gomes Dantas, M.; Andrade Nogueira, F.H.; Ferreira da Silva-Júnior, E.; de Araújo-Júnior, J.X.; Mendonça de Aquino, T.; Nogueira Ribeiro, Ê.A.; da Silva Solon, L.; Soares Aragão, C.F.; Barreto Gomes, A.P. Development and validation of HPLC-DAD and UHPLC-DAD methods for the simultaneous determination of guanylhydrazone derivatives employing a factorial design. Molecules 2017, 22, 1394. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Villaverde, J.J.; Silva, C.M.; Neto, C.P.; Silvestre, A.J.D. Supercritical fluid extraction of phenolic compounds from Eucalyptus globulus Labill bark. J. Supercrit. Fluids 2012, 71, 71–79. [Google Scholar] [CrossRef]
- Villaverde, J.J.; Ligero, P.; Vega, A.d. Bleaching Miscanthus x giganteus acetosolv pulps with hydrogen peroxide/acetic acid. Part 1: Behaviour in aqueous alkaline media. Bioresour. Technol. 2009, 100, 4731–4735. [Google Scholar] [CrossRef] [PubMed]
- Bruzzoniti, M.C.; Checchini, L.; De Carlo, R.M.; Orlandini, S.; Rivoira, L.; Del Bubba, M. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: A critical review. Anal. Bioanal. Chem. 2014, 406, 4089–4116. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548–2560. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Nawaz, S.; Barker, H.; Ahmad, I.; Ashraf, M. Development of a simple extraction and clean-up procedure for determination of organochlorine pesticides in soil using gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Correia-Sá, L.; Fernandes, V.C.; Carvalho, M.; Calhau, C.; Domingues, V.F.; Delerue-Matos, C. Optimization of QuEChERS method for the analysis of organochlorine pesticides in soils with diverse organic matter. J. Sep. Sci. 2012, 35, 1521–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, V.C.; Domingues, V.F.; Mateus, N.; Delerue-Matos, C. Multiresidue pesticides analysis in soils using modified QuEChERS with disposable pipette extraction and dispersive solid-phase extraction. J. Sep. Sci. 2013, 36, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Ndongo, G.A.; Boyomo, M.O.; Owono, P.A. Ab initio study of polar and non-polar aprotic solvents effects on some 3-hydroxychromones and 3-hydroxyquinolones derivatives. J. Mol. Model. 2018, 24, 92. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Ashokkumar, B.; Mohammed Siddiq, A. The density, dynamic viscosity and kinematic viscosity of protic polar solvents (pure and mixed systems) studies: A theoretical insight of thermophysical properties. J. Mol Liq. 2018, 251, 458–469. [Google Scholar] [CrossRef]
- De Carlo, R.M.; Rivoira, L.; Ciofi, L.; Ancillotti, C.; Checchini, L.; Del Bubba, M.; Bruzzoniti, M.C. Evaluation of different QuEChERS procedures for the recovery of selected drugs and herbicides from soil using LC coupled with UV and pulsed amperometry for their detection. Anal. Bioanal. Chem. 2015, 407, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Delerue-Matos, C. A throughput method using the quick easy cheap effective rugged safe method for the quantification of ibuprofen and its main metabolites in soils. J. Sep. Sci. 2016, 39, 3436–3444. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2016; p. 856. [Google Scholar]
- Vilela, C.; Santos, S.A.O.; Villaverde, J.J.; Oliveira, L.; Nunes, A.; Cordeiro, N.; Freire, C.S.R.; Silvestre, A.J.D. Lipophilic phytochemicals from banana fruits of several Musa species. Food Chem. 2014, 162, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Mantzos, N.; Karakitsou, A.; Zioris, I.; Leneti, E.; Konstantinou, I. QuEChERS and solid phase extraction methods for the determination of energy crop pesticides in soil, plant and runoff water matrices. Int. J. Environ. Anal. Chem. 2013, 93, 1566–1584. [Google Scholar] [CrossRef]
- Mei, M.; Du, Z.-X.; Cen, Y. QuEChERS-Ultra-performance liquid chromatography tandem mass spectrometry for determination of five currently used herbicides. Chin. J. Anal. Chem. 2011, 39, 1659–1664. [Google Scholar] [CrossRef]
- Kwon, H.; Lehotay, S.J.; Geis-Asteggiante, L. Variability of matrix effects in liquid and gas chromatography-mass spectrometry analysis of pesticide residues after QuEChERS sample preparation of different food crops. J. Chromatogr. A 2012, 1270, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kmellár, B.; Fodor, P.; Pareja, L.; Ferrer, C.; Martínez-Uroz, M.A.; Valverde, A.; Fernández-Alba, A.R. Validation and uncertainty study of a comprehensive list of 160 pesticide residues in multi-class vegetables by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2008, 1215, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Sandín-España, P.; Sevilla-Morán, B.; Calvo, L.; Mateo-Miranda, M.; Alonso-Prados, J.L. Photochemical behavior of alloxydim herbicide in environmental waters. Structural elucidation and toxicity of degradation products. Microchem. J. 2013, 106, 212–219. [Google Scholar] [CrossRef]
- Sheldrick, B.H.; Wang, C. Particle size distribution. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 499–511. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |


| Fitting Parameters | Alloxydim | Sethoxydim | Profoxydim | Deallyloxylated-alloxydim | Deethoxylated-sethoxydim | Sethoxydim-oxazole |
|---|---|---|---|---|---|---|
| b0 | 101.6 a | 106.9 a | 105.8 a | 100.3 a | 103.0 a | 103.7 a |
| bSW | −0.8269 | 1.751 | −2.371 a | −0.5147 | 0.3115 | −1.607 c |
| bAM | −4.959 a | −4.854 a | −6.367 a | −2.014 a | −2.067 a | 10.12 a |
| bEV | 0.8366 | 1.933 c | 3.194 a | 0.9733 d | 1.417 c | 4.717 a |
| bWC | −0.6412 | −0.3913 | −1.231 c | 0.5237 | 0.5356 | 1.105 |
| bET | 0.9447 d | 0.3545 | 0.8743 | 1.373 c | 1.370 c | 0.3711 |
| bSW-AM | −0.02532 | 0.2443 | −0.3971 | 0.6546 | 0.5940 | 3.398 a |
| bSW-EV | 0.6864 d | 2.109 c | −0.5446 | 1.497 c | 1.537 c | 2.265 b |
| bSW-WC | −1.263 c | 0.006918 | −0.6714 | −0.9634 | −0.7483 | 0.03950 |
| bSW-ET | 1.381 c | 0.8230 | 2.471 a | 1.489 c | 1.656 c | 0.8718 |
| bAM-EV | −1.804 b | −1.713 d | −2.164 a | −1.146 d | −1.162 d | −4.017 a |
| bAM-WC | 1.396 c | 1.685 d | 2.381 a | 1.710 b | 1.882 b | 0.8537 |
| bAM-ET | −0.6283 | −0.6910 | −0.8073 | −0.4672 | −0.3677 | 0.3240 |
| bEV-WC | 0.06119 | 1.099 | −0.4256 | −0.6932 | −0.7470 | −0.9536 |
| bEV-ET | −0.8625 | −1.158 | −0.7665 | −1.217 c | −1.651 c | −1.030 |
| bWC-ET | 0.3770 | 0.7965 | 0.02497 | 0.6367 | 0.7979 | 1.111 |
| bSW-AM-EV | −0.5365 | −0.5793 | −0.7477 | −0.2786 | 0.001259 | −0.5633 |
| bSW-AM-WC | 0.1645 | −0.09995 | 1.413 c | 0.7606 | 1.248 d | 1.142 |
| bSW-AM-ET | −0.6347 | −0.6291 | −0.6368 | −0.5067 | −0.4016 c | −0.2854 |
| bSW-EV-WC | 0.6929 | 1.650 d | 0.2431 | 0.2161 | 0.1957 | 0.4587 |
| bSW-EV-ET | −0.8404 | −1.135 | −0.04247 | −1.062 d | −1.513 d | −0.6957 |
| bSW-WC-ET | −0.2049 | 0.1874 | −0.6880 | −0.2849 | −0.2937 | 0.1272 |
| bAM-EV-WC | −1.505 c | −2.514 b | −2.980 a | −1.443 c | −2.404 a | −2.534 a |
| bAM-EV-ET | 0.09265 | −0.5385 | 0.03543 | −0.09737 | −0.5561 | −0.6266 |
| bAM-WC-ET | −0.6797 | −0.3485 | −0.5506 | −0.6236 | −0.6813 | −0.2181 |
| bEV-WC-ET | −0.3661 | −0.3285 | −0.1668 | −0.5718 | −0.7661 | −0.3618 |
| bSW-SW | −0.05830 | −6.134 d | 5.027 c | −0.6877 | −1.293 | −2.781 |
| bAM-AM | −4.073 d | −3.410 | −5.206 c | −2.465 | −2.349 | −10.91 a |
| bEV-EV | −1.734 | −2.347 | −3.375 | −1.115 | −2.028 | −1.976 |
| bWC-WC | −2.133 | −1.699 | −3.023 | −1.159 | −1.306 | −0.9245 |
| bET-ET | −0.1019 | −0.2822 | −0.8741 | −0.9409 | −0.5995 | −1.470 |
| R | 0.9228 | 0.8905 | 0.9520 | 0.8761 | 0.8837 | 0.9674 |
| Fitting Parameters | Alloxydim | Sethoxydim | Profoxydim | Deallyloxylated-alloxydim | Deethoxylated-sethoxydim | Sethoxydim-oxazole |
|---|---|---|---|---|---|---|
| b0 | 98.50 a | 96.30 a | 99.42 a | 96.52 a | 96.65 a | 93.59 a |
| bSW | −2.046 a | −1.623 a | −0.9979 a | −1.397 a | −1.233 a | −5.374 a |
| bAM | −4.199 a | −6.918 a | −5.900 a | −0.5917 c | −0.6606 c | 14.45 a |
| bEV | −0.3586 | −0.4540 c | −0.2489 | −0.3171 | −0.8767 b | 3.544 a |
| bWC | 0.7559 c | 0.4174 | 0.7932 b | 0.7751 b | 1.075 a | 2.006 a |
| bET | 0.2162 | 0.3104 | −0.04629 | 0.7781 b | 0.5730 d | 0.6799 c |
| bSW-AM | −1.001 c | 0.006950 | 1.500 a | 0.4094 | 0.1525 | 2.818 a |
| bSW-EV | −0.7845 c | −1.148 a | 0.2673 | −0.6982 c | −0.6762 c | −0.4862 d |
| bSW-WC | 1.452 a | 1.084 a | 1.852 a | 1.181 a | 0.9806 a | 1.086 a |
| bSW-ET | 0.1732 | −0.08985 | 0.1410 | −0.03353 | −0.05905 | −0.05778 |
| bAM-EV | −0.5958 | −0.7734 b | −0.5355 c | −1.098 a | −1.141 a | −5.220 a |
| bAM-WC | −0.8033 c | −0.7921 b | 0.3970 | −0.5502 | −0.4931 | −1.827 a |
| bAM-ET | −1.327 a | −1.159 a | −0.8263 a | −0.9402 b | −1.046 a | −1.023 a |
| bEV-WC | 0.7362 d | 0.8608 c | 0.6544 c | 0.5351 d | 0.6066 c | −0.08351 |
| bEV-ET | 0.3184 | −0.1492 | −0.2950 | 0.09183 | −0.1445 | −0.2852 |
| bWC-ET | 0.2434 | −0.06107 | −0.1582 | 0.07877 | 0.1107 | 0.7931 b |
| bSW-AM-EV | −0.08380 | −0.6281 c | −1.232 a | −0.2973 | −0.6082 c | −0.7774 b |
| bSW-AM-WC | −0.4183 | −0.8071 b | −1.498 a | −0.6732 c | −0.8846 b | −0.8068 b |
| bSW-AM-ET | 0.2012 | −0.04719 | −0.4896 d | −0.08773 | −0.1503 | −0.5721 c |
| bSW-EV-WC | −0.2389 | 0.1304 d | 0.6455 c | 0.07467 d | 0.02193 d | 0.4043 |
| bSW-EV-ET | 0.1344 | 0.5538 | 0.4635 | 0.8739 b | 0.5816 | 0.02332 |
| bSW-WC-ET | 0.2797 | 0.5672 d | 0.7497 b | 0.2862 | 0.5585 d | −0.1483 |
| bAM-EV-WC | 0.05389 | −0.2829 | −0.4543 d | −0.5252 d | −0.5960 c | 0.2494 |
| bAM-EV-ET | 0.2714 | 0.04895 | 0.1469 | −0.1186 | −0.3965 | −0.1905 |
| bAM-WC-ET | 0.2759 | −0.1020 | 0.1953 | 0.09603 | 0.2365 | −0.2606 |
| bEV-WC-ET | −0.3355 | −0.07306 | 0.1628 | −0.1769 | −0.08688 | 0.2886 |
| bSW-SW | 0.1189 | −0.1245 | 1.538 d | −1.291 | −1.249 | −0.7618 |
| bAM-AM | −7.183 a | −8.683 a | −7.731 a | −3.467 a | −3.124 b | −12.42 a |
| bEV-EV | 3.876 b | 3.331 a | 0.7742 | 2.272 c | 2.575 c | 1.251 |
| bWC-WC | −1.693 | −0.7284 | −0.1338 | −0.5212 | −0.9923 | −0.3232 |
| bET-ET | 1.262 | 1.740 d | 1.222 | 2.566 c | 1.767 d | 1.881 d |
| R | 0.9458 | 0.9829 | 0.9830 | 0.9002 | 0.9085 | 0.9964 |
| Target Compound | Citrate QuEChERS–Confidence Levels | Acetate QuEChERS–Confidence Levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% | 90% | 80% | 70% | 0% | 95% | 90% | 80% | 70% | 0% | ||
| Alloxydim | Deviation | 25.5−0.0 | 23.7−0.0 | 18.2−0.3 | 15.5−0.0 | 6.5−0.1 | 6.3−0.1 | 6.1−0.0 | 5.8−0.0 | 5.1−0.0 | 4.4−0.0 |
| R | 0.6156 | 0.6529 | 0.7336 | 0.8623 | 0.9228 | 0.8617 | 0.8820 | 0.9187 | 0.9254 | 0.9458 | |
| Sethoxydim | Deviation | 26.2−0.1 | 26.2−0.1 | 22.1−0.2 | 15.9−0.0 | 13.0−0.1 | 5.4−0.0 | 5.0−0.0 | 4.3−0.0 | 3.1−0.0 | 2.8−0.0 |
| R | 0.4977 | 0.4977 | 0.5606 | 0.8385 | 0.8905 | 0.9511 | 0.9696 | 0.9723 | 0.9796 | 0.9829 | |
| Profoxydim | Deviation | 14.4−0.4 | 14.4−0.4 | 10.2−0.1 | 10.2−0.1 | 6.5−0.0 | 5.5−0.0 | 5.5−0.0 | 5.5−0.0 | 4.2−0.0 | 2.5−0.1 |
| R | 0.8404 | 0.8404 | 0.9177 | 0.9177 | 0.9520 | 0.9487 | 0.9592 | 0.9663 | 0.9769 | 0.9830 | |
| Deallyloxylated-alloxydim | Deviation | 24.8−0.1 | 23.1−0.0 | 16.1−0.0 | 11.9−0.0 | 6.8−0.1 | 6.7−0.1 | 4.9−0.1 | 3.7−0.0 | 3.7−0.0 | 3.0−0.0 |
| R | 0.3113 | 0.4033 | 0.6230 | 0.6975 | 0.8761 | 0.5839 | 0.7345 | 0.8388 | 0.8730 | 0.9002 | |
| Deethoxylated-sethoxydim | Deviation | 23.9−0.1 | 22.1−0.0 | 12.9−0.1 | 10.5−0.0 | 6.9−0.2 | 6.7−0.0 | 5.3−0.3 | 3.9−0.1 | 3.5−0.1 | 2.7−0.0 |
| R | 0.4120 | 0.4775 | 0.6800 | 0.7143 | 0.8837 | 0.6288 | 0.7322 | 0.8390 | 0.8887 | 0.9085 | |
| Sethoxydim-oxazole | Deviation | 14.4−0.0 | 12.2−0.1 | 11.4−0.0 | 11.4−0.0 | 11.1−0.1 | 4.3−0.1 | 4.3−0.0 | 4.3−0.0 | 4.0−0.0 | 4.2−0.0 |
| R | 0.9280 | 0.9380 | 0.9434 | 0.9434 | 0.9674 | 0.9906 | 0.9933 | 0.9945 | 0.9955 | 0.9964 | |
| QuEChERS | Optimized Conditions a | Recovery (%) b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alloxydim | Sethoxydim | Profoxydim | Deallyloxylated-alloxydim | Deethoxylated-sethoxydim | Sethoxydim-oxazole | ||||||||
| Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | ||
| Citrate | SW = 3.23 g AM = 52.8% EV = 8 mL WC = 3.8 mL ET = 1.8 min | 98.9 | 100.5 | 100.0 | 98.8 | 100.1 | 97.1 | 99.3 | 102.7 | 100.0 | 99.4 | 99.7 | 97.5 |
| Acetate | SW = 3.96 g AM = 57.0% EV = 9.4 mL WC = 4.0 mL ET = 2.8 min | 99.9 | 101.2 | 97.6 | 101.9 | 99.8 | 100.5 | 99.9 | 102.1 | 99.9 | 100.3 | 97.7 | 96.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villaverde, J.J.; Sevilla-Morán, B.; López-Goti, C.; Alonso-Prados, J.L.; Sandín-España, P. Computational-Based Study of QuEChERS Extraction of Cyclohexanedione Herbicide Residues in Soil by Chemometric Modeling. Molecules 2018, 23, 2009. https://doi.org/10.3390/molecules23082009
Villaverde JJ, Sevilla-Morán B, López-Goti C, Alonso-Prados JL, Sandín-España P. Computational-Based Study of QuEChERS Extraction of Cyclohexanedione Herbicide Residues in Soil by Chemometric Modeling. Molecules. 2018; 23(8):2009. https://doi.org/10.3390/molecules23082009
Chicago/Turabian StyleVillaverde, Juan José, Beatriz Sevilla-Morán, Carmen López-Goti, José Luis Alonso-Prados, and Pilar Sandín-España. 2018. "Computational-Based Study of QuEChERS Extraction of Cyclohexanedione Herbicide Residues in Soil by Chemometric Modeling" Molecules 23, no. 8: 2009. https://doi.org/10.3390/molecules23082009