1. Introduction
Yuanhuzhitong tablet (YHZT), formally incorporated into Chinese Pharmacopoeia and Chinese National Essential Medicine List, consists of two herbs including 223 g of Radix Angelicae dahuricae and 445 g of vinegar-processed
Corydalis yanhusuo W.T. Wang (
C. yanhusuo) [
1]. As a classic Chinese Patent Drug, YHZT has been frequently applied to alleviate various pains including hypochondriac pain, headache, stomachache and dysmenorrhea [
1]. Chemically, alkaloids and coumarins are the main active constituents of YHZT, which possess a variety of pharmacological actions, such as analgesia, spasmolysis, anti-inflammatory, antianxiety and vasodilatation [
2]. The vinegar-quenching processing technology has been utilized for the extraction of the active ingredient of
C. yanhusuo due to a better solubility and to increase the curative effect [
3]. The processing method of vinegar
C. yanhusuo has a long application history in traditional Chinese medicines (TCM). Previous study showed that the alkaloid content of
C. yanhusuo was higher with vinegar in its decoction process than that without it [
4]. TCM is often composed of two or more herbs to obtain synergistic effects or to reduce possible adverse reactions [
5,
6,
7]. Clinical practice and previous pharmacological studies have proven that Radix Angelicae dahuricae could enhance the analgesic effect of
C. yanhusuo [
8].
C. yanhusuo, which is also called
yuanhu in China, is a key constituent herb in YHZT. It is one of the most significant dried medicinal herbs used in TCM [
9].
C. yanhusuo was recorded in the Pharmacopoeia of the People’s Republic of China in 2015 [
1]. It has been used for centuries for analgesic, sedation, anti-arrhythmic. It has been studied more and more nowadays since the recent discovery of its cancer pain relief effect [
10,
11,
12,
13,
14]. A number of its constituents have been isolated and reported, including alkaloids, aliphatic acid, ecdysterone, etc [
15]. Alkaloids were identified as the main effective components in
C. yanhusuo according to the pharmacological study results. Meanwhile, tetrahydropalmatine is the one alkaloid that has been selected as a phytochemical marker for the quality control of
C. yanhusuo in the Chinese Pharmacopoeia [
1]. Tetrahydropalmatine, corydaline, tetrahydrobeberine and berberine have a significant analgesic effect [
16,
17]. Dehydrocorydaline and coptisine have the effect of anti-inflammatory and anti-coronary heart disease [
18]. Tetrahydrobeberine have protection on cardiac cerebral vessel and antipsychotic effects [
19]. Prptopine exhibits significant antihepatotoxic effects [
20]. Palmatine, berberine and berberrubine are reported to possess antibacterial activity [
21].
For a better understanding of pharmacokinetic behavior of alkaloids in the animal and human body, a sensitive analytical method needs to be established to quantitatively analyze the alkaloids in
C. yanhusuo and YHZT. Several methods have been reported, including high-performance liquid chromatography with ultra-violet detection (HPLC-UV) [
22,
23], high-performance liquid chromatography with DAD detection (HPLC-DAD) [
24], and high-performance liquid chromatography electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS) [
25] for quantitative analysis of alkaloids in
C. yanhusuo extract. The effect of the co-administration of
C. yanhusuo and Radix
Angelicae dahuricae on pharmacokinetic behavior of tetrahydropalmatine was investigated and the result showed that the plasma concentration of tetrahydropalmatine was higher in the combination pair group than that in the
C. yanhusuo single herb group [
26]. Many pharmacokinetic studies have been reported about the alkaloids from
C. yanhusuo extract in rat plasma [
27,
28,
29].
In this article, a new UHPLC-ESI-MS/MS method has been developed for simultaneous analysis of 10 alkaloids in beagle dog plasma after the oral administration of C. yanhusuo extraction and YHZT. It is the first article that analyse 10 alkaloids from C. yanhuso in biological fluids in a single run. According to the calculation, the dosage of C. yanhusuo extract and YHZT for beagle dog could be confirmed accurately. In the fasting state, the beagle dogs were given the equal amount of the two medicines. The absorption of alkaloids in beagle dog plasma was compared and their pharmacokinetic characteristics were elucidated, which would hopefully establish the basic groundwork for the further study and clinical application of C. yanhusuo and YHZT.
4. Materials and Methods
4.1. Materials
Corydaline (151118), dehydrocorydaline (16051707), tetrahydropalmatine (151123), protopine (17062106), palmatine (140821), tetrahydroberberine (151111), columbamine (141108), berberine (141128), coptisine (140430), berberrubine (140407) and theophyline (17011102) with over 98% purity were purchased from Chengdu Pufei De Biotech Co. Ltd. (Chengdu, Sichuan Province, China). HPLC grade methanol and acetonitrile were purchased from Dikma Technologies Inc (Beijing, China). All other reagents including ethyl acetate, ether, dichloromethane and acetone belonged to analytical grade. Ultra-pure water was prepared by using a Milli-Q water purification system (Millipore, Molsheim, France). The blank plasma samples were achieved from the blood of healthy beagle dogs. Yuanhuzhitong tablet (20151201) was purchased from Sunflower Pharmaceutical Industry (Harbin, China). Corydalis yanhusuo W.T. Wang was obtained from the Panan Traditional Chinese Crude Drug Market, located in Zhejiang Province, China, and it was identified by Professor Zhenyue Wang of Heilongjiang University of Chinese Medicine in May, 2017.
4.2. UHPLC-ESI-MS/MS Conditions
The UHPLC-ESI-MS/MS was performed on an Agilent 1290 ultra-high performance liquid chromatography (UHPLC) system and an Agilent 6430 QQQ-MS mass spectrometer with an electrospray ionization (ESI) source interface (Agilent Technologies, Santa Clara, CA, USA). The UHPLC-ESI-MS/MS was performed on an Agilent Eclipse Plus C18 RRHD column (1.8 µm, 50 × 2.1 mm). In order to obtain the maximum sensitivity of the MRM, parameters such as fragmentor, collision energy and the nitrogen flow rate were optimized. The results showed in the
Table 6. The mobile phases used 0.1% formic acid/water (A) and acetonitrile (B) as follows: 0–7.5 min, 22–32% (B); 7.5–8.5 min, 32–43% (B); 8.5–10.5 min, 43–55% (B). The flow rate was 0.3 mL/min. The column temperature was 35 °C. 5 μL sample solution was injected with a needle wash process. High-purity N
2 was used as the nebulizing gas, and N
2 was used as drying gas at a flow rate of 11 L/min. The mass spectrometer was operated in positive-ion mode at a capillary voltage of 4500 V. The source temperature was kept at 100 °C. The desolvation temperature was kept at 350 °C.
4.3. Preparation of Standard and QC Solutions
A stock standard solution with compound I–X mixing together was prepared in methanol with a concentration of 223.0, 206.0, 200.0, 113.0, 216.0, 200.0, 202.0, 201.0, 200.0 and 230.0 µg/mL, respectively. Theophyline (I.S.) was prepared with methanol at concentration of 1050.0 ng/mL. A series of calibration standards solutions were prepared at seven different concentration levels, I (557.0, 139.3, 34.8, 8.7, 2.2, 1.1 and 0.5 ng/mL), II (257.5, 64.4, 16.1, 4.0, 1.0, 0.5 and 0.3 ng/mL), III (507.0, 126.75, 31.68, 7.920, 1.980, 0.9902 and 0.4951 ng/mL ), IV (540.0, 135.0, 33.8, 8.5, 2.1, 1.1 and 0.5 ng/mL), V (525.0, 131.3, 32.8, 8.2, 2.1, 1.0 and 0.5 ng/mL), VI (420.0, 105.0, 26.3, 6.6, 1.6, 0.8 and 0.4 ng/mL), VII (400.0, 100.0, 25.0, 6.3, 1.6, 0.8 and 0.4 ng/mL), VIII (540.0, 135.0, 33.8, 8.4, 2.1, 1.1 and 0.5 ng/mL), IX (500.0, 125.0, 31.3, 7.8, 2.0, 1.0 and 0.5 ng/mL) and X (230.0, 57.5, 14.4, 3.6, 0.9, 0.4 and 0.2 ng/mL) respectively. Three different concentration levels of the QC samples were prepared for this assay, high QC (445.6/206.0/405.6/432.0/420.0/336.0/320.0/432.0/400.0/184.0 ng/mL), medium QC (34.8/16.1/31.7/33.8/32.8/26.3/25.0/33.8/31.3/14.4 ng/mL), low QC (1.1/0.5/1.0/1.1/1.0/0.9/0.8/1.0/1.0/0.4 ng/mL), and LLOQ (0.5/0.3/0.5/0.5/0.5/0.4/0.4/0.5/0.5/0.2 ng/mL), for I–X, respectively. All above samples were kept at 4 °C until use.
4.4. Preparation of C. yanhusuo Extract and YHZT
After soaking for 24 h, the C. yanhusuo was extracted twice with 60% ethanol (1:4, w/v) at 75 °C, 3 h for the first time and 2 h for the second time. The combined decoction was filtered and it was concentrated. Then, the paste rate is calculated. The contents of C. yanhusuo extract for I–X were 0.73, 1.50, 1.12, 0.92, 1.51, 1.03, 1.01, 0.81, 0.89 and 0.47 mg/g, respectively.
According to Chinese Pharmacopoeia, the dosage of
C. yanhusuo medicinal materials for human is 10 g [
1]. Specific surface area of human to dog equivalent dose ratio of body surface area conversion betwwen the human and dog is 0.32. The dosage of
C. yanhusuo medicinal materials for dog is 0.27 g/kg. After a series of extraction and concentration operations, the extraction ratio of
C. yanhusuo medicinal materials is 18%. Thus, the dosage of
C. yanhusuo extract for beagle dog is 0.0486 g/kg.
The formulation illustration of YHZT was prescribed in the Chinese Pharmacopoeia [
1]. 1000 Yuanhuzhitong tablets were made of 223 g of Radix Angelicae dahuricae and 445 g of vinegar-processed
Corydalis yanhusuo W.T. Wang medicinal materials by a series of extraction and concentration operations. Each tablet was equivalent to 0.445 g
C.yanhusuo medicinal materials. And according to Chinese Pharmacopoeia, the weight of the tablet core is 0.25 g [
1]. Therefore, every gram YHZT powders was equivalent to 1.78 g
C.yanhusuo medicinal materials. After taking off the Sugar coating, the tablets were lapped into powder. Then, the YHZT powder was weighed precisely. As mentioned above, the dosage of
C. yanhusuo medicinal materials for beagle dog is 0.27 g/kg. It was equivalent to 0.15 g YHZT powders.
4.5. Biosample Preparation
A liquid-liquid extraction method was selected for the beagle dog plasma sample preparation. Fifty µL of internal standard solution (1050 ng/mL), 100 µL aliquot of plasma samples, and 100 µL of methanol were pipetted into a 10 mL glass tube. The mixture solution was vortex-mixed in glass tubes for 60 s. Then it was extracted with 3 mL ethyl acetate, vortex-mixed for 120 s, centrifuged at 877× g for 5 min. The organic layer was transferred into another spotless tube. Then, it was dried under a flow of nitrogen at 40 °C. The residue was re-dissolved by 100 µL mobile phase, vortex-mixed for 120 s, filtered by a 0.22 μm nylon 66 membrane. A 5 µL aliquot was injected into the UHPLC-ESI-MS/MS system.
The calibration standards and the QC samples were prepared under the same conditions as the plasma samples, which collected from the beagle dog after oral administration C. yanhusuo and YHZT powders. The spiked samples were handled by the following steps. 50 µL of internal standard solution (1050 ng/mL), 100 µL aliquot of blank plasma samples, and 100 µL of calibration standards or the QC methanol solution were pipetted into a 10 mL glass tube. And the mixture solution was vortex-mixed in glass tubes for 60 s. Then it was extracted with 3 mL ethyl acetate, vortex-mixed for 120 s, centrifuged at 877× g for 5 min. The organic layer was transferred into another spotless tube. Then, it was dried under a flow of nitrogen at 40 °C. The residue was re-dissolved by 100 µL mobile phase, vortex-mixed for 120 s, filtered by a 0.22 μm nylon66 membrane. The 5 µL samples was detected by the UHPLC-ESI-MS/MS system.
4.6. Method Validation
4.6.1. Selectivity
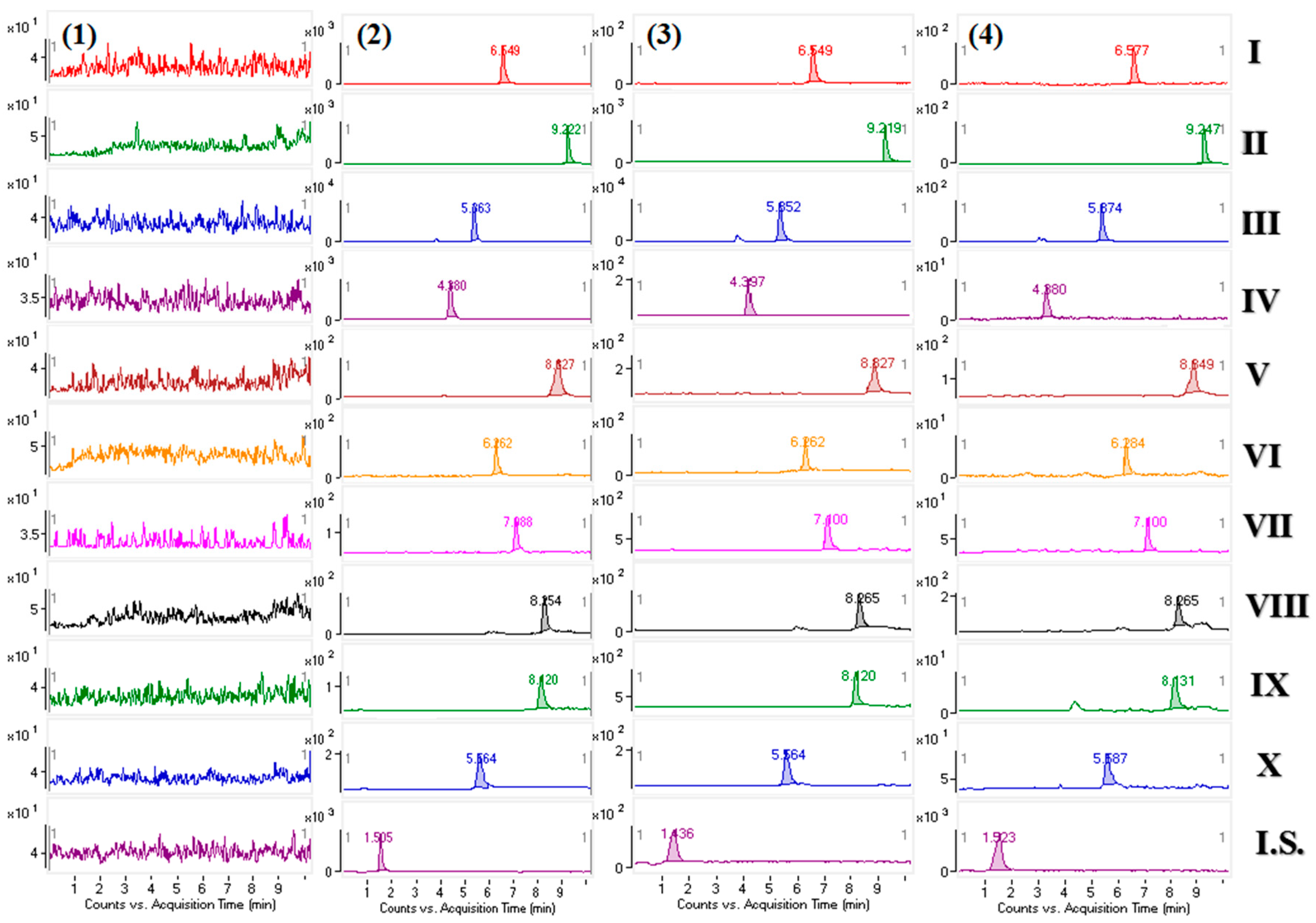
In order to rule out the interference from endogenous matrix compounds with 10 analytes and I.S., method specificity was determined in beagle dog plasma. Six different blank plasma were obtained from different beagle dog plasma, and spiked with the alkaloids and I.S. at LLOQ level, then analyzed by UHPLC-MS/MS system. The response of co-eluting interferences was evaluated by the comparison of the drug free plasma chromatograms, the blank plasma chromatograms which spiked with analytes and I.S., and the plasma samples from the beagle dogs after oral administration of the C. yanhusuo extract and YHZT.
4.6.2. Linearity and LLOQ
Each calibration curve was assessed by plotting the peak area ratio of the analytes and I.S. (Y) versus each nominal concentration (X) of the analytes. The calibration model was selected based on the analysis of the data by linear regression with weighting factors (1/x2). The LLOQ was determined as the lowest concentration point of the calibration curve with sufficient precision and the accuracy. The LLOQ could be quantified with S/N, which should be bigger than 10. Six replicate samples were used for evaluation.
4.6.3. Precision and Accuracy
The precision and accuracy of the intra-day and inter-day were determined by the analysis of LLOQ, LQC, MQC and HQC samples (n = 6) on the one day and on three different days, respectively. Accuracy was evaluated as the relative error (RE) of the measured mean value. And the precision was determined as the RSD of the measured concentration. The concentration of each sample was calculated via a calibration curve that constructed on the same day. The criteria for precision and accuracy of evaluation are as follows: the accuracy should be less than 15% of the actual value for QC samples and the RSD should no more than 15%. The RSD of LLOQ samples should not exceed 20%.
4.6.4. Extraction Recovery and I.S.-Normalized Matrix Factor
The extraction efficiency of the ten analytes was investigated by the comparison of six replicates beagle dog plasma samples at low, medium and high QC levels. The relative recoveries of the 10 analytes and the I.S. were determined by comparing the peak areas of an extracted sample against a post-extraction spiked sample and calculated by the ratio of the peak responses. The matrix factor was evaluated by comparing the absolute peak areas of blank matrix samples spiked after extraction with analytes to pure solution of the analytes. The I.S.-normalized matrix factor was calculated by the matrix factor ratio of the analyte to the internal standard.
4.6.5. Stability
The stability test included three freeze-thaw cycles stability (−40 to 23 °C), room temperature stability (storage for 4 h at ambient temperature), long-term sample storage stability (−20 °C for two weeks) and ready-to-injection of extracted sample stability (4 °C for 12 h). LQC, MQC, HQC samples with five replicates at each level were kept at the above conditions and analyzed against freshly prepared calibrators as the reference.
4.7. Application to Pharmacokinetic Studies
Animal experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, and the study was approved by the Animal Ethics Committee of the Institution. Twelve healthy male beagle dogs (body weight 12 ± 2 kg) were obtained from Shenyang Kangping Institute of Medical Laboratory Animals (SCXK (Liao) 2014-0003). Then it was randomly divided into two groups. All experimental procedures were approved by the ethics committees of Harbin Medical University. The blood samples of beagle dog were achieved at specific time points after oral administration of C. yanhusuo extraction with a dose of 0.0486 g/kg and YHZT powders with a dose of 0.15 g/kg. The beagle dogs were fasted overnight before experiment, and had free water supply even during the experiment. For animals, after oral administration of C. yanhusuo extraction and YHZT powders, 0.5 mL blood samples were withdrawn from the forearm vein into heparinized tubes at 0, 0.083, 0.25, 0.5, 0.75, 1.0, 2.0, 3.0, 4.0, 8.0, 12.0 and 24.0 h. Blood samples were centrifuged at 4582× g for 6 min and the upper layer plasma were kept at −20 °C until detection.
4.8. Data Analysis
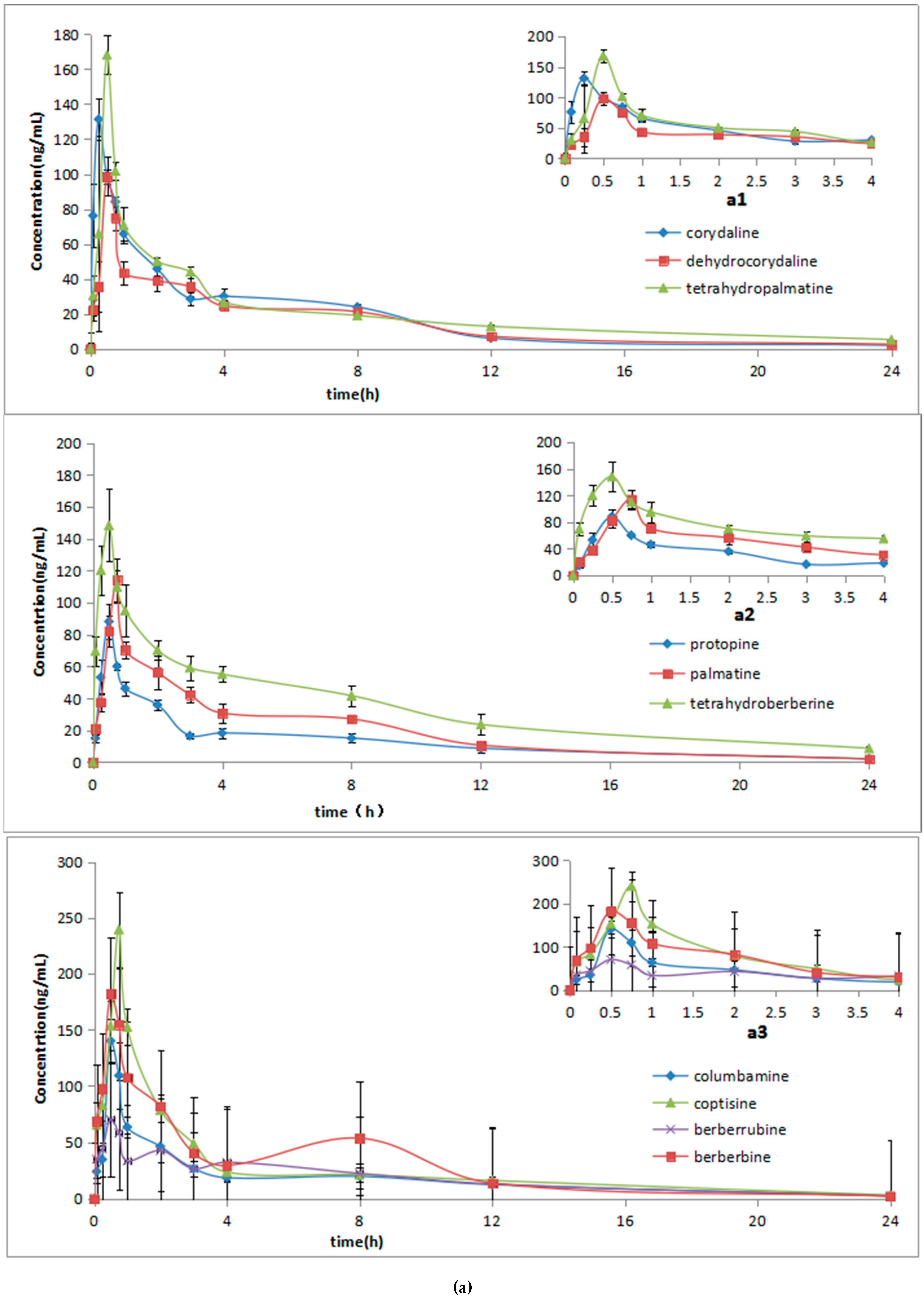
In order to determine the pharmacokinetics parameters of 10 alkaloids, all data were processed by non-compartmental analysis using the DAS 2.0 software package (Chinese Pharmacological Society, Shanghai, China). The plasma concentration at different times was expressed as mean ± SD, and the mean concentration-time curves were plotted. The corresponding pharmacokinetic parameters (the elimination half-life, t1/2; area under the peak area-time curve from time zero to last sampling time AUC0→t and AUC0→∞) were tested. The elimination rate constant (Ke) was calculated by linear regression of the terminal points in a semi-log plot of the plasma concentration against time. The elimination half-life was calculated using the formula t1/2 = 0.693/Ke. The area under plasma concentration-time curve (AUC0→t) to the last measurable plasma concentration (Ct) was estimated by using the linear trapezoidal rule. The area under the plasma concentration-time curve to time infinity (AUC0→∞) was calculated as AUC0→∞ = AUC0→t + Ct/Ke. Moreover, the maximum observed plasma concentration (Cmax) and the time of first occurrence of Cmax (time for maximal concentration, Tmax) were also determined.