Comparative Analysis of Pigments, Phenolics, and Antioxidant Activity of Chinese Jujube (Ziziphus jujuba Mill.) during Fruit Development
Abstract
:1. Introduction
2. Results and Discussion
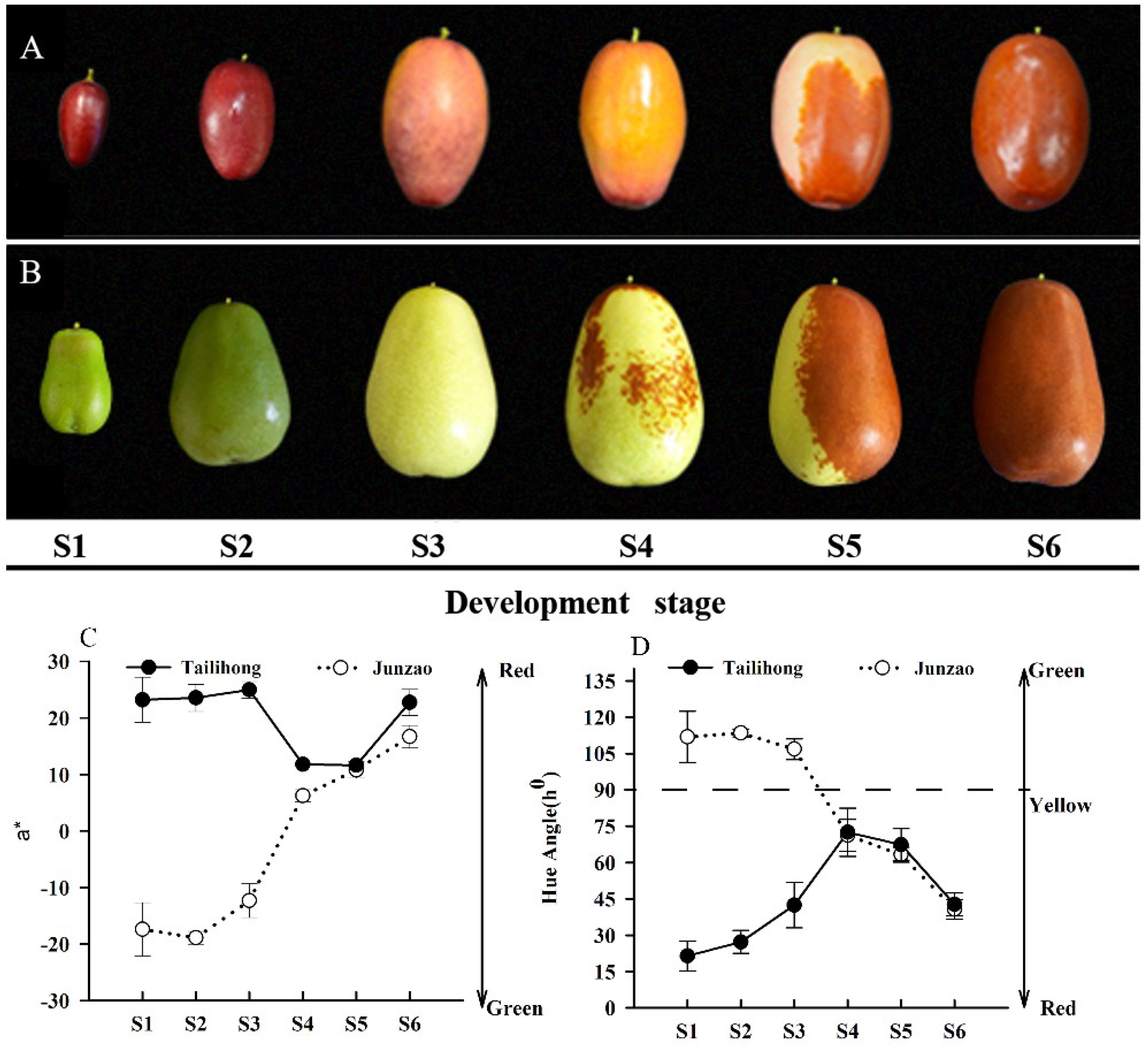
2.1. Skin Color Changes during Fruit Development and Ripening in ‘Tailihong’ and ‘Junzao’
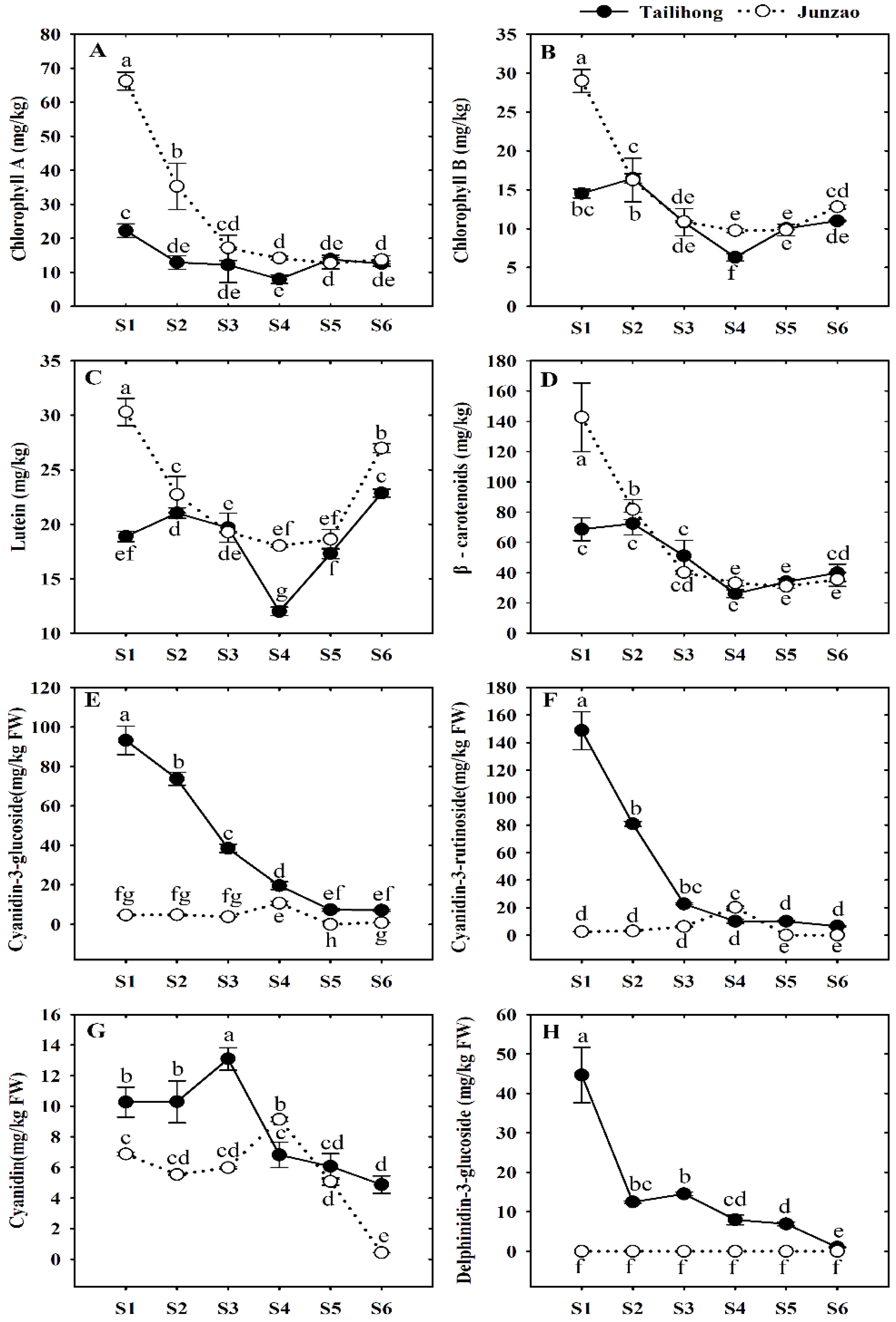
2.2. Pigment Components in the Skins of ‘Tailihong’ and ‘Junzao’
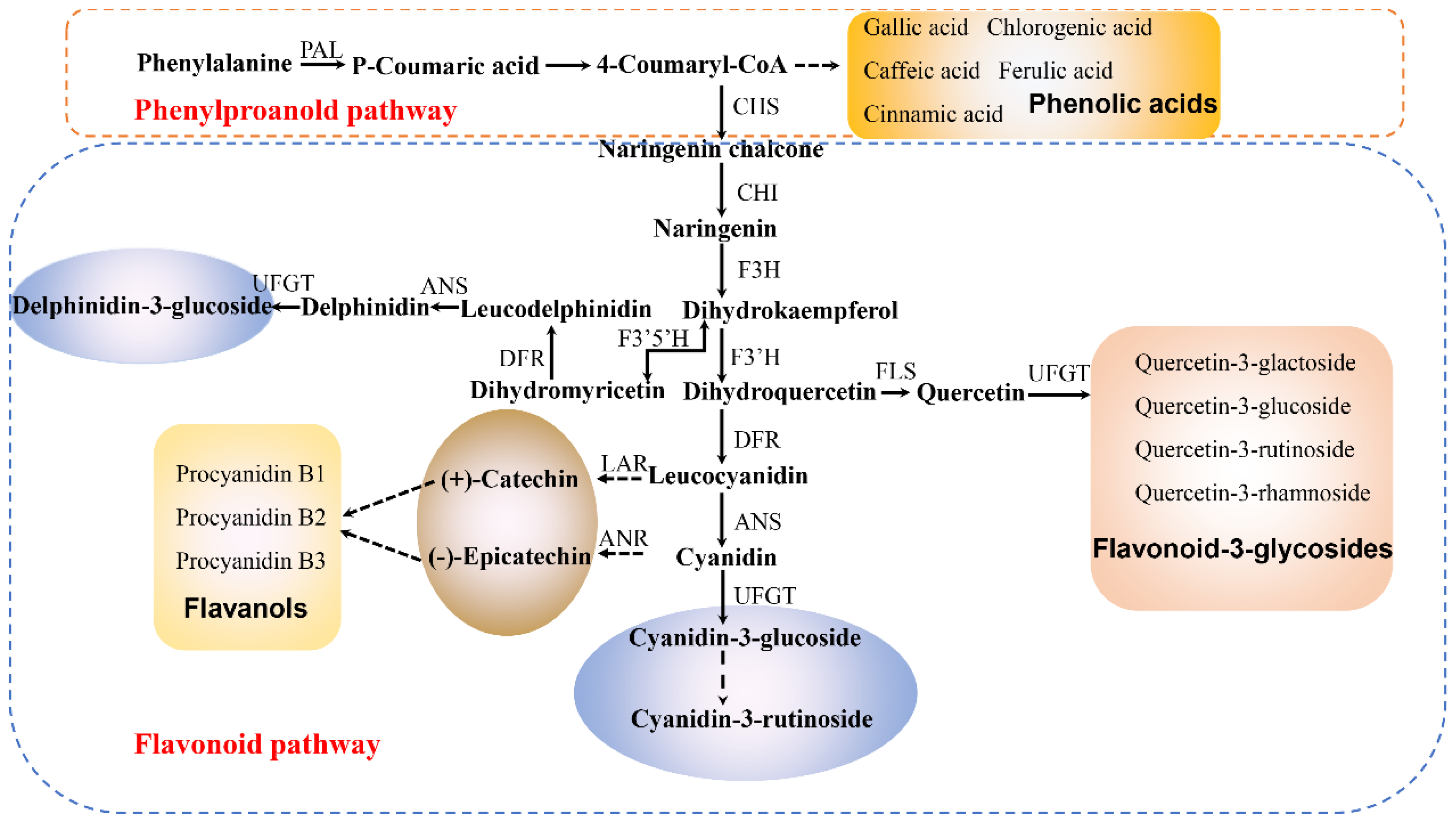
2.3. Contents of Individual Phenolic in the Skins of ‘Tailihong’ and ‘Junzao’
2.4. Total Content of Phenolics, Flavonoids, Flavanols, and Anthocyanins in the Skins of ‘Tailihong’ and ‘Junzao’
2.5. Antioxidant Activity in the Skins of ‘Tailihong’ and ‘Junzao’
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Color Phenotypic Measurement
3.3. Identification and Quantification of Chlorophylls and Carotenoids
3.4. Identification and Quantification of Anthocyanins Extraction
3.5. Analyses of Individual Phenolic Compounds
3.6. Phenolics of Analyses
3.7. Antioxidant Activity of Analyses
3.7.1. Free-Radical Scavenging of DPPH
3.7.2. Ferric Reducing Antioxidant Power (FRAP)
3.7.3. Free-Radical Scavenging of ABTS
3.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Zhang, C.M.; Zhao, X.; Fei, Z.J.; Wan, K.K.; Zhang, Z.; Pang, X.M.; Yin, X.; Bai, Y.; Sun, X.Q.; et al. The Jujube Genome Provides Insights into Genome Evolution and the Domestication of Sweetness/Acidity Taste in Fruit Trees. PLoS Genet. 2016, 12, e1006433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Li, C.L. (Eds.) Forestry Statistical Yearbook 2016; China Forestry Publishing House: Beijing, China, 2017; Volume 70, pp. 83–87. [Google Scholar]
- Pahuja, M.; Mehla, J.; Reeta, K.H.; Joshi, S.; Gupta, Y.K. Hydroalcoholic extract of Zizyphus jujuba ameliorates seizures, oxidative stress, and cognitive impairment in experimental models of epilepsy in rats. Epilepsy Behav. 2011, 21, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Plastina, P.; Bonofiglio, D.; Vizza, D.; Fazio, A.; Rovito, D.; Giordano, C.; Barone, I.; Catalano, S.; Gabriele, B. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J. Ethnopharmacol. 2012, 140, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.F.; Chung, K.F.; Poon, M.M.; Ho, F.Y.; Zhang, S.P.; Zhang, Z.J.; Ziea, E.T.; Wong, V.T. Chinese herbal medicine for insomnia: A systematic review of randomized controlled trials. Sleep Med. Rev. 2012, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.; Maiwulanjiang, M.; Zhang, W.L.; Zhan, J.Y.; Lam, C.T.; Zhu, K.Y.; Yao, P.; Choi, R.C.; Lau, D.T.; et al. Chemical and biological assessment of Ziziphus jujuba fruits from China: Different geographical sources and developmental stages. J. Agric. Food Chem. 2013, 61, 7315–7324. [Google Scholar] [CrossRef] [PubMed]
- Du, L.J.; Gao, Q.H.; Ji, X.L.; Ma, Y.J.; Xu, F.Y.; Wang, M. Comparison of flavonoids, phenolic acids, and antioxidant activity of explosion-puffed and sun-dried jujubes (Ziziphus jujuba Mill.). J. Agric. Food Chem. 2013, 61, 11840–11847. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Zhang, D.; Wang, Y.Q.; Li, P.M.; Ma, F.W. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Wang, B.; Huang, Q.; Venkitasamy, C.; Chai, H.; Gao, H.; Cheng, N.; Cao, W.; Lv, X.; Pan, Z. Changes in phenolic compounds and their antioxidant capacities in jujube (Ziziphus jujuba Miller.) during three edible maturity stages. LWT Food Sci. Technol. 2016, 66, 56–62. [Google Scholar] [CrossRef]
- Wu, C.S.; Gao, Q.H.; Guo, X.D.; Yu, J.G.; Wang, M. Effect of ripening stage on physicochemical properties and antioxidant profiles of a promising table fruit ‘pear-jujube’ (Zizyphus jujuba Mill.). Sci. Hortic. 2012, 148, 177–184. [Google Scholar] [CrossRef]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.L.; Escribano-Bailon, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Rivera-Lopez, J.; Ordorica-Falomir, C.; Wesche-Ebeling, P. Changes in anthocyanin concentration in Lychee (Litchi chinensis Sonn.) pericarp during maturation. Food Chem. 1999, 65, 195–200. [Google Scholar] [CrossRef]
- Kevresan, Z.S.; Mastilovic, J.S.; Mandic, A.I.; Torbica, A.M. Effect of Different Ripening Conditions on Pigments of Pepper for Paprika Production at Green Stage of Maturity. J. Agric. Food Chem. 2013, 61, 9125–9130. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Brahem, M.; Dubois-Laurent, C.; Huet, S.; Jourdan, M.; Geoffriau, E.; Peltier, D.; Gagne, S. Differential Pigment Accumulation in Carrot Leaves and Roots during Two Growing Periods. J. Agric. Food Chem. 2016, 64, 906–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, P.J.; You, F.; Huang, L.X.; Zhang, C.H. Comprehensive assessment of phenolic compounds and antioxidant performance in the developmental process of jujube (Ziziphus jujuba Mill.). J. Funct. Foods 2017, 36, 233–242. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Ye, S.; Ye, Y.; Ren, F. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol. 2010, 48, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Almansa, S.; Hernandez, F.; Legua, P.; Nicolas-Almansa, M.; Amoros, A. Physico-chemical and physiological changes during fruit development and on-tree ripening of two Spanish jujube cultivars (Ziziphus jujuba Mill.). J. Sci. Food Agric. 2016, 96, 4098–4105. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.E.; Lister, C.E.; Reay, P.F.; Triggs, C.M. Influence of pigment composition on skin color in a wide range of fruit and vegetables. J. Am. Soc. Hortic. Sci. 1997, 122, 594–598. [Google Scholar]
- Alniami, J.H.; Saggar, R.A.M.; Abbas, M.F. The physiology of ripening of jujube (Ziziphus jujuba Mill.). Sci. Hortic. 1992, 51, 303–308. [Google Scholar]
- Bastos, V.J.; Neves, L.C.; da Silva, P.M.C.; Shahab, M.; Colombo, R.C.; Roberto, S.R. Harvest point determination of indian jujube fruit (Ziziphus mauritiana L.) based on physicochemical and functional parameters. Sci. Hortic. 2016, 213, 392–402. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S.; et al. Flavonid intake and long-term risk of coronary-heart-disease and cancer in the 7 countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Ahn, J.B.; Kim, H.J.; Im, N.K.; Kozukue, N.; Levin, C.E.; Friedman, M. Changes in free amino acid, protein, and flavonoid content in jujube (Ziziphus jujuba) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J. Agric. Food Chem. 2012, 60, 10245–10255. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ding, T.; Zhang, N.; Jiang, P.; Liu, D. Identification of bitter compounds from dried fruit of Ziziphus jujuba cv. Junzao. Int. J. Food Prop. 2017, 20, S26–S35. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, H.; Liu, Q.C.; Zhao, Y.; Cui, X.Q.; Guo, S.; Zhang, L.; Ho, C.T.; Bai, N.S. Chemical characterization of the main bioactive constituents from fruits of Ziziphus jujuba. Food Funct. 2016, 7, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, Y.; Vainstein, A.; Chen, S.; Ma, H. Regulation of Fig (Ficus carica L.) Fruit Color: Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway. Front. Plant Sci. 2017, 8, 1990. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Wu, C.S.; Wang, M.; Xu, B.N.; Du, L.J. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, alpha-tocopherol, beta-carotene, and phenolic compounds. J. Agric. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef] [PubMed]
- Zozio, S.; Servent, A.; Cazal, G.; Mbeguie, A.M.D.; Ravion, S.; Pallet, D.; Abel, H. Changes in antioxidant activity during the ripening of jujube (Ziziphus mauritiana Lamk.). Food Chem. 2014, 150, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bohn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, F.; Huang, L.X.; Zhang, C.H.; Xie, P.J.; Zhang, Y.L. Changes in phenolic content and DPPH radical scavengingactivity during the development in the skin of ‘Dongzao’ jujube. Food Sci. Biotechnol. 2014, 43, 65. [Google Scholar]
- Bi, X.; Zhang, J.; Chen, C.; Zhang, D.; Li, P.; Ma, F. Anthocyanin contributes more to hydrogen peroxide scavenging than other phenolics in apple peel. Food Chem. 2014, 152, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Ahn, J.B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of Jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Chen, Q.; Li, X.; Li, M.; Kan, C.; Chen, B.; Zhang, Y.; Xue, Z. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chem. 2015, 173, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R.G. Repoting of objective color measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar]
- Zhao, X.Q.; Yuan, Z.H.; Yin, Y.L.; Feng, L.J. Patterns of Pigment Changes in Pomegranate (Punica granatum L.) Peel during Fruit Ripening. In Proceedings of the III International Symposium on Pomegranate and Minor Mediterranean Fruits; Yuan, Z., Wilkins, E., Wang, D., Eds.; International Society for Horticultural Science: Leuven, Belgium, 2015; Volume 1089, pp. 83–89. [Google Scholar]
- Wang, H.; Chen, F.; Yang, H.; Chen, Y.; Zhang, L.; An, H. Effects of ripening stage and cultivar on physicochemical properties and pectin nanostructures of jujubes. Carbohydr. Polym. 2012, 89, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free-radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Developmental Stage | Cultivar | Flavanol Component (mg/kg FW) | Phenolic Acids Component (mg/kg FW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procyanidin B1 | Procyanidin B2 | Procyanidin B3 | (−)-Epicatechin | (+)-Catechin | Quercetin | Gallic Acid | Chlorogenic Acid | Caffeic Acid | Ferulic Acid | Cinnamic Acid | ||
| S1 | Tailihong | 107.52 ± 4.33 c | 374.16 ± 31.47 c | 237.35 ± 15.6 c | 27.94 ± 2.57 a | 58.19 ± 3.62 bc | 16.19 ± 1.19 g | 61.22 ± 2.8 fg | 94.75 ± 3.25 a | 22.65 ± 0.66 a | 14.98 ± 1.45 b | 44.64 ± 1.34 a |
| Junzao | 80.51 ± 7.38 d | 300.67 ± 21.94 d | 112.03 ± 5.04 d | 20.23 ± 0.8 b | 33.42 ± 1.05 de | 26.21 ± 1.35 cd | 77.37 ± 3.73 de | 73.67 ± 4.77 b | 15.45 ± 0.13 d | 3.31 ± 0.2 d | 18.29 ± 0.35 cd | |
| S2 | Tailihong | 100.26 ± 4.45 cd | 391.15 ± 15.44 c | 341.56 ± 7.81 b | 10.91 ± 0.25 d | 56 ± 4.18 bc | 22.12 ± 0.24 de | 119 ± 1.33 b | 61.29 ± 3.09 c | 16.69 ± 0.21 cd | 50 ± 2.66 a | 36.5 ± 2.84 a |
| Junzao | 31.19 ± 0.66 e | 491.29 ± 11.38 b | 128.97 ± 0.69 d | 15.57 ± 0.77 c | 73.36 ± 0.07 ab | 23.53 ± 0.83 de | 69.54 ± 1.67 ef | 46.86 ± 3.71 d | 20.22 ± 1.21 b | 2.77 ± 0.01 d | 40.24 ± 3.2 a | |
| S3 | Tailihong | 286.06 ± 15.65 a | 888.18 ± 15.94 a | 476.48 ± 15.65 a | 8.16 ± 0.73 e | 82.08 ± 6.49 a | 28.33 ± 1 c | 157.01 ± 6.58 a | 48.96 ± 2.66 d | 17.67 ± 1.17 c | 16.84 ± 1.25 b | 42.41 ± 1.04 a |
| Junzao | 31.97 ± 0.28 e | 228.8 ± 8.3 e | 38.88 ± 3.36 e | 12.98 ± 0.84 d | 21.78 ± 0.02 e | 42.79 ± 0.7 a | 74.39 ± 0.22 de | 57.4 ± 0.62 c | 7.42 ± 0.6 fg | 7.91 ± 0.08 c | 8.56 ± 0.67 e | |
| S4 | Tailihong | 187.63 ± 29.07 b | 417.54 ± 63.91 c | 248.42 ± 9.99 c | 7.29 ± 0.38 ef | 45.37 ± 4.24 cd | 16.62 ± 1.96 g | 83.87 ± 9.86 d | 44.57 ± 5.15 d | 13.3 ± 0.56 e | 14.15 ± 0.7 b | 28.5 ± 1.03 b |
| Junzao | 290.3 ± 0.82 a | 200.07 ± 0.53 ef | 491.94 ± 36.18 a | 11.39 ± 0.07 d | 20 ± 1.69 e | 24.87 ± 0.39 cde | 69.64 ± 2.28 ef | 19.43 ± 0.01 f | ND | 3.01 ± 0.08 d | 27.31 ± 1.99 b | |
| S5 | Tailihong | 47.43 ± 6.1 e | 406.89 ± 15.52 c | 44.51 ± 4.92 e | 5.46 ± 0.23 f | 27.25 ± 0.66 de | 21.48 ± 1.32 ef | 75.2 ± 4.49 de | 21.19 ± 4.46 f | 5.34 ± 1.13 h | 4.38 ± 0.27 d | 12.24 ± 0.13 de |
| Junzao | 25.71 ± 1.14 e | 85.37 ± 3.2 h | 52.16 ± 10.73 e | 7.57 ± 0.59 ef | 18.69 ± 0.6 e | 22.51 ± 0.94 de | 93.8 ± 5.2 c | 31.17 ± 1.18 e | 6.14 ± 0.45 gh | 3.54 ± 0.26 d | 12.54 ± 0.97 de | |
| S6 | Tailihong | 76.94 ± 4.58 d | 129.38 ± 2.16 gh | 66.07 ± 5.98 e | 7.89 ± 0.64 e | 32.34 ± 2.25 de | 17.59 ± 1.46 fg | 55.34 ± 4.51 g | 42.54 ± 4.92 d | 8.53 ± 1.28 f | 7.46 ± 0.53 c | 24.04 ± 1.51 bc |
| Junzao | 49.12 ± 7.7 e | 153.26 ± 12.32 fg | 48.63 ± 7.25 e | 8.32 ± 0.02 e | 16.38 ± 0.24 e | 32.56 ± 7.8 b | 63.96 ± 4.41 fg | 47.24 ± 0.13 d | 7.56 ± 0.3 fg | 2.89 ± 0.04 d | 14.65 ± 1.06 de | |
| Developmental Stage | Cultivar | Flavonol Component (mg/kg FW) | |||
|---|---|---|---|---|---|
| Quercetin-3-galactoside | Quercitrin-3-glucoside | Quercetin-3-rutinose | Quercetin-3-rhamnoside | ||
| S1 | Tailihong | 37.53 ± 2.75 bc | 29.27 ± 2.43 e | 1.39 ± 0.11 g | 30.2 ± 1.61 ef |
| Junzao | 81.36 ± 6.95 a | 324.31 ± 19.51 a | 8.37 ± 0.61 a | 244.9 ± 11.62 a | |
| S2 | Tailihong | 19.88 ± 1.22 def | 105.08 ± 2.07 c | 2.1 ± 0.16 f | 51.25 ± 0.34 de |
| Junzao | 34.24 ± 0.25 c | 161.72 ± 19.79 b | 4.61 ± 0.26 c | 151.03 ± 3.02 b | |
| S3 | Tailihong | 27.09 ± 0.75 d | 155.1 ± 7.47 b | 2.64 ± 0.19 ef | 69.89 ± 2.16 cd |
| Junzao | 25.04 ± 2.23 de | 35.37 ± 4.41 e | 3.02 ± 0.44 e | 87.17 ± 5.33 c | |
| S4 | Tailihong | 19.49 ± 1.66 ef | 76.8 ± 5.01 d | 2.81 ± 0.34 e | 39.4 ± 3.93 ef |
| Junzao | 42.87 ± 2.88 b | 97.1 ± 2.61 c | 5.86 ± 0.13 b | 155.8 ± 1.01 b | |
| S5 | Tailihong | 13.73 ± 1.01 fg | 64.94 ± 17.7 d | 3.8 ± 0.45 d | 48.36 ± 2.07 e |
| Junzao | 5.7 ± 0.8 hi | 7.67 ± 0.19 f | 1.19 ± 0.18 g | 30.08 ± 2.68 ef | |
| S6 | Tailihong | 12.13 ± 0.97 gh | 9.5 ± 2.83 f | 3.08 ± 0.27 e | 24.79 ± 1.04 f |
| Junzao | 3.52 ± 0.29 i | 40.94 ± 2.16 e | 1.21 ± 0.1 g | 166.27 ± 1.23 b | |
| DPPH | FRAP | ABTS+ | |
|---|---|---|---|
| TPC | −0.618 * | 0.591 * | −0.585 * |
| TFC | −0.615 * | 0.515 | −0.401 |
| TFAC | −0.753 ** | 0.789 ** | −0.653 * |
| TAC | −0.714 ** | 0.889 ** | −0.730 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Zhang, Z.; Su, J.; Zhou, J.; Li, X. Comparative Analysis of Pigments, Phenolics, and Antioxidant Activity of Chinese Jujube (Ziziphus jujuba Mill.) during Fruit Development. Molecules 2018, 23, 1917. https://doi.org/10.3390/molecules23081917
Shi Q, Zhang Z, Su J, Zhou J, Li X. Comparative Analysis of Pigments, Phenolics, and Antioxidant Activity of Chinese Jujube (Ziziphus jujuba Mill.) during Fruit Development. Molecules. 2018; 23(8):1917. https://doi.org/10.3390/molecules23081917
Chicago/Turabian StyleShi, Qianqian, Zhong Zhang, Juanjuan Su, Jun Zhou, and Xingang Li. 2018. "Comparative Analysis of Pigments, Phenolics, and Antioxidant Activity of Chinese Jujube (Ziziphus jujuba Mill.) during Fruit Development" Molecules 23, no. 8: 1917. https://doi.org/10.3390/molecules23081917
APA StyleShi, Q., Zhang, Z., Su, J., Zhou, J., & Li, X. (2018). Comparative Analysis of Pigments, Phenolics, and Antioxidant Activity of Chinese Jujube (Ziziphus jujuba Mill.) during Fruit Development. Molecules, 23(8), 1917. https://doi.org/10.3390/molecules23081917




