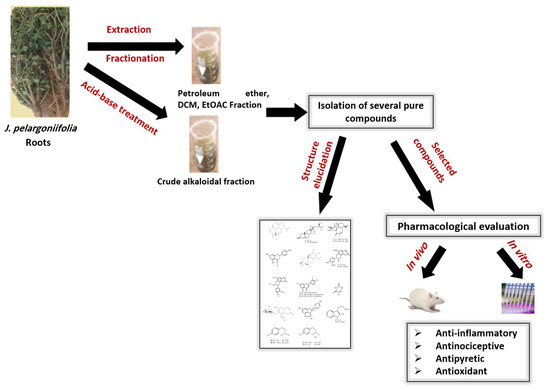
The Phytochemical and Biological Investigation of Jatropha pelargoniifolia Root Native to the Kingdom of Saudi Arabia
Abstract
:1. Introduction
2. Result and Discussion
2.1. Isolation of Compounds
2.2. Structure Elucidation
2.3. Biological Activity
3. Materials and Methods
3.1. Chemicals and Analytical Instruments
3.2. Plant Material
3.3. Animals
3.4. Extraction, Fractionation, and Purification
3.5. Antinociceptive Activity Test
3.5.1. Hot-plate Method
3.5.2. Acetic Acid-induced Writhing in Mice Test
3.5.3. Tail-Flick Method
3.6. Anti-Inflammatory Activity Test
Carrageenan-Induced Edema in the Rat Paw Method
3.7. Antipyretic Activity Screening
Yeast-Induced Hyperthermia in Rats
3.8. Antioxidant Effect
Nitric Oxide Radical-Scavenging Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Alves, M.V. Checklist das espѐcies de Euphorbiaceae Juss. Ocorrentes no semi-áridopernambucano, Brasil. Acta Bot. Bras. 1998, 12, 485–495. [Google Scholar] [CrossRef]
- Webster, G.L. Classification of the Euphorbiaceae. Ann. Mo. Bot. Gard. 1994, 81, 3–143. [Google Scholar] [CrossRef]
- Sabandar, C.W.; Ahmat, N.; Jaafar, F.M.; Sahidin, I. Medicinal property, phytochemistry and pharmacology of several Jatropha species (Euphorbiaceae): A review. Phytochemistry 2013, 85, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Félix-Silva, J.; Giordani, R.B.; Silva, A.A., Jr.; Zucolotto, S.M.; Fernandes-Pedrosa, M.F. Jatropha gossypiifolia L. (Euphorbiaceae): A review of traditional uses, phytochemistry, pharmacology, and toxicology of this medicinal plant. Evid-Based Complement. Altern. Med. 2014, 2014, 1–32. [Google Scholar]
- Oskoueian, E.N.; Saad, W.Z.; Omar, A.; Ahmad, S.; Kuan, W.B.; Zolkifli, N.A.; Hendra, R.; Ho, Y.W. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Jatropha curcas Linn. J. Med. Plants Res. 2011, 5, 49–57. [Google Scholar]
- Yusuf, S.O.; Maxwell, I.E. Analgesic activity of the methanolic leaf extract of Jatropha Curcas (Linn). Afr. J. Biomed. Res. 2010, 13, 149–152. [Google Scholar]
- Schmelzer, G.H.; Fakim, G.A. Jatropha pelargoniifolia; Record from Prota4u; PROTA (Plant Resources of Tropical Africa/RessourcesVégétales de l’AfriqueTropicale): Wageningen, The Netherlands, 2007. [Google Scholar]
- Zhang, X.P.; Zhang, M.L.; Sua, X.H.; Huoa, C.H.; Gub, Y.C.; Shi, Q.W. Chemical constituents of the plants from genus Jatropha. Chem. Biodivers. 2009, 6, 2166–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Zhang, C.Y.; Dai, J.J.; Rahman, K.; Zhang, H. Diterpenoids with thioredoxin reductase inhibitory activities from Jatropha multifida. Nat. Prod. Res. 2017, 31, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Yang, X.Y. New coumarin glucopyranosides from roots of Angelica dahurica. Chin. Herb. Med. 2018, 10, 103–106. [Google Scholar] [CrossRef]
- Konishi, T.; Wada, S.; Kiyosawa, S. Constituents of the leaves of Daphne pseudo-mezereum. Yakugaku Zasshi: J. Pharm. Soc. Jpn. 1993, 113, 670–675. [Google Scholar] [CrossRef]
- Jung, M.; Geiger, H.; Zinsmeister, H.D. Tri- and tetrahydroxycoumarin derivatives from Tetraphis pellucida. Phytochemistry 1995, 39, 379–381. [Google Scholar] [CrossRef]
- Liu, J.Q.; Yang, Y.F.; Wang, C.F.; Li, Y.; Qiu, M.H. Three new diterpenes from Jatropha curcas. Tetrahedron 2012, 68, 972–976. [Google Scholar] [CrossRef]
- Habib, M.R.; Nikkon, F.; Rahman, M.; Haque, M.E.; Karim, M.R. Isolation of stigmasterol and β-sitosterol from methanolic extract of root bark of Calotropis gigantean (Linn). Pak. J. Biol. Sci. 2007, 10, 4174–4176. [Google Scholar] [PubMed]
- Oskoueian, E.; Abdullah, N.; Ahmad, S.; Saad, W.Z.; Omar, A.; Ho, Y.W. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. Int. J. Mol. Sci. 2011, 12, 5955–5970. [Google Scholar] [CrossRef] [PubMed]
- Naengchomnong, W.; Thebtaranonth, Y. Isolation and structure determination of four novel diterpenes from Jatropha curcus. Tetrahedron Lett. 1986, 27, 2439–2442. [Google Scholar] [CrossRef]
- Maltese, F.; Erkelens, C.; Kooy, F.V.D.; Choi, Y.H. Identification of natural epimeric flavanone glycosides by NMR spectroscopy. Food Chem. 2009, 116, 575–579. [Google Scholar] [CrossRef]
- Mutheeswarana, S.; Saravana, P.; Kumarb, P.; Yuvaraj, V.; Duraipandiy, N.A.; Balakrishnab, K.; Ignacimuthu, S. Screening of some medicinal plants for anticariogenic activity: An investigation on bioactive constituents from Jatropha gossypifolia (L.) root. Biocatal. Agric. Biotech. 2017, 10, 161–166. [Google Scholar] [CrossRef]
- Hufford, C.D.; Oguntimein, B.O. Non-polar constituents of Jatropha curcas. Lloydia 1978, 41, 161–165. [Google Scholar]
- Denton, W.R.; Harding, W.W.; Anderson, C.I.; Jacobs, H.; McLean, S.; Reynolds, W.F. New diterpenes from Jatropha divaricata. J. Nat. Prod. 2001, 64, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Alimboyoguen, A.B.; De Castro-Cruz, K.A.; Shen, C.C.; Li, W.T.; Ragasa, C.Y. Chemical constituents of the bark of Aleurites moluccana L. Willd. J. Chem. Pharm. Res. 2014, 6, 1318–1320. [Google Scholar]
- Akbar, E.; Sadiq, Z. Coumarinolignoid rare natural product: A review. Asian J. Chem. 2012, 24, 4831–4842. [Google Scholar]
- Begum, S.A.; Sahai, M.; Ray, A.B. Non-conventional lignans: coumarinolignans, flavonolignans, and stilbenolignans. Prog. Chem. Org. Nat. Prod. 2010, 93, 1–70. [Google Scholar]
- Das, B.; Venkataiah, B. A minor coumarino-lignoid from Jatropha gossypifolia. Biochem. Syst. Ecol. 2001, 2, 213–214. [Google Scholar] [CrossRef]
- Biswanath, D.; Kashinatham, A.; Venkataiah, B.; Srinivas, K.V.N.S.; Mahender, G.; Reddy, M.R. Cleomiscosin A, a coumarino-lignoid from Jatropha gossypifolia. Biochem. Syst. Ecol. 2003, 31, 1189–1191. [Google Scholar]
- Xu, J.F.; Feng, Z.M.; L, J.; Zhang, P.C. New hepatoprotective coumarinolignoids from Mallotus apelta. Chem. Biodivers. 2008, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Mok, S.Y.; Choi, C.; Cho, E.J.; Kim, H.Y.; Lee, S. Analysis of apigenin in Blumea balsamifera Linn DC. and its inhibitory activity against aldose reductase in rat lens. J. Agric. Chem. Environ. 2012, 1, 28–33. [Google Scholar]
- Abdelgadir, H.A.; Staden, J.V. Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L. (Euphorbiaceae): A review. S. Afr. J. Bot. 2013, 88, 204–218. [Google Scholar] [CrossRef]
- Hurd, R.E.; Reid, B.R. NMR spectroscopy of the ring nitrogen protons of uracil and substituted uracils; relevance to a base pairing in the solution structure of transfer RNA. Nucleic Acids Res. 1977, 4, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Staubmann, R.; Schubert-Zsilavecz, M.; Hiermann, A.; Kartnig, T. A complex of 5-hydroxypyrrolidin-2-one and pyrimidine-2,4-dione isolated from Jatropha curcas. Phytochemistry 1999, 50, 337–338. [Google Scholar] [CrossRef]
- Elhawary, S.S.; Eltantawy, M.E.; Sleem, A.A.; Abdallah, H.M.; Mohamed, N.M. Investigation of phenolic content and biological activities of Scabiosa atropurpurea L. World Appl. Sci. J. 2011, 3, 311–317. [Google Scholar]
- Zhang, T.; Zhou, J.; Wang, Q. Flavonoids from aerial part of Bupleurum chinense DC. Biochem. Syst. Ecol. 2007, 35, 801–804. [Google Scholar] [CrossRef]
- Fernández, S.; Wasowski, C.; Paladini, A.C.; Marder, M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol. Biochem. Behav. 2004, 77, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.; Kamperdick, C.; Ninh, P.T.; Lien, T.P.; Thao, T.T.P.; Sung, T.V. Immunosuppressive auronol glycosides from Artocarpus tonkinensis. Pharmazie 2004, 59, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Grina, J.A.; Ratcliff, M.R.; Stermitz, F.R. Old and new alkaloids from Zanthoxylum arborescens. J. Org. Chem. 1982, 47, 2648–2651. [Google Scholar] [CrossRef]
- Yokoo, Y.; Kohda, H.; Kusumoto, A.; Naoki, H.; Matsumoto, N.; Amachi, T.; Suwa, Y.; Fukazawa, H.; Ishida, H.; Tsuji, K.; et al. Isolation from beer and structural determination of a potent stimulant of gastrin release. Alcohol Alcohol. 1999, 34, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.S.; Kassem, F.F.; Abdallah, R.M. Two alkaloids from Ephedra aphylla growing in Egypt. Nat. Prod. Sci. 2003, 9, 1–4. [Google Scholar]
- Neal, J.M.; Sato, P.T.; Johnson, C.L.; McLaughlin, J.L. Cactus alkaloids X: Isolation of hordenine and N-methyltyramine from Ariocarpus kotschoubeyanus. J. Pharm. Sci. 1971, 60, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Hanan, A.; Ali, E.-G.; Oliver, K. A comparative study of the biological activities of Jatropha pelargoniifolia and Jatropha glauca native to Saudi Arabia. Phytomedicine 2018. submitted. [Google Scholar]
- Fana, Y.; Li, X.; Zhang, L.; Duana, P.; Li, F.; Zhao, D.; Wang, Y.; Wub, H. Ether-functionalized ionic liquids: Highly efficient extractants for hordenine. Chem. Eng. Res. Des. 2017, 124, 66–73. [Google Scholar] [CrossRef]
- Ma, J.; Wang, S.; Huang, X.; Geng, P.; Wen, C.; Zhou, Y.; Yu, L.; Wang, X. Validated UPLC-MS/MS method for determination of hordenine in rat plasma and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 111, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Christine, S.; Bell, A.; Stewart-Johnsox, E. N-methyltyramine, a biologically active amine in Acacia seeds. Phytochemistry 1979, 18, 2022–2023. [Google Scholar]
- Jadeja, R.N.; Devkar, R.V. Polyphenols and flavonoids in controlling non-alcoholic steatohepatitis. In Polyphenols in Human Health and Disease, 1st ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 615–623. [Google Scholar]
- Begum, S.; Saxena, B.; Goyal, M.; Ranjan, R.; Joshi, V. B.; Rao, V.; Krishnamurthy, S.; Sahai, M. Study of anti-inflammatory, analgesic and antipyretic activities of seeds of Hyoscyamusniger and isolation of a new coumarinolignan. Fitoterapia 2010, 81, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Picha, P.; Naengchomnong, W.; Promratanapongse, P.; Kano, E.; Hayashi, S.; Ohtsubo, T.; Zhang, S.W.; Shioura, H.; Kitai, R.; Matsumoto, H.; et al. Effect of natural pure compounds curcusones A and C from tropical herbal plant Jatropha curcas on thermo sensitivity and development of thermotolerance in Chinese hamster V-79 cells in vitro. J. Exp. Clin. Cancer Res. 1996, 15, 177–183. [Google Scholar]
- Skoog, D.; Holler, F.J.; Nieman, T.A. An introduction to chromatographic separations. In Principles of instrumental analysis, 5th ed.; Skoog, D., Holler, F.J., Nieman, T.A., Eds.; Saunders College Publishing: Philadelphia, PA, USA, 1992; pp. 674–700. [Google Scholar]
- Madhumitha, G.; Fowsiya, J. A. Hand Book on: Semi Micro Technique for Extraction of Alkaloids; International E-Publication: Indore, India, 2015; p. 9. [Google Scholar]
- Turner, R.A. Analgesics, in Screening Methods in Pharmacology; Academic Press: London, UK, 1965; p. 100. [Google Scholar]
- Koster, R.; Anderson, M.; De Beer, E.J. Acetic acid for analgesic screening. Fed. Proc. 1959, 18, 412–417. [Google Scholar]
- D’amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carregeenan-induced oedema in hind paw of the rats as an assay for anti-inflammatory drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Loux, J.J.; Depalma, D.D.; Yankell, S.L. Antipyretic testing of aspirin in rats. Toxicol. Appl. Pharmacol. 1972, 22, 672–675. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–22 are available from the authors. |

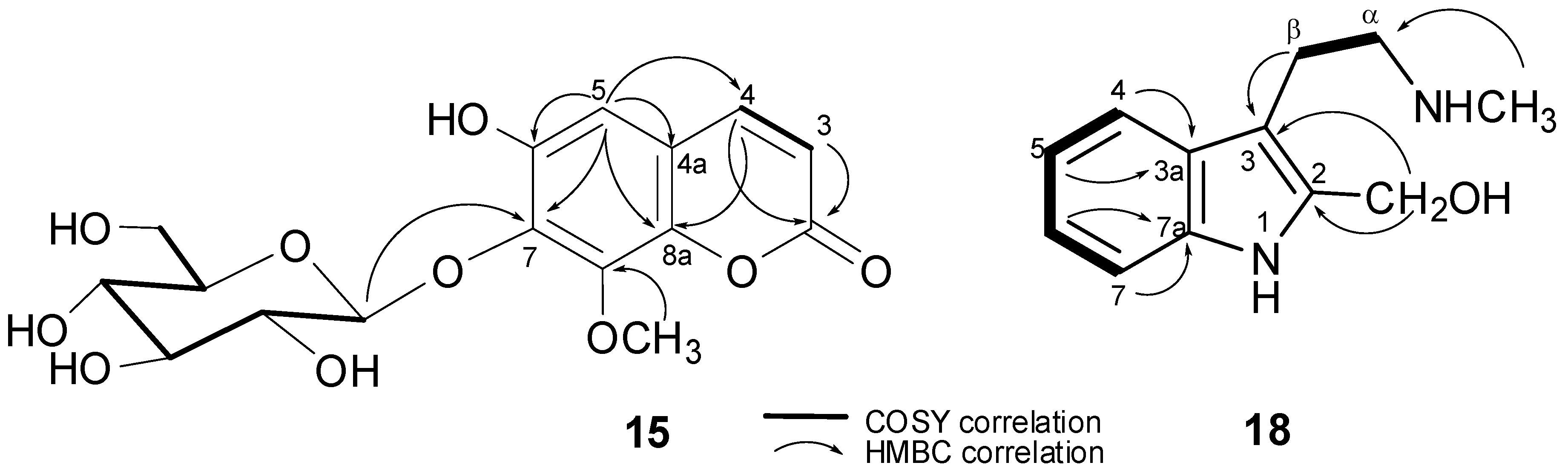
| Position | δH | δC |
|---|---|---|
| 2 | - | 163.5 |
| 3 | 6.26 (d, J = 9.5 Hz, 1H) | 116.2 |
| 4 | 7.88 (d, J = 9.5 Hz, 1H) | 146.5 |
| 5 | 7.00 (s, 1H) | 106.1 |
| 6 | - | 145.7 |
| 7 | - | 133.2 |
| 8 | - | 147.5 |
| 4a | - | 112.7 |
| 8a | - | 144.4 |
| 1′ | 4.99 (d, J = 7.8 Hz, 1H) | 106.2 |
| 2′ | 3.57 (dd, J = 9.4, 9.4, 1H) | 75.5 |
| 3′ | 3.46 (d, J = 1.9, 1H) | 77.8 |
| * 4′ | 3.47 (brs, 1H) | 71.0 |
| * 5′ | 3.30 (brs, 1H) | 78.5 |
| 6′ | 3.72 (d, J = 4.9, 1H) 3.80 (d, J = 2.4, 1H) | 62.2 |
| OCH3-8 | 3.91 (s, 3H) | 57.0 |
| OH-6 | 10.53 | - |
| Position | δH | δC |
|---|---|---|
| 2 | - | 128.0 |
| 3 | - | 107.2 |
| 3a | - | 130.6 |
| 4 | 7.29 (d, J = 7.8 Hz, 1H) | 112.0 |
| 5 | 7.07 (t, J = 7.8 Hz, 1H) | 122.4 |
| 6 | 7.00 (t, J = 7.8 Hz, 1H) | 120.0 |
| 7 | 7.41 (d, J = 7.8 Hz, 1H) | 118.6 |
| 7a | - | 138.1 |
| αCH2 | 3.10 (t, J = 5.8 Hz, 2H) | 54.1 |
| βCH2 | 3.00 (t, J = 5.8 Hz, 2H) | 21.5 |
| CH3 | 2.69 (s, 3H) | 44.9 |
| CH2OH | 3.91 (s, 2H) | 52.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aati, H.Y.; El-Gamal, A.A.; Kayser, O.; Ahmed, A.F. The Phytochemical and Biological Investigation of Jatropha pelargoniifolia Root Native to the Kingdom of Saudi Arabia. Molecules 2018, 23, 1892. https://doi.org/10.3390/molecules23081892
Aati HY, El-Gamal AA, Kayser O, Ahmed AF. The Phytochemical and Biological Investigation of Jatropha pelargoniifolia Root Native to the Kingdom of Saudi Arabia. Molecules. 2018; 23(8):1892. https://doi.org/10.3390/molecules23081892
Chicago/Turabian StyleAati, Hanan Y., Ali A. El-Gamal, Oliver Kayser, and Atallah F. Ahmed. 2018. "The Phytochemical and Biological Investigation of Jatropha pelargoniifolia Root Native to the Kingdom of Saudi Arabia" Molecules 23, no. 8: 1892. https://doi.org/10.3390/molecules23081892