Synthesis and Biological Evaluation of Pyrimidine-oxazolidin-2-arylimino Hybrid Molecules as Antibacterial Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antimicrobial Evaluation
2.3. Biological Results
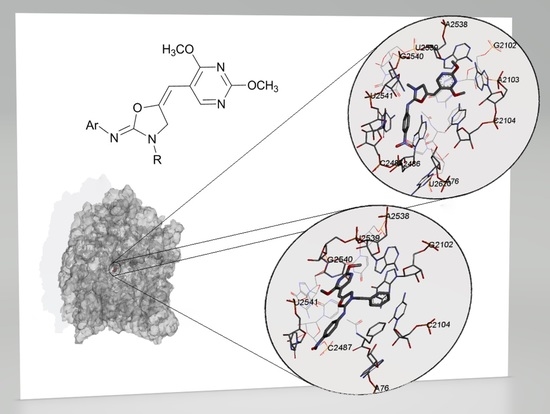
2.4. Molecular Docking Study
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.2. General Procedure for the Preparation of (2Z,5E)-3-substituted-5-((2,4-dimethoxypyrimidin-5-yl)methylidene)-N-(aryl)oxazolidin-2-imino (8a–i)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, G. Synthesis and Antibacterial Properties of Oxazolidinones and Oxazinanones. Anti-Infect. Agents Med. Chem. 2008, 7, 32–49. [Google Scholar] [CrossRef]
- Renslo, A.R.; Luehr, G.W.; Gordeev, M.F. Recent developments in the identification of novel oxazolidinone antibacterial agents. Bioorg. Med. Chem. Lett. 2006, 14, 4227–4240. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.A.; Wright, G.D. Streptogramins, Oxazolidinones, and Other Inhibitors of Bacterial Protein Synthesis. Chem. Rev. 2005, 105, 529–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravestock, M.B. Recent developments in the discovery of novel oxazolidinone antibacterials. Curr. Opin. Drug Discov. Dev. 2005, 8, 469–477. [Google Scholar]
- Bush, K.; Macielag, M.; Weidner-Wells, M. Taking inventory: Antibacterial agents currently at or beyond phase 1. Curr. Opin. Microbiol. 2004, 7, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Bozdogan, M.; Appelbaum, P.C. Oxazolidinones: Activity, mode of action, and mechanism of resistance. Int. J. Antimicrob. Agents 2004, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Barbachyn, M.R.; Ford, C.W. Oxazolidinone structure-activity relationships leading to linezolid. Angew. Chem. Int. Ed. 2003, 42, 2010–2023. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, D.K. Oxazolidinone antibacterial agents: A critical review. Curr. Top. Med. Chem. 2003, 3, 1021–1042. [Google Scholar] [CrossRef] [PubMed]
- Leach, K.L.; Brickner, S.J.; Noe, M.C.; Miller, P.F. Linezolid, the first oxazolidinone antibacterial agent. Ann. N. Y. Acad. Sci. 2011, 1222, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Kanyo, Z.F.; Wang, D.; Franceschi, F.J.; Moore, P.B.; Steitz, T.A.; Duffy, E.M. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J. Med. Chem. 2008, 51, 3353–3356. [Google Scholar] [CrossRef] [PubMed]
- Dickema, D.J.; Jones, R.N. Oxazolidinone antibiotics. Lancet 2001, 358, 1975–1982. [Google Scholar] [CrossRef]
- McKee, E.E.; Ferguson, M.; Bentley, A.T.; Marks, T.A. Inhibition of mammalian mitochondrial protein synthesis by oxazolidinones. Antimicrob. Agents Chemother. 2006, 50, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Nagiec, E.E.; Wu, L.; Swaney, S.M.; Chosay, J.G.; Ross, D.E.; Brieland, J.K.; Leach, K.L. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob. Agents Chemother. 2005, 49, 3896–3902. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.Z.E.; Fleming, P.; Eyermann, C.J.; Gravestock, M.B.; Ramsay, R.R. Orientation of oxazolidinones in the active site of monoamine oxidase. Biochem. Pharmacol. 2005, 70, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Kim, J.; Jo, S.; Yeom, M.; Moon, B.; Choo, I.H.; Lee, J.; Lim, E.J.; Park, K.D.; Min, S.-J.; et al. Oxazolopyridines and thiazolopyridines as monoamine oxidase B inhibitors for the treatment of Parkinson’s disease. Bioorg. Med. Chem. 2013, 21, 5480–5487. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.R.; Adra, M.; Gillman, P.K. Serotonin toxicity associated with the use of linezolid: A review of postmarketing data. Clin. Infect. Dis. 2006, 42, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Meck, J.V.; Martin, D.S.; D’Aunno, D.S.; Waters, W.W. Pressor response to intravenous tyramine is a marker of cardiac, but not vascular, adrenergic function. J. Cardiovasc. Pharmacol. 2003, 41, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.A. Antibacterial agents: Patent highlights July to December 2002. Curr. Opin. Investig. Drugs 2003, 4, 117–127. [Google Scholar] [PubMed]
- Hubschwerlen, C.; Specklin, J.-L.; Sigwalt, C.; Schroeder, S.; Locher, H.H. Design, synthesis and biological evaluation of oxazolidinone-quinolone hybrids. Bioorg. Med. Chem. 2003, 11, 2313–2319. [Google Scholar] [CrossRef]
- Jalander, L.F.; Longquist, J.E. Synthesis of 1,3-Dialkyl- and 1,3-Diphenyl-5-cyano-2-thiouracil Derivatives. Heterocycles 1998, 48, 743–747. [Google Scholar] [CrossRef]
- Srivastava, K.; Agarwal, A.; Chauhan, P.M.; Agarwal, S.K.; Bhaduri, A.P.; Singh, S.N.; Fatima, N.; Chatterjee, R.K. Potent 1,3-Disubstituted-9H-pyrido[3,4-b]indoles as New Lead Compounds in Antifilarial Chemotherapy. J. Med. Chem. 1999, 42, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Ban, M.; Taguchi, H.; Katsushima, T.; Aoki, S.; Watanabe, A. Novel antiallergic agents. Part I: Synthesis and pharmacology of pyrimidine amide derivatives. Bioorg. Med. Chem. 1998, 6, 1057–1067. [Google Scholar] [CrossRef]
- Wright, G.E.; Gambino, J.J. Quantitative structure-activity relationships of 6-anilinouracils as inhibitors of Bacillus subtilis DNA polymerase III. J. Med. Chem. 1984, 27, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Kotadiya, G.M.; Trivedi, A.R. Studies on molecular properties prediction, antitubercular and antimicrobial activities of novel quinoline based pyrimidine motifs. Bioorg. Med. Chem. Lett. 2014, 24, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jeong, K.W.; Shin, S.; Lee, J.U.; Kim, Y. Discovery of novel selective inhibitors of Staphylococcus aureus β-ketoacyl acyl carrier protein synthase III. Eur. J. Med. Chem. 2012, 47, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.C.; Sun, J.; Luo, Y.; Yang, Y.; Zhu, H.L. Design, synthesis, and structure-activity relationships of pyrazole derivatives as potential FabH inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 4657–4660. [Google Scholar] [CrossRef] [PubMed]
- More, P.G.; Karale, N.N.; Lawand, A.S.; Narang, N.; Patil, R.H. Synthesis and anti-biofilm activity of thiazole Schiff bases. Med. Chem. Res. 2014, 23, 790–799. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Kalam Khan, F.A.; Patil, R.H.; Marathe, S.D.; Gade, W.N.; Shinde, D.B. Biofilm inhibition of linezolid-like Schiff bases: Synthesis, biological activity, molecular docking and in silico ADME prediction. Bioorg. Med. Chem. Lett. 2015, 25, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, S.; Beccalli, E.M.; Borelli, T.; Castellano, C.; Chiacchio, M.A.; Diamante, D.; Broggini, G. Copper(II)-Catalyzed Alkoxyhalogenation of Alkynyl Ureas and Amides as a Route to Haloalkylidene-Substituted Heterocycles. J. Org. Chem. 2015, 80, 7226–7234. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.P.; Yang, G.Z.; Zhang, C.; Cheng, J.R.; Liang, J.P.; Shang, R.F. Synthesis and evaluation of novel pleuromutilin derivatives with a substituted pyrimidine moiety. Eur. J. Med. Chem. 2015, 101, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Vipra, A.; Desai, S.N.; Junjappa, R.P.; Roy, P.; Poonacha, N.; Ravinder, P.; Sriram, B.; Padmanabhan, S. Determining the Minimum Inhibitory Concentration of Bacteriophages: Potential Advantages. Adv. Microbiol. 2013, 3, 181–190. [Google Scholar] [CrossRef]
- Ager, S.; Gould, K. Clinical update on linezolid in the treatment of Gram-positive bacterial infections. Infect. Drug Res. 2012, 5, 87–102. [Google Scholar]
- Shaw, K.J.; Barbachyn, M.R. The oxazolidinones: Past, present, and future. Ann. N. Y. Acad. Sci. 2011, 1241, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, D.; Lee, J.H.; Seo, S.H.; Nam, G.; Choo, H.; Kang, S.B.; Kwak, J.H.; Oh, T.; Cho, S.N.; Pae, A.N.; et al. Synthesis and in vitro evaluation of the antitubercular and antibacterial activity of novel oxazolidinones bearing octahydrocyclopenta[c]pyrrol-2-yl moieties. Chem. Pharm. Bull. 2014, 62, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Scwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.; Miglani, R.; Purnapatre, K.P.; Mathur, T.; Singhal, S.; Khan, S.; Voleti, S.R.; Upadhyay, D.J.; Saini, K.S.; Rattan, A.; et al. Mode of action of Ranbezolid against staphylococci and structural modeling studies of its interaction with ribosomes. Antimicrob. Agents Chemother. 2009, 53, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds 8a–i are available from the authors. |






| Entry | Urea | R | Ar | Product (Yield %) |
|---|---|---|---|---|
| 1 | 6a | Me | Ph | 7a (63%)23 |
| 2 | 6b | Me | 1-naphthyl | 7b (73%)23 |
| 3 | 6c | Me | 4-NO2-C6H4 | 7c (68%)23 |
| 4 | 6d | Me | 3-Cl-C6H4 | 7d (75%) |
| 5 | 6e | Me | 4-MeO-C6H4 | 7e (60%) |
| 6 | 6f | Bn | Bn | 7f (63%) |
| 7 | 6g | Bn | 1-naphthyl | 7g (79%) |
| 8 | 6h | Bn | 4-NO2-C6H4 | 7h (76%) |
| 9 | 6i | Bn | 4-MeO-C6H4 | 7i (42%) |
| Entry | Oxazolidines | R | Ar | Product (Yield %) |
|---|---|---|---|---|
| 1 | 7a | Me | Ph | 8a (52%) |
| 2 | 7b | Me | 1-naphthyl | 8b (70%) |
| 3 | 7c | Me | 4-NO2-C6H4 | 8c (77%) |
| 4 | 7d | Me | 3-Cl-C6H4 | 8d (72%) |
| 5 | 7e | Me | 4-MeO-C6H4 | 8e (44%) |
| 6 | 7f | Bn | Bn | 8f (45%) |
| 7 | 7g | Bn | 1-naphthyl | 8g (74%) |
| 8 | 7h | Bn | 4-NO2-C6H4 | 8h (87%) |
| 9 | 7i | Bn | 4-MeO-C6H4 | 8i (50%) |
| Compounds | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|---|
| Bs | Sa | Pa | St | Kb | Ca | Ct | |
| 8a (R=Me; Ar=Ph) | 36 | 45 | 48 | 40 | 120 | 250 | 800 |
| 8b (R=Me; Ar=1-Naft) | 14 | 13 | 24 | 13 | 48 | 140 | 140 |
| 8c (R=Me; Ar=p-NO2) | 4.2 | 4.8 | 13 | 21 | 13 | 24 | 100 |
| 8d (R=Me; Ar=3-Cl) | 300 | 600 | 800 | 400 | 80 | 1000 | 1000 |
| 8e (R=Me; Ar=p-OMe) | 400 | 800 | 800 | 1000 | 600 | 800 | 1000 |
| 8f (R=Bn; Ar=Bn) | 9.5 | 7.5 | 24 | 24 | 14 | 140 | 140 |
| 8g (R=Bn; Ar=1-Naft) | 10 | 12 | 48 | 48 | 24 | 110 | 140 |
| 8h (R=Bn; Ar=p-NO2) | 3.2 | 2.8 | 9.5 | 12 | 12 | 14 | 100 |
| 8i (R=Bn; Ar=p-OMe) | 24 | 19 | 48 | 28 | 30 | 85 | 105 |
| Linezolid | 3 | 4 | 3 | 4 | - | - | - |
| Ciprofloxacin | 3.5 | 3.5 | 3 | 3 | - | - | - |
| Fluconazole | - | - | - | - | - | 20 | 12 |
| Compound | CC50 (μg/mL) |
|---|---|
| 8b | 98 |
| 8c | >200 |
| 8f | 120 |
| 8g | 120 |
| 8h | >200 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, R.; Chiacchio, M.A.; Campisi, A.; Monciino, G.; Veltri, L.; Iannazzo, D.; Broggini, G.; Giofrè, S.V. Synthesis and Biological Evaluation of Pyrimidine-oxazolidin-2-arylimino Hybrid Molecules as Antibacterial Agents. Molecules 2018, 23, 1754. https://doi.org/10.3390/molecules23071754
Romeo R, Chiacchio MA, Campisi A, Monciino G, Veltri L, Iannazzo D, Broggini G, Giofrè SV. Synthesis and Biological Evaluation of Pyrimidine-oxazolidin-2-arylimino Hybrid Molecules as Antibacterial Agents. Molecules. 2018; 23(7):1754. https://doi.org/10.3390/molecules23071754
Chicago/Turabian StyleRomeo, Roberto, Maria A. Chiacchio, Agata Campisi, Giulia Monciino, Lucia Veltri, Daniela Iannazzo, Gianluigi Broggini, and Salvatore V. Giofrè. 2018. "Synthesis and Biological Evaluation of Pyrimidine-oxazolidin-2-arylimino Hybrid Molecules as Antibacterial Agents" Molecules 23, no. 7: 1754. https://doi.org/10.3390/molecules23071754