Isolation and Identification of the Anti-Oxidant Constituents from Loropetalum chinense (R. Brown) Oliv. Based on UHPLC–Q-TOF-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Extraction
2.3. DPPH Assay
2.4. UHPLC–Q-TOF-MS/MS Analysis of the Crude Extract
2.5. Isolation of the Crude Extract
3. Results
3.1. UHPLC–Q-TOF-MS/MS Analysis of the Crude Extract
3.1.1. Fragmentation of Gallic Acid Tannins
3.1.2. Fragmentation of Flavonoids Compounds
3.1.3. Fragmentation of Quinine Acid Compounds
3.2. Structural Identification of Purified Samples
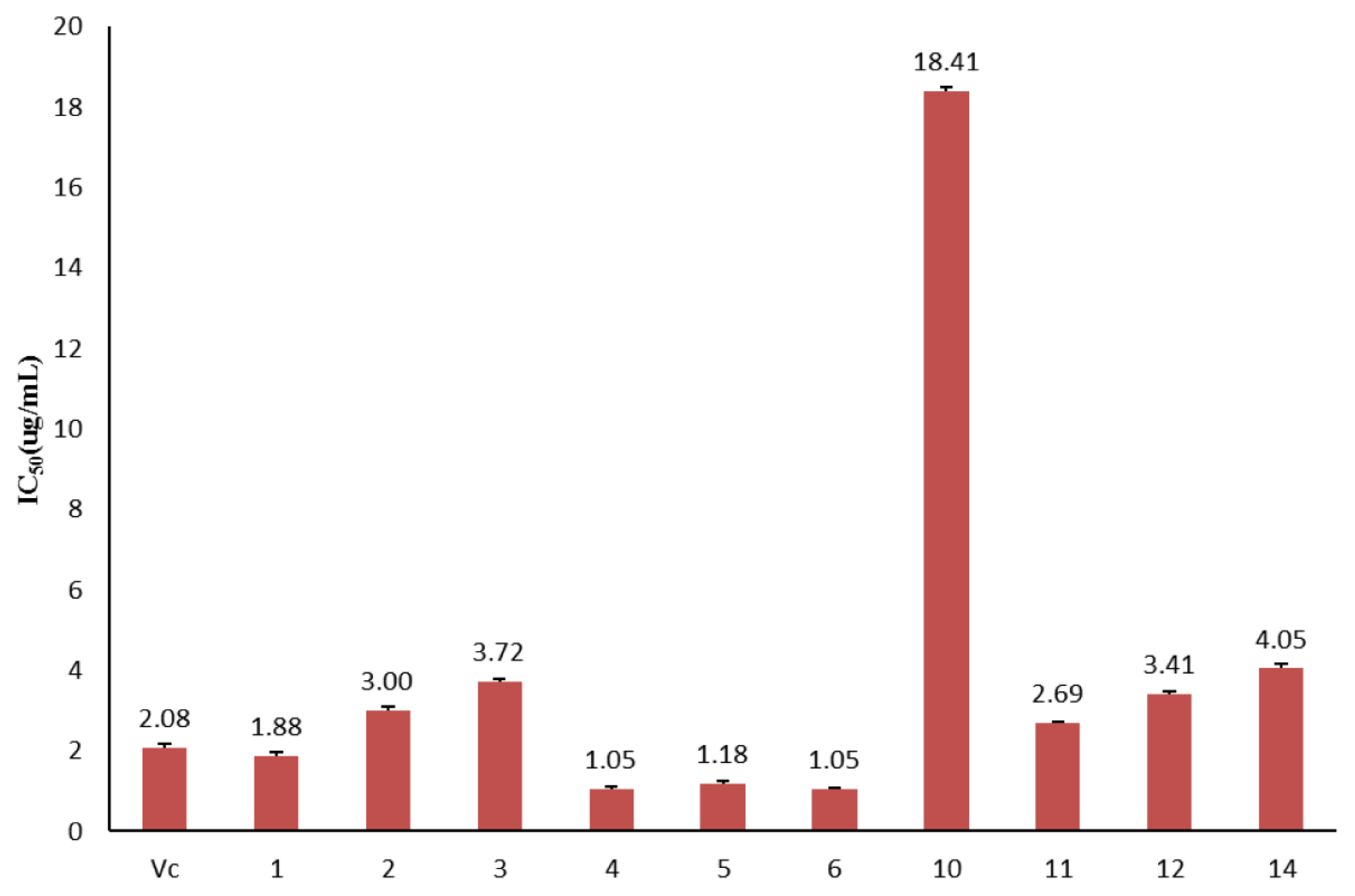
3.3. Antioxidant Activity Analysis of Purified Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Robert, L.; Jia, Z.W.; Michael, A.T. Defining ROS in biology and medicine. React. Oxyg. Species (Apex) 2016, 1, 9–21. [Google Scholar]
- Zhu, H.; Kassim, T.; Arben, S.; Michael, A.T.; Robert, L.Y. Oxygen and oxygen toxicity: The birth of concepts. React. Oxyg. Species (Apex) 2016, 1, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, Z.W; Li, H.M.; Yan, X.F.; Feng, B. AGE/RAGE-Induced EMP release via the Nox-derived ROS pathway. J. Diabetes Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ana, L.S.; Sanchari, S.; Ariel, B.L. The good, the bad, and the ugly of ROS: New insights on aging and aging-related diseases from eukaryotic and prokaryotic model organisms. Oxid. Med. Cell. Longev. 2018, 2018, 1941285. [Google Scholar] [CrossRef]
- Gabriella, A.; Ulla, G.K. NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal. Immunol. 2018. [Google Scholar] [CrossRef]
- Miura, M.; Taguchi, Y.; Handoh, T.; Hasegawa, T.; Takahashi, Y.; Morita, N.; Matsumoto, A.; Sato, H.; Shindoh, C. Regional increase in ROS within stretched region exacerbates arrhythmias in rat trabeculae with nonuniform contraction. Pflugers Arch. 2018. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Gahruie, H.H.; Niakousari, M. Antioxidant, antimicrobial, cell viability and enzymatic inhibitory of antioxidant polymers as biological macromolecules. Int. J. Biol. Macromol. 2017, 104, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Bara, C.I.; Popa, V.I. Cytogenetical effect of some polyphenol compounds separated from industrial by-products on maize (Zea mays L.) plants. Cellul. Chem. Technol. 2015, 49, 799–805. [Google Scholar]
- Miles, E.A.; Zou, B.L.; Calder, P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Cosarca, S.; Toma, F.; Mare, A.; Man, A.; Miklos, A.; Imre, S.; Boz, I. Antibacterial activities of beech bark (Fagus sylvatica L.) polyphenolic extract. Environ. Eng. Manag. J. 2018, 17, 877–884. [Google Scholar]
- Committee for the Pharmacopoeia of People’s Republic of China. Pharmacopoeia of People’s Republic of China, 1977 ed.; Chemical Industry Publishing House: Beijing, China, 1977; p. 528. [Google Scholar]
- Zhang, Y.; Ren, F.C.; Yang, Y.; Wei, L.; Xu, R.; Zhao, Y.M. Chemical constituents and biological activities of Loropetalum chinense and Loropetalum chinense var, rubrum: Research advances. J. Int. Pharm. Res. 2014, 41, 307–312. [Google Scholar]
- Takashi, Y.; Shigeharu, T.; Yanze, L.; Yuan, K.; Ji, C.R.; Takuo, O. Hydrolysable tannins from Loropetalum Chinense. Phytochemistry 1993, 32, 1287–1292. [Google Scholar]
- Takashi, Y.; Osamu, N.; Chen, L.; Liu, Y.Z.; Takuo, O. Ellagitannin monomers and oligomers from Euphorbia prostrate Ait. and oligomers from Loropetalum chinense oliv. Chem. Pharm. Bull. (Tokyo) 1990, 38, 3296–3302. [Google Scholar]
- Liu, Y.Z.; Wu, Y.Y.; Yuan, K.; Ji, C.R.; Hou, A.J.; Takashi, Y.; Takuo, O. Astragalin 2′′,6′′-Di-O-gallate from Loropetalum chinense. Phytochemistry 1997, 46, 389–391. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Fan, D.; Xiong, B.J.; Kong, L.B.; Zhu, X.D. Isolation of new flavan-3-ol and lignin glucoside from Loropetalum chinense and their antimicrobial activities. Fitoterapia 2013, 90, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.H.; He, M.Z.; Zhang, W.G.; Ou, Y.H.; Feng, Y.L.; Yang, S.L. Determination of the Antioxidant Activity of Ethanol Extract and Its Difierent Polarity Fractions from Loropetalum chinense with DPPH Assay in vitro. Lishizhen Med. Mater. Med. Res. 2013, 23, 1577–1579. [Google Scholar]
- Xie, Y.; Shao, H.H.; Song, Y.G.; Liu, Y.T.; Zhang, W.G.; Feng, Y.L.; Yang, S.L. Extraction technology of gallic acid and total polyphenols from Loropetalum chinense. Chin. Med. Pharmacol. Clin. 2012, 18, 9–12. [Google Scholar]
- Bakhta, A.; Amira, M.S.; Hamadi, F.; Monique, S.J.S.; Mohamed, B. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomed. Pharmacother. 2016, 84, 1088–1098. [Google Scholar]
- Isabel, M.; Leandro, L.; Teresa, Q.; Sofia, K.; Helena, P. The bark of Eucalyptus sideroxylon as a source of phenolic extractswith anti-oxidant properties. Ind. Crop. Prod. 2016, 82, 81–87. [Google Scholar]
- Henrique, S.A.; Gustavo, A.P.; Damila, R.M.; Marcos, N.E.; Glaucia, M.P. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food. Chem. 2018, 245, 738–749. [Google Scholar]
- Wang, L.L.; Sang, M.M.; Liu, E.W.; Prince, O.B.; Zhang, Y.; Wang, T.; Han, L.F.; Gao, X.M. Rapid profiling and pharmacokinetic studies of major compounds incrude extract from Polygonum multiflorum by UHPLC-Q-TOF-MS and UPLC–MS/MS. J. Pharm. Biomed. 2017, 140, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Y.; Bushra, S.; Matthew, A. Biological activities of phenolic compounds extracted from Amaranthaceae plants and their LC/ESI-MS/MS profiling. J. Funct. Foods 2016, 26, 645–656. [Google Scholar]
- Zhang, L.; Tu, Z.C.; Xie, X.; Lu, Y.; Wang, Z.X.; Wang, H.; Sha, X.M. Antihyperglycemic, antioxidant activities of two Acer palmatum cultivars, and identification of phenolics profile by UPLC-QTOF-MS/MS: New natural sources of functional constituents. Ind. Crop. Prod. 2016, 89, 522–532. [Google Scholar] [CrossRef]
- Sunil, K.; Awantika, S.; Brijesh, K. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. Chin. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar]
- Qin, Y.; Gao, B.Y.; Shi, H.M.; Cao, J.; Yin, C.L.; Lu, W.Y.; Yu, L.L.; Cheng, Z.H. Characterization of flavonol mono-, di-, tri- and tetra-O-glycosides byultra-performance liquid chromatography-electrosprayionization-quadrupole time-of-flight mass spectrometry and itsapplication for identification of flavonol glycosides in Violatianschanica. J. Pharm. Biomed. 2017, 142, 113–124. [Google Scholar]
- Shan, L.L.; Wu, Y.Y.; Yuan, L.; Zhang, Y.N.; Xu, Y.Y.; Li, Y.B. Rapid screening of Chemical constituents in Rhizoma anemarrhenae by UPLC-Q-TOF/MS combined with data postprocessing techniques. Evid. Based Complement. Altern. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Yu, Y.; Wang, Z.Z.; Geng, J.L.; Dai, Y.; Xiao, W.; Yao, X.S. Chemical profiling of Re-Du-Ning injection by ultra-performance liquid chromatography coupled with electrospray ionization tandem quadrupole time-of-flight mass spectrometry through the screening of diagnostic ions in MSE mode. PLoS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Baskaran, S.I.; Chen, B.H. Determination of phenolic acids and flavonoids in Taraxacum formosanum kitam by liquid chromatography tandem mass spectrometry coupled with a post column derivatization technique. Int. J. Mol. Sci. 2012, 13, 260–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Long, X.H.; Liu, Z.Q.; Shao, H.B.; Liu, L. Analysis of phenolic acids of jerusalem artichoke (Helianthus tuberosus L.) responding to salt-stress by liquid chromatography/tandem mass spectrometry. Sci. World J. 2014, 1–8. [Google Scholar]
- Shao, X.; Zhao, J.; Wang, X.; Tao, Y. Rapid screening and quantitative determination of active components in qing-hua-yu-re-formula using UHPLC-Q-TOF/MS and HPLC-UV. J. Anal. methods Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.G.; Chen, H.F.; Shao, H.H.; Feng, Y.L.; Zhong, Y.Q.; Yang, S.L. Hemostatic activity and ingredients of Loropetalum chinense. Chin. J. Exp. Tradit. Med. Formul. 2017, 23, 47–52. [Google Scholar]
- Mohan, C.G.; Viswanatha, G.L.; Savinay, G.; Rajendra, C.E.; Halemani, P.D. 1,2,3,4,6 Penta-O-galloyl--d-glucose, a bioactivity guided isolated compound from Mangifera indica inhibits 11β-HSD-1 and ameliorates high fat diet-induced diabetes in C57BL/6 mice. Phytomedicine 2013, 20, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Luisella, V.; Mario, D.A.; Andrea, G.; Marco, G.; Pierre, C.; Enrica, B. In vitro antiplasmodial activity of extracts of Tristaniopsis species and identification of the active constituents: Ellagic acid and 3,4,5-Trimethoxyphenyl-(6′-O-galloyl)-O-a-d-glucopyranoside. J. Nat. Prod. 2001, 64, 603–607. [Google Scholar]
- Klaus, P.L.; Maki, K.; Andreas, S.; Herbert, K. An ellagitannin, n-butyl gallate, two aryltetralin lignans, and an unprecedented diterpene ester from Pelargonium reniforme. Phytochemistry 2008, 69, 820–826. [Google Scholar]
- Liu, M.C.; Yang, S.J.; Jin, L.H.; Hu, D.Y.; Wu, Z.B.; Yang, S. Chemical constituents of the ethyl acetate extract of Belamcanda chinensis (L.) DC roots and their antitumor activities. Molecules 2012, 17, 6156–6169. [Google Scholar] [CrossRef] [PubMed]
- Yasmine, C.; Samir, B.; Eric, M.; Zhao, M.J.; Paul, M.; Fadila, B. On-line screening, isolation and identification of antioxidant compounds of Helianthemum ruficomum. Molecules 2017, 22, 239. [Google Scholar]
- Li, K.; Lin, Y.; Li, B.; Pan, T.W.; Wang, F.; Yuan, R.Q.; Ji, J.J.; Diao, Y.P.; Wang, S.Y. Antibacterial constituents of fructus chebulae immaturus and their mechanisms of action. BMC Complement. Altern. Med. 2016, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Serge, L.; Isabelle, C.; André, P.; Charles, G.; Michaël, O.; Francine, N.L.; Vakhtang, M.; Jean, L. Chemical composition and anti-herpes simplex virus type 1 (HSV-1) activity of extracts from Cornus canadensis. BMC Complement. Altern. Med. 2017, 17, 123. [Google Scholar]
- Wan, C.P.; Li, S.S.; Liu, L.; Chen, C.Y.; Fan, S.Y. Caffeoylquinic Acids from the Aerial Parts of Chrysanthemum coronarium L. Plants (Basel) 2017, 6, E10. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1,2,3,4,6-penta-O-galloyl-β-d-glucose, 3,4,5-trimethoxyphenyl-(6′-O-galloyl)-O-β-d-glucopyranoside, 6′-O-galloylsalidroside, gallic acid, protocatechuic acid, ethyl gallate, tiliroside, 3-O-coumaroylquinicacid, kaempferol-3-O-β-d-galactopyranosyl-(1→6)-β-d-glucopyranoside, kaempferol-3-O-β-d-galactopyranoside, quercetin-3-O-β-d-glucopyranoside, 5-O-caffeoylquinic acid, 3-O-coumaroylquinic acid methyl ester, 3-O-caffeoylquinic acid, 5-O-coumaroylquinic acid, 3,5-O-diocaffeoylquinic acid, 4,5-O-diocaffeoylquinic acid, 3,4-O-diocaffeoylquinic acid are available from the authors. |


| No. | tR/Min | Formula | Error/ppm | Adduct | Found at Mass/Da | MS2 Ions | Indentification |
|---|---|---|---|---|---|---|---|
| 1 | 1.21 | C14H16O10 | −0.1 | [M + H]+ | 345.0815 | 171.0275, 153.0179, 125.0235 | Theogallin |
| 2 | 1.32 | C13H16O10 | 0.2 | [M − H]− | 331.0671 | 331.0671, 169.0144, 125.0243 | Monogalloyl glucose |
| 3 | 1.32 | C7H12O6 | −0.8 | [M + H]+ | 193.0706 | 175.0608, 147.0656, 129.0551 | Quinic acid |
| 4 | 1.66 | C14H16O10 | 0.5 | [M + H]+ | 345.0818 | 327.0726, 171.0289, 153.0185, 125.0232 | Theogallin |
| 5 | 1.75 | C7H6O5 | −2.4 | [M + H]+ | 171.0284 | 153.0180, 135.0074, 125.0234, 109.0286, 107.0128, 97.0283 | Gallic acid |
| 6 | 1.97 | C13H16O10 | 0.7 | [M − H]− | 331.0673 | 331.0673, 169.0130, 125.0241 | Monogalloyl glucose |
| 7 | 2.35 | C13H16O10 | −0.1 | [M − H]− | 331.0672 | 331.0672, 169.0144, 125.0242 | Monogalloyl glucose |
| 8 | 2.86 | C13H16O10 | −0.1 | [M − H]− | 331.0671 | 331.0671, 169.0142, 125.0249 | Monogalloyl glucose |
| 9 | 3.63 | C7H6O4 | −1.7 | [M + H]+ | 155.0336 | 137.0230, 109.0288, 107.0112, 93.0343, 81.0343 | Protocatechuic acid |
| 10 | 3.63 | C27H22O18 | 2.6 | [M − H]− | 633.0750 | 633.0750, 481.0649, 300.0992, 169.0153, 125.0232 | Corilagin |
| 11 | 3.64 | C13H16O10 | 0.9 | [M − H]− | 331.0674 | 331.0685, 169.0148, 151.0031, 125.0257 | Monogalloyl glucose |
| 12 | 4.06 | C20H20O14 | 1 | [M − H]− | 483.0785 | 483.0786, 331.0673, 313.0568, 169.0145, 151.0038, 125.0249 | Digalloylglucose |
| 13 | 4.07 | C19H26O15 | 0.3 | [M − H]− | 493.1201 | 493.1201, 331.0648, 169.0142, 125.0248 | Gallic acid diglucoside |
| 14 | 4.26 | C13H16O10 | −0.1 | [M − H]− | 331.0670 | 331.0670, 169.0141, 125.0250 | Monogalloyl glucose |
| 15 | 4.49 | C19H26O15 | 1 | [M − H]− | 493.1204 | 493.1204, 331.0688, 313.0565, 169.0149, 125.0243 | Gallic acid diglucoside |
| 16 | 4.52 | C27H22O18 | 3.4 | [M − H]− | 633.0752 | 633.0752, 481.0608, 463.0505, 300.0992, 169.0138, 125.0255 | Corilagin |
| 17 | 4.77 | C8H8O5 | −3.8 | [M + H]+ | 185.0437 | 125.0305, 107.0121, 81.0340 | Methyl gallate |
| 18 | 4.79 | C20H20O14 | 0.8 | [M − H]− | 483.0784 | 483.0784, 331.0676, 313.0566, 169.0150, 151.0041, 125.0254 | Digalloylglucose |
| 19 | 5.31 | C27H22O18 | 3.5 | [M − H]− | 633.0755 | 633.0755, 463.0597, 300.0980, 169.0152 | Corilagin |
| 20 | 5.33 | C20H20O14 | 1.2 | [M − H]− | 483.0786 | 483.0786, 331.0677, 313.0571, 169.0143, 151.0035, 125.0249 | Digalloylglucose |
| 21 | 5.81 | C27H22O18 | 2.8 | [M − H]− | 633.0751 | 633.0751, 481.0757, 463.0505, 300.0989, 169.0143, 125.0246 | Corilagin |
| 22 | 5.89 | C16H18O9 | 0.7 | [M − H]− | 353.0889 | 191.0565, 179.0347, 173.0445, 161.0249, 135.0447 | Caffeoylquinic acid |
| 23 | 5.99 | C27H24O18 | −3 | [M − H]− | 635.0955 | 635.0955, 483.0799, 465.0670, 331.0694, 313.0574, 169.0143, 125.0253 | Trigalloylglucopyranose |
| 24 | 6.02 | C14H10O9 | −0.8 | [M + H]+ | 323.0395 | 153.0179, 125.0233, 79.0185 | Digallic acid |
| 25 | 6.84 | C8H8O5 | −1.1 | [M + H]+ | 185.0442 | 153.0183, 135.0078, 125.0236, 107.0103, 97.0282 | Methyl gallate |
| 26 | 6.88 | C27H24O18 | 2.9 | [M − H]− | 635.0908 | 635.0908, 483.0788, 465.0700, 331.0683, 313.0579, 169.0149, 125.0252 | Trigalloylglucopyranose |
| 27 | 6.92 | C27H22O18 | 3.5 | [M − H]− | 633.0749 | 633.0749, 300.0981, 169.0150, 125.0239 | Corilagin |
| 28 | 7.09 | C16H18O9 | 0.3 | [M − H]− | 353.0878 | 191.0554, 179.0344, 173.0446, 161.0242, 135.0447 | Chlorogenic acid |
| 29 | 7.43 | C27H30O16 | −1.1 | [M + H]+ | 611.1784 | 611.1784, 449.1066, 287.0559 | Panasenoside |
| 30 | 7.50 | C27H24O18 | 3.3 | [M − H]− | 635.0910 | 635.0913, 483.0789, 465.0687, 331.0698, 313.0567, 169.0145, 125.0241 | Trigalloylglucopyranose |
| 31 | 7.51 | C27H22O18 | 3.2 | [M − H]− | 633.0753 | 633.0753, 481.0729, 300.0991, 169.0145, 125.0255 | Corilagin |
| 32 | 7.63 | C9H10O5 | −2 | [M + H]+ | 199.0597 | 181.0502, 153.0190, 140.0470, 125.0232, 107.0121, 97.0288 | Ethyl gallate |
| 33 | 7.87 | C15H10O5 | 0.2 | [M + H]+ | 271.0599 | 271.0599, 215.0699, 177.0559, 169.0641, 153.0569, 149.0246, 119.0509 | Apigenin |
| 34 | 7.87 | C22H18O11 | −1.4 | [M + H]+ | 459.0915 | 459.0915, 307.0381, 289.0714, 163.0391, 153.0178, 151.0391, 139.0389 | Epigallocatechin Gallate |
| 35 | 7.97 | C27H30O16 | −2.2 | [M + H]+ | 611.1593 | 449.1059, 303.0484, 287.0534, 267.0019, 145.0489, 85.0278 | Rutin |
| 36 | 8.14 | C16H18O8 | 0.3 | [M + H]+ | 339.1073 | 165.0545, 147.0441, 119.0489, 91.0548, 65.0396 | Coumaroylquinic acid |
| 37 | 8.18 | C16H18O9 | 0.4 | [M − H]− | 353.0878 | 191.0558, 179.0328, 173.0328, 161.0243, 135.0456 | Caffeoylquinic acid |
| 38 | 8.18 | C27H24O18 | 3.3 | [M − H]− | 635.0911 | 635.0911, 483.0789, 465.0690, 313.0567, 169.0143, 125.0247 | Trigalloylglucopyranose |
| 39 | 8.97 | C21H24O11 | −1.1 | [M + H]+ | 453.1386 | 453.1386, 406.9998, 315.0727, 297.0602, 255.0507, 171.0283, 153.0178, 127.0393 | Galloylsalidroside |
| 40 | 9.08 | C17H20O9 | −0.8 | [M + H]+ | 369.1177 | 195.0656, 177.0547, 149.0588, 145.0283, 134.0349, 117.0344, 89.0394 | Feruloylquinic acid |
| 41 | 9.10 | C27H24O18 | 1.7 | [M − H]− | 635.0901 | 635.0901, 483.0800, 465.0679, 331.0694, 313.0566, 169.0140, 125.0253 | Trigalloylglucopyranose |
| 42 | 9.20 | C34H28O22 | 2.8 | [M − H]− | 787.1021 | 787.1021, 635.0919, 617.0798, 483.0752, 465.0682, 313.0566, 169.0142, 125.0249 | Tetrakisgalloylglucopyranose |
| 43 | 9.35 | C16H18O8 | 0.3 | [M + H]+ | 339.1073 | 147.0440, 119.0492, 91.0548, 65.0395 | Coumaroylquinic acid |
| 44 | 9.85 | C41H32O26 | −6.3 | [M − H]− | 939.1050 | 939.1050, 787.0948, 769.0879, 635.0942, 617.0738, 313.0576, 169.0147 | Pentagalloylglucopyranose |
| 45 | 10.00 | C34H28O22 | 3.4 | [M − H]− | 787.1026 | 787.1026, 635.0921, 617.0811, 483.0785, 465.0679, 313.0555, 169.0141, 125.0245 | Tetrakisgalloylglucopyranose |
| 46 | 10.21 | C22H26O13 | −0.9 | [M + H]+ | 499.1442 | 315.0719, 297.0616, 275.0925, 255.0505, 185.0814, 171.0294, 153.0189, 127.0389 | 1-O-3′,4′,5′-trimethoxyphenyl-(6-O-galloyl)-β-d-glucopyranoside |
| 47 | 10.22 | C28H24O16 | −0.9 | [M + H]+ | 617.1131 | 617.1131, 447.0910, 303.0503, 297.0610, 153.0184 | Galloylhyperin |
| 48 | 10.29 | C34H28O22 | 3.5 | [M − H]− | 787.1027 | 787.1027, 635.0934, 617.0827, 483.0827, 465.0697, 313.0571, 169.0152, 125.0255 | Tetrakisgalloylglucopyranose |
| 49 | 10.51 | C28H24O16 | −1.9 | [M + H]+ | 617.1129 | 617.1147, 447.0920, 237.0385, 153.0189 | Galloylhyperin |
| 50 | 10.51 | C22H18O10 | −0.9 | [M + H]+ | 443.0969 | 273.0757, 165.0549, 153.0179, 151.0386, 147.0431, 139.0385, 123.0438 | Catechin gallate |
| 51 | 10.52 | C15H12O5 | −0.3 | [M + H]+ | 273.0757 | 273.0760, 163.0402, 153.0192, 147.0424, 135.0436, 123.0440, 105.0326 | Naringenin |
| 52 | 10.63 | C21H18O13 | −1.2 | [M + H]+ | 479.0814 | 317.0294, 285.0024, 257.0061 | Shikimic acid-O-digallate |
| 53 | 10.63 | C34H28O22 | 3.6 | [M − H]− | 787.1028 | 787.1028, 635.0925, 617.0823, 483.0829, 465.0656, 313.0557, 169.0143, 125.0246 | Tetrakisgalloylglucopyranose |
| 54 | 10.76 | C14H6O8 | 0.3 | [M + H]+ | 303.0140 | 303.0140, 285.0035, 275.0189, 257.0082, 229.0130, 201.0179, 173.0232, 145.0284 | Ellagic acid |
| 55 | 10.78 | C15H10O8 | 0.1 | [M + H]+ | 319.0449 | 319.0450, 301.0346, 290.0422, 273.0393, 245.0444, 217.0503, 165.0176, 153.0182 | Myricetin |
| 56 | 10.94 | C21H20O12 | −0.6 | [M + H]+ | 465.1028 | 319.0453, 303.0507, 285.0388, 257.0455, 229.0497, 145.0498, 127.0387, 97.0290 | Myricitrin |
| 57 | 10.96 | C15H10O7 | −0.7 | [M + H]+ | 303.0497 | 303.0497, 285.0037, 275.0176, 257.0436, 229.0493, 153.0181 137.0229 | Isomer of Quercetin |
| 58 | 11.21 | C21H20O12 | −0.7 | [M + H]+ | 465.1025 | 303.0508, 257.0446, 229.0492, 165.0176, 145.0491, 127.0389, 97.0289, 85.0289 | Hyperoside |
| 59 | 11.22 | C15H10O7 | 0.2 | [M + H]+ | 303.0503 | 303.0503, 285.0389, 257.0457, 229.0498, 153.0181 137.0232 | Isomer of Quercetin |
| 60 | 11.40 | C21H20O11 | −1.1 | [M + H]+ | 449.1073 | 287.0545, 153.0178 | Luteoloside |
| 61 | 11.66 | C41H32O26 | 2.4 | [M − H]− | 939.1131 | 939.1131, 787.0943, 769.0877, 635.0796, 617.0789, 313.0644, 169.0141, 125.0259 | Pentagalloylglucopyranose |
| 62 | 11.73 | C15H10O7 | −0.7 | [M + H]+ | 303.0497 | 303.0497, 285.0367, 257.0453, 229.0478, 153.0186, 137.0591 | Isomer of Quercetin |
| 63 | 11.96 | C20H18O11 | −1 | [M + H]+ | 435.092 | 303.0500, 285.0415, 257.0448, 229.0493, 153.0179, 137.0217 | Isomer of guaijaverin |
| 64 | 12.06 | C15H10O6 | 0.2 | [M + H]+ | 287.0542 | 287.0542, 258.0511, 241.0482, 213.0550, 165.0167, 153.0178, 137.0218, 121.0282 | Isomer of Kaempferol |
| 65 | 12.07 | C21H20O11 | −0.3 | [M + H]+ | 449.1075 | 287.0560, 165.0177, 153.0182 | Isomer of luteoloside |
| 66 | 12.16 | C28H24O15 | 6.6 | [M − H]− | 599.1088 | 599.1088, 447.0969, 313.0582, 285.0414, 169.0147, 151.0036, 125.0245 | Astragalin-O-gallate |
| 67 | 12.22 | C41H32O26 | 2.6 | [M − H]− | 939.1133 | 939.1133, 787.1020, 769.0979, 635.0901, 617.0780, 313.0514, 169.0142, 125.0262 | Pentagalloylglucopyranose |
| 68 | 12.54 | C25H24O12 | 0.6 | [M − H]− | 515.1195 | 515.1195, 353.0883, 191.0556, 179.0343, 135.0449 | Dicaffeoylquinic acids |
| 69 | 12.55 | C15H10O6 | 0.3 | [M + H]+ | 287.0552 | 287.0552, 258.0525, 241.0483, 213.0533, 165.0188, 157.0469, 153.0183, 121.0285 | Isomer of Kaempferol |
| 70 | 12.63 | C21H20O11 | −0.7 | [M + H]+ | 449.1075 | 303.0511, 287.0562, 165.0178, 145.0496, 129.0548, 127.0387 | Quercitrin |
| 71 | 12.67 | C15H10O7 | 0.6 | [M + H]+ | 303.0505 | 303.0505, 285.0395, 257.0445, 229.0494, 153.0182, 137.0231 | Isomer of Quercetin |
| 72 | 12.97 | C16H12O7 | 1.8 | [M + H]+ | 317.0653 | 317.0653, 302.0437, 285.0415, 274.0482, 246.0531, 229.0483, 153.0182 | Isomer of isorhamnetin |
| 73 | 12.99 | C28H24O15 | 7.6 | [M − H]− | 599.1064 | 599.1064, 447.0947, 313.0577, 285.0408, 169.0136, 151.0035, 125.0238 | Astragalin-O-gallate |
| 74 | 13.02 | C15H10O6 | 0.4 | [M + H]+ | 287.0552 | 287.0552, 258.0533, 231.0647, 213.0545, 165.0171, 153.0175, 137.0236, 121.0286 | Isomer of Kaempferol |
| 75 | 13.04 | C35H28O19 | −1.8 | [M + H]+ | 753.1284 | 467.0822.449.0706, 315.0705, 287.0552, 153.0181, 125.0236 | Astragalin-O-digallate |
| 76 | 13.30 | C15H10O6 | 0.4 | [M + H]+ | 287.0561 | 287.0561, 258.0566, 213.0567, 165.0193, 153.0175, 147.0429, 137.0240 | Isomer of Kaempferol |
| 77 | 13.68 | C23H24O12 | −1.6 | [M + H]+ | 493.1332 | 493.1332, 331.0819, 315.0505, 270.0515 | Tricin-O-glucopyranoside |
| 78 | 13.72 | C35H28O19 | −2 | [M + H]+ | 753.1283 | 753.1261, 601.1206, 467.0812, 449.0707, 287.0547, 237.0393, 153.0185 | Astragalin-O-digallate |
| 79 | 14.27 | C15H10O6 | 0.5 | [M + H]+ | 287.0546 | 287.0546, 258.0527, 241.0491, 213.0551, 165.0180, 153.0181, 137.0233, 121.0285 | Isomer of Kaempferol |
| 80 | 14.27 | C21H20O10 | −1 | [M + H]+ | 433.1125 | 287.0553, 165.0183, 129.0542, 85.0285, 71.0498 | Kaempferol-O-rhamnoside |
| 81 | 14.32 | C23H24O12 | −1.5 | [M + H]+ | 493.3333 | 331.0814, 315.0496, 270.0519 | Isomer of tricin-O-glucopyranoside |
| 82 | 14.32 | C35H28O19 | −2.5 | [M + H]+ | 753.1279 | 753.1366, 601.1066, 467.0804, 449.0691, 287.0543, 237.0403, 153.0183 | Isomer of astragalin-di-O-gallate |
| 83 | 14.64 | C16H12O7 | 1.3 | [M + H]+ | 317.0649 | 317.0649, 302.0442, 285.0392, 246.0505, 175.9679, 153.0188, 139.0399 | Isomer of isorhamnetin |
| 84 | 14.69 | C30H26O13 | 9.4 | [M − H]− | 593.1360 | 593.1360, 447.0949, 307.0825, 285.0413, 163.0403, 151.0030, 145.0290, 119.0508 | Isomer of Tribuloside |
| 85 | 16.27 | C15H10O7 | 0.4 | [M + H]+ | 303.0503 | 303.0503, 285.0399, 257.0445, 229.0491, 201.0552, 153.0182, 137.0230 | Quercetin |
| 86 | 16.36 | C15H10O6 | −0.6 | [M + H]+ | 287.0553 | 287.0553, 161.0234, 153.0183, 135.0434 | Isomer of Kaempferol |
| 87 | 16.72 | C16H12O7 | −0.7 | [M + H]+ | 317.0569 | 317.0569, 302.0420, 274.0469, 228.0420, 153.0170, 147.0435 | Isorhamnetin |
| 88 | 16.88 | C15H10O6 | 0.8 | [M + H]+ | 287.0547 | 287.0547, 258.0507, 241.0461, 213.0539, 165.0182, 153.0179, 121.0281 | Isomer of Kaempferol |
| 89 | 16.89 | C30H26O13 | 6.8 | [M − H]− | 593.1341 | 593.1341, 447.0955, 307.0835, 285.0399, 163.0398, 151.0038, 145.0296, 119.0506 | Tribuloside |
| 90 | 16.89 | C22H22O10 | −1.3 | [M + H]+ | 447.128 | 301.0705, 286.0479, 258.0536, 153.0179 | Methylluteolin-O-rhamnopyranosid |
| 91 | 17.34 | C17H14O7 | −0.2 | [M + H]+ | 331.0812 | 331.0820, 315.0498, 286.0462, 270.0522, 258.0520 | Quercetin-dimethyl ether |
| 92 | 17.39 | C15H10O6 | 0.8 | [M + H]+ | 287.055 | 287.0550, 258.0539, 241.0463, 213.0522, 165.0175, 153.0190, 121.0279 | Isomer of Kaempferol |
| 93 | 17.39 | C30H26O13 | 5.8 | [M − H]− | 593.1354 | 593.1354, 447.0938, 307.0828, 285.0403, 163.0396, 151.0031, 145.0290, 119.0505 | Isomer of Tribuloside |
| 94 | 17.48 | C22H22O10 | −0.9 | [M + H]+ | 447.1281 | 301.0710, 286.0490, 258.0527 | Methylluteolin-O-rhamnopyranosid |
| 95 | 18.11 | C15H12O5 | −0.8 | [M + H]+ | 273.0755 | 273.0736, 164.8737, 153.0183, 147.0432, 121.0273, 91.0557 | Isomer of naringenin |
| 96 | 19.05 | C15H10O6 | 0.9 | [M + H]+ | 287.0557 | 287.0557, 258.0527, 241.0494, 231.0652, 213.0550, 165.0185, 153.0184, 121.0286 | Kaempferol |
| 97 | 19.52 | C17H14O7 | 0.7 | [M + H]+ | 331.0183 | 331.0819, 315.0505, 286.0477, 270.0529, 258.0529, 242.0583 | Quercetin-dimethyl ether |
| 98 | 19.76 | C16H12O7 | −0.9 | [M + H]+ | 317.0657 | 317.0657, 302.0424, 274.0464, 153.0185 | Isomer of isorhamnetin |
| 99 | 19.97 | C20H18O11 | −1.1 | [M + H]+ | 435.0924 | 435.0924, 237.0398, 153.0175, 127.0406 | Guaijaverin |
| 100 | 20.71 | C17H14O7 | −0.3 | [M + H]+ | 331.0809 | 331.0807, 315.0495, 286.0538, 270.0528, 242.0549, 168.0608 | Quercetin-dimethyl ether |
| Vc | Concentration (µg/mL) | 0.73 | 1.16 | 1.45 | 2.18 | 2.90 |
| Inhibition rate (%) | 18.71 ± 0.17 | 24.26 ± 0.36 | 34.79 ± 0.47 | 46.19 ± 0.28 | 69.15 ± 0.45 | |
| 1 | Concentration (µg/mL) | 0.88 | 1.75 | 2.63 | 2.98 | 3.50 |
| Inhibition rate (%) | 20.03 ± 0.15 | 37.09 ± 0.22 | 63.64 ± 0.26 | 69.15 ± 0.40 | 85.93 ± 0.11 | |
| 2 | Concentration (µg/mL) | 2.31 | 2.78 | 3.24 | 3.70 | 4.63 |
| Inhibition rate (%) | 29.08 ± 0.20 | 45.43 ± 0.31 | 52.63 ± 0.11 | 62.32 ± 0.35 | 87.81 ± 0.13 | |
| 3 | Concentration (µg/mL) | 2.33 | 3.72 | 4.65 | 5.58 | 6.98 |
| Inhibition rate (%) | 25.99 ± 0.14 | 46.53 ± 0.15 | 59.59 ± 0.31 | 71.43 ± 0.24 | 87.76 ± 0.16 | |
| 4 | Concentration (µg/mL) | 0.52 | 0.87 | 1.21 | 1.56 | 2.08 |
| Inhibition rate (%) | 22.33 ± 0.25 | 38.75 ± 0.27 | 52.23 ± 0.36 | 67.66 ± 0.40 | 82.14 ± 0.43 | |
| 5 | Concentration (µg/mL) | 0.76 | 1.01 | 1.68 | 1.89 | 2.10 |
| Inhibition rate (%) | 26.66 ± 0.37 | 38.02 ± 0.33 | 67.52 ± 0.28 | 78.21 ± 0.19 | 81.00 ± 0.40 | |
| 6 | Concentration (µg/mL) | 0.61 | 1.01 | 1.41 | 1.82 | 2.02 |
| Inhibition rate (%) | 26.25 ± 0.27 | 42.90 ± 0.32 | 59.54 ± 0.33 | 76.50 ± 0.27 | 86.47 ± 0.33 | |
| 10 | Concentration (µg/mL) | 10.63 | 21.25 | 25.5 | 31.88 | 42.50 |
| Inhibition rate (%) | 27.55 ± 0.29 | 50.94 ± 0.19 | 61.29 ± 0.26 | 78.36 ± 0.41 | 87.37 ± 0.37 | |
| 11 | Concentration (µg/mL) | 1.38 | 3.22 | 3.68 | 4.14 | 9.20 |
| Inhibition rate (%) | 25.40 ± 0.27 | 47.85 ± 0.26 | 63.16 ± 0.29 | 75.07 ± 0.33 | 88.54 ± 0.33 | |
| 12 | Concentration (µg/mL) | 0.99 | 2.46 | 7.39 | 9.85 | 12.31 |
| Inhibition rate (%) | 22.33 ± 0.39 | 46.55 ± 0.40 | 68.06 ± 0.50 | 72.53 ± 0.31 | 74.79 ± 0.25 | |
| 14 | Concentration (µg/mL) | 2.23 | 3.56 | 4.45 | 6.68 | 8.01 |
| Inhibition rate (%) | 27.06 ± 0.19 | 39.51 ± 0.51 | 46.28 ± 0.37 | 66.85 ± 0.24 | 88.77 ± 0.31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Li, M.; Zhang, C.; Du, W.; Shao, H.; Feng, Y.; Zhang, W.; Yang, S. Isolation and Identification of the Anti-Oxidant Constituents from Loropetalum chinense (R. Brown) Oliv. Based on UHPLC–Q-TOF-MS/MS. Molecules 2018, 23, 1720. https://doi.org/10.3390/molecules23071720
Chen H, Li M, Zhang C, Du W, Shao H, Feng Y, Zhang W, Yang S. Isolation and Identification of the Anti-Oxidant Constituents from Loropetalum chinense (R. Brown) Oliv. Based on UHPLC–Q-TOF-MS/MS. Molecules. 2018; 23(7):1720. https://doi.org/10.3390/molecules23071720
Chicago/Turabian StyleChen, Haifang, Mulan Li, Chen Zhang, Wendi Du, Haihua Shao, Yulin Feng, Wugang Zhang, and Shilin Yang. 2018. "Isolation and Identification of the Anti-Oxidant Constituents from Loropetalum chinense (R. Brown) Oliv. Based on UHPLC–Q-TOF-MS/MS" Molecules 23, no. 7: 1720. https://doi.org/10.3390/molecules23071720






