Controlled Release of DEET Loaded on Fibrous Mats from Electrospun PMDA/Cyclodextrin Polymer
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Polymer Synthesis
2.3. Polymer Processing and Curing
2.4. Fibrous-Nanosponge DEET Loading and Extraction Procedures
2.5. Cotton Samples’ Loading Procedure
2.6. Nanosponge-Powder Loading Procedure
2.7. Fibrous-Nanosponge DEET Release Study Setup
2.8. Scanning Electron Microscopy Analysis
2.9. Thermogravimetric Analysis Measurements
2.10. HPLC Measurements
3. Results and Discussion
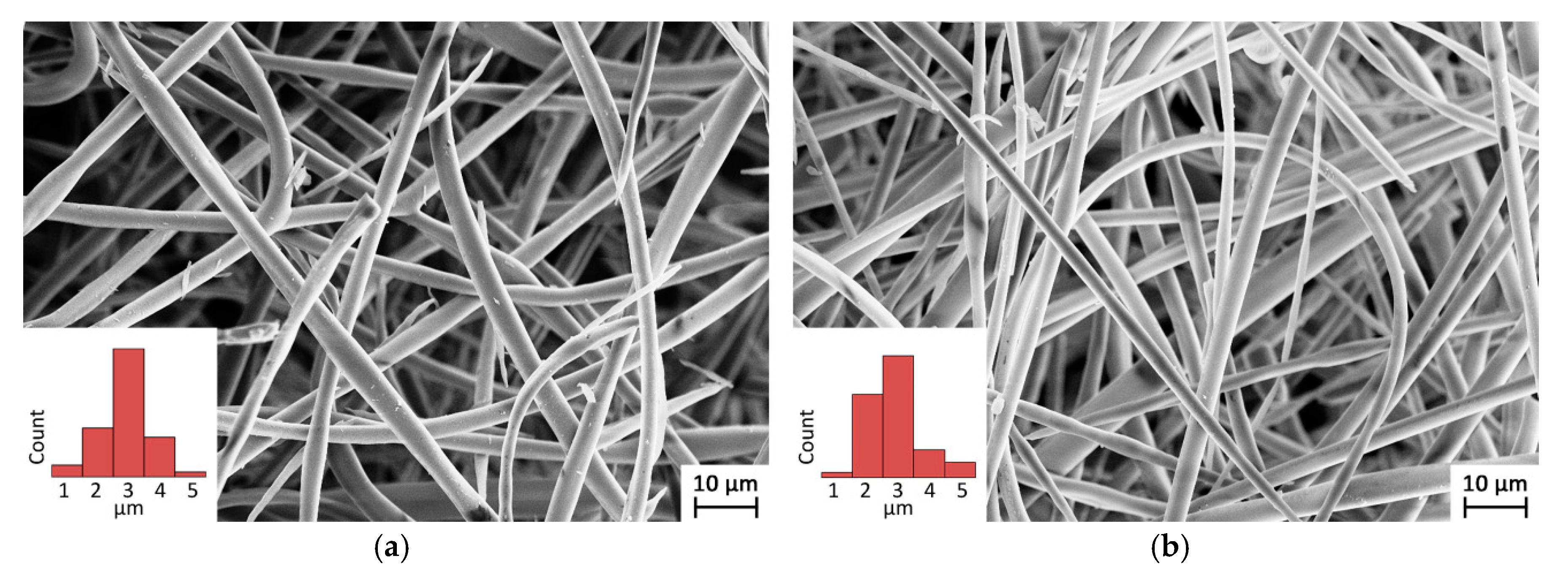
3.1. Polymer Processing: Analysis of the Morphology
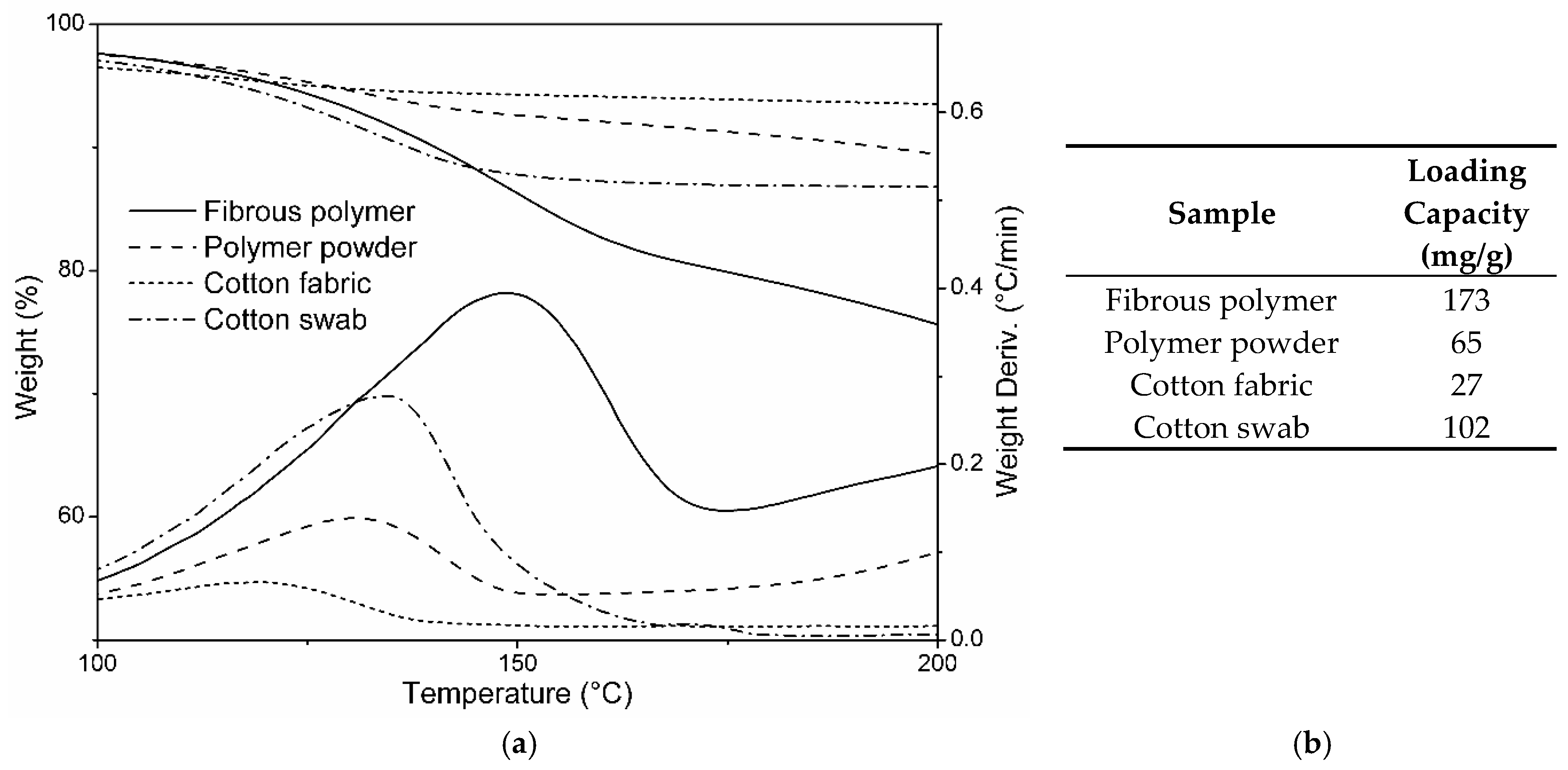
3.2. DEET Loading Capacity Assessment
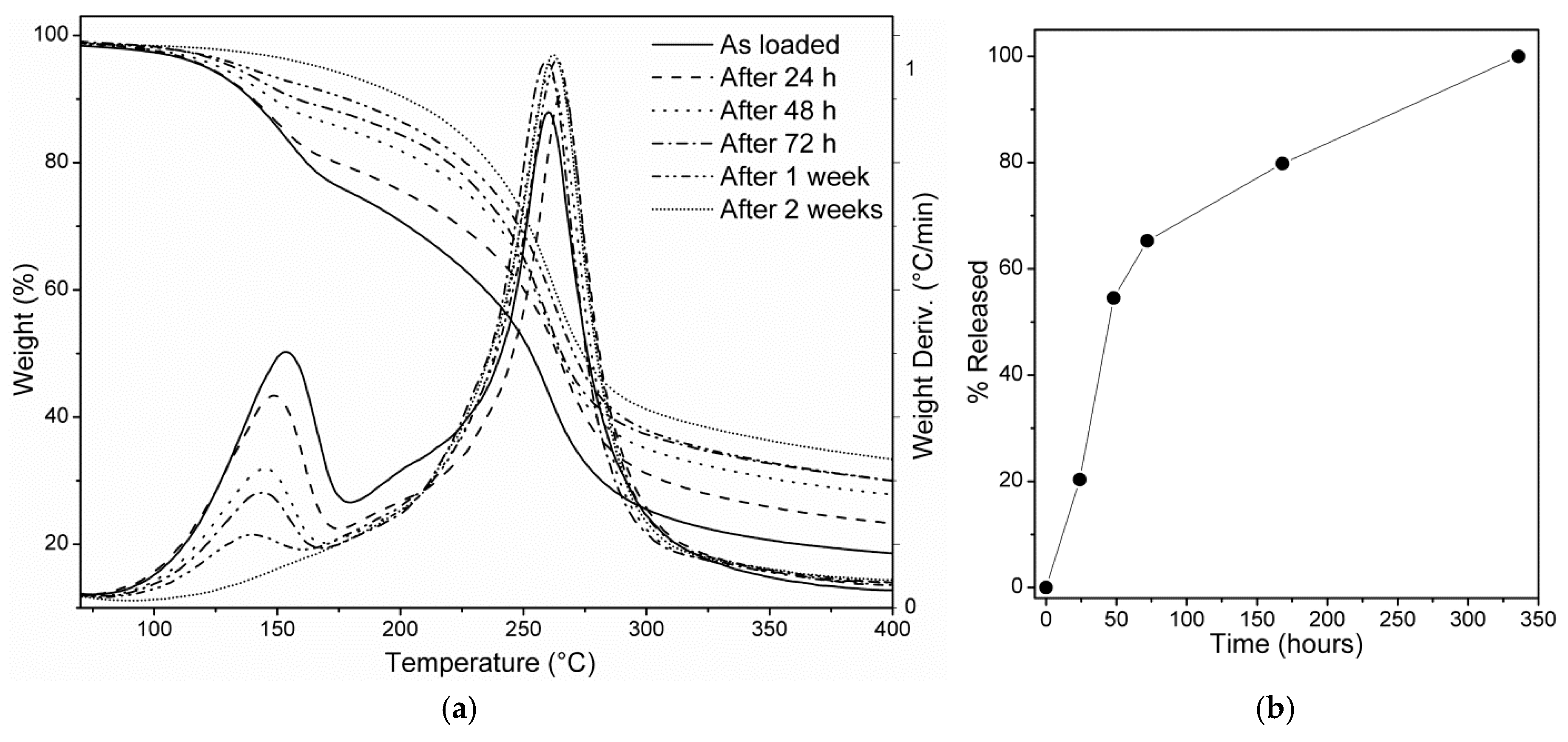
3.3. Thermogravimetric Analysis
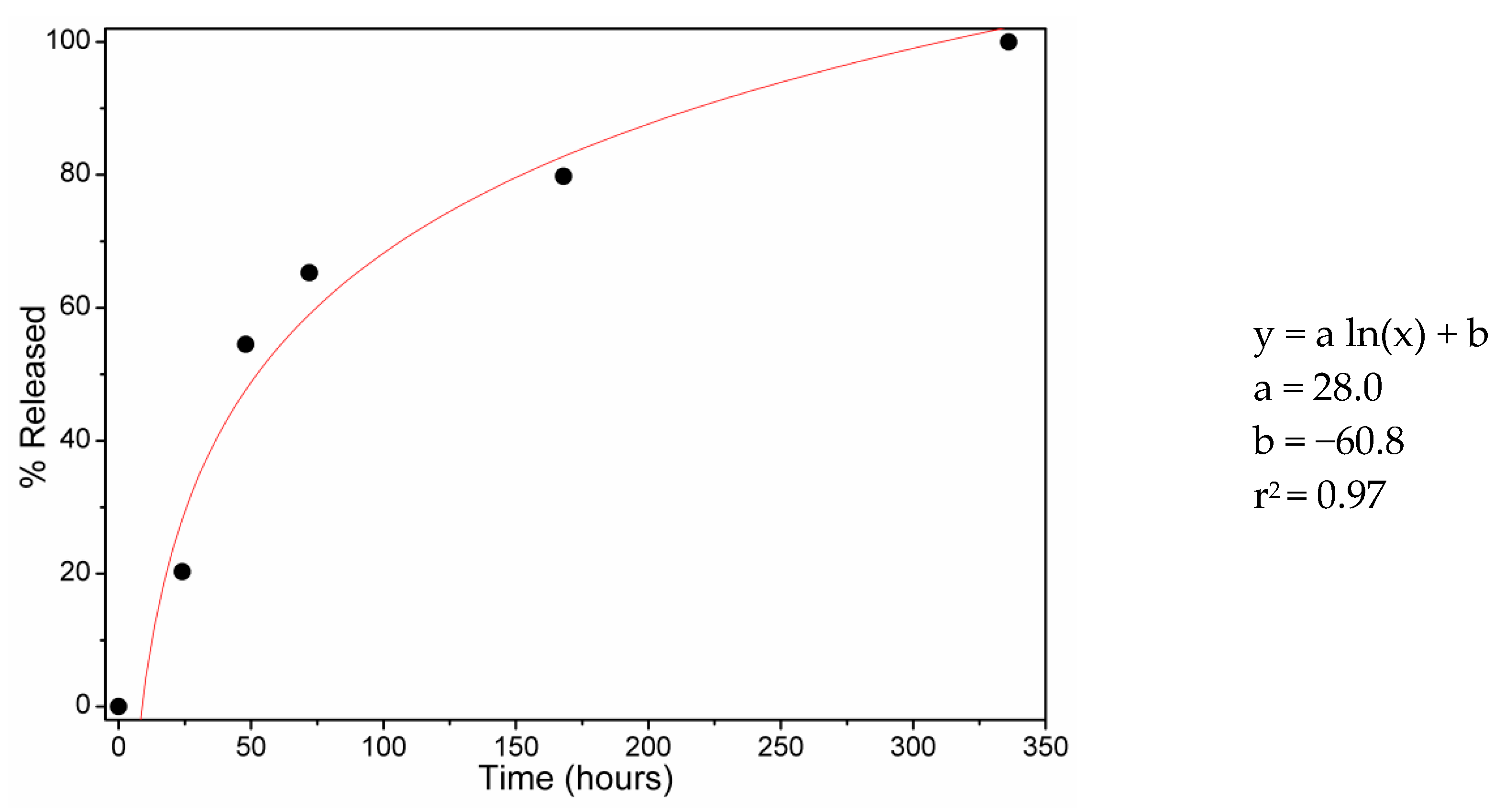
3.4. DEET Release Study
3.5. DEET Loading Capacity with Respect to the Morphology and Chemical Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blitvich, B. Arboviruses: Molecular Biology, Evolution and Control. Nikos Vasilakis and Duane J. Gubler. Am. J. Trop. Med. Hyg. 2016, 95, 488–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, J.H. Chemical and plant-based insect repellents: Efficacy, safety, and toxicity. Wilderness Environ. Med. 2016, 27, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Alpern, J.D.; Dunlop, S.J.; Dolan, B.J.; Stauffer, W.M.; Boulware, D.R. Personal Protection Measures Against Mosquitoes, Ticks, and Other Arthropods. Med. Clin. N. Am. 2016, 100, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.; da Silva, M.R.M.; de Oliveira de Siqueira, L.B.; Rodrigues, R.A.S.; Bodjolle-d’Almeira, L.; dos Santos, E.P.; Ricci-Júnior, E. Trends in insect repellent formulations: A review. Int. J. Pharm. 2018, 539, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Barradas, T.N.; Lopes, L.M.A.; Ricci, E.; De Holanda E Silva, K.G.; Mansur, C.R.E. Development and characterization of micellar systems for application as insect repellents. Int. J. Pharm. 2013, 454, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Islam, J.; Zaman, K.; Duarah, S.; Raju, P.S.; Chattopadhyay, P. Mosquito repellents: An insight into the chronological perspectives and novel discoveries. Acta Trop. 2017, 167, 216–230. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.K.; Kim, K.C.; Cho, Y.; Gwon, Y.D.; Cho, H.S.; Heo, Y.; Park, K.; Lee, Y.W.; Kim, M.; Oh, Y.K.; et al. Comparison of Repellency Effect of Mosquito Repellents for DEET, Citronella, and Fennel Oil. J. Parasitol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Antwi, F.B.; Shama, L.M.; Peterson, R.K.D. Risk assessments for the insect repellents DEET and picaridin. Regul. Toxicol. Pharmacol. 2008, 51, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications. Cyclodext. Pharm. Cosmet. Biomed. Curr. Futur. Ind. Appl. 2011, 17, 323–342. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, S.; Cavalli, R.; Trotta, F. Cyclodextrin-based nanosponges: A versatile platform for cancer nanotherapeutics development. Wiley Interdiscip. 2016, 8, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Romi, R.; Nostro, P.L.; Bocci, E.; Ridi, F.; Baglioni, P. Bioengineering of a Cellulosic Fabric for Insecticide Delivery via Grafted Cyclodextrin. Biotechnol. Prog. 2005, 21, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Cecone, C.; Caldera, F.; Anceschi, A.; Scalarone, D.; Trotta, F.; Bracco, P.; Zanetti, M. One-step facile process to obtain insoluble polysaccharides fibrous mats from electrospinning of water-soluble PMDA/cyclodextrin polymer. J. Appl. Polym. Sci. 2018, 135, 46490. [Google Scholar] [CrossRef]
- Trotta, F.; Caldera, F.; Cavalli, R.; Mele, A.; Punta, C.; Melone, L.; Castiglione, F.; Rossi, B.; Ferro, M.; Crupi, V.; et al. Synthesis and characterization of a hyper-branched water-soluble β-cyclodextrin polymer. Beilstein J. Org. Chem. 2014, 10, 2586–2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peila, R.; Scordino, P.; Shanko, D.B.; Caldera, F.; Trotta, F.; Ferri, A. Synthesis and characterization of β-cyclodextrin nanosponges for N,N-diethyl-meta-toluamide complexation and their application on polyester fabrics. React. Funct. Polym. 2017, 119, 87–94. [Google Scholar] [CrossRef]
- Saenger, B.W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chem. Int. Ed. Engl. 1980, 8, 344–362. [Google Scholar] [CrossRef]
- Proniuk, S.; Liederer, B.M.; Dixon, S.E.; Rein, J.A.; Kallen, M.A.; Blanchard, J. Topical Formulation Studies with DEET (N,N-Diethyl-3-methylbenzamide) and Cyclodextrins. J. Pharm. Sci. 2002, 91, 101–110. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecone, C.; Caldera, F.; Trotta, F.; Bracco, P.; Zanetti, M. Controlled Release of DEET Loaded on Fibrous Mats from Electrospun PMDA/Cyclodextrin Polymer. Molecules 2018, 23, 1694. https://doi.org/10.3390/molecules23071694
Cecone C, Caldera F, Trotta F, Bracco P, Zanetti M. Controlled Release of DEET Loaded on Fibrous Mats from Electrospun PMDA/Cyclodextrin Polymer. Molecules. 2018; 23(7):1694. https://doi.org/10.3390/molecules23071694
Chicago/Turabian StyleCecone, Claudio, Fabrizio Caldera, Francesco Trotta, Pierangiola Bracco, and Marco Zanetti. 2018. "Controlled Release of DEET Loaded on Fibrous Mats from Electrospun PMDA/Cyclodextrin Polymer" Molecules 23, no. 7: 1694. https://doi.org/10.3390/molecules23071694





