A High-Content Screen Reveals New Small-Molecule Enhancers of Ras/Mapk Signaling as Probes for Zebrafish Heart Development
Abstract
:1. Introduction
2. Results
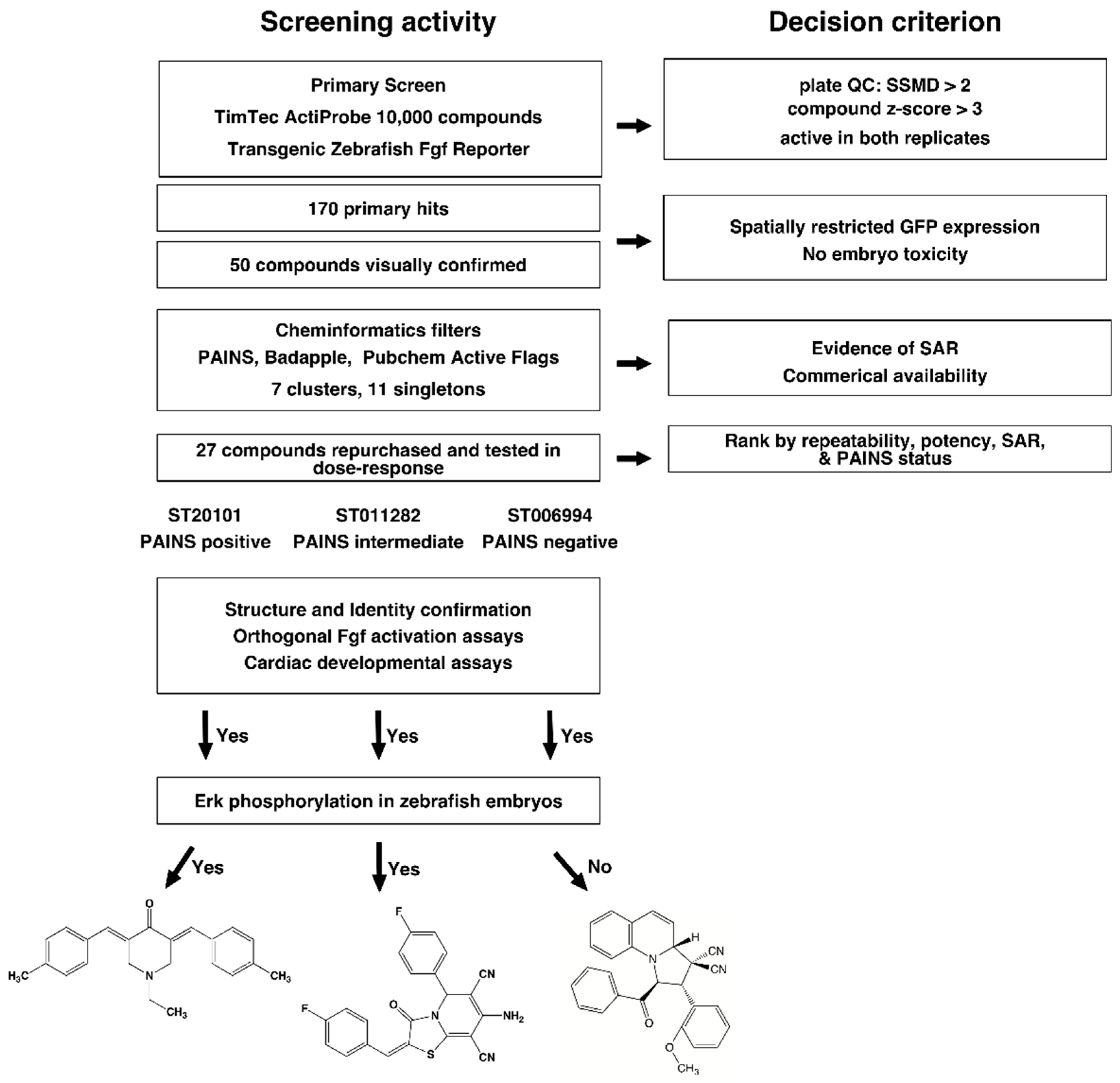
2.1. High Throughput Screening and Hit Triage
2.2. Cheminformatics Analysis
2.3. Hit Confirmation
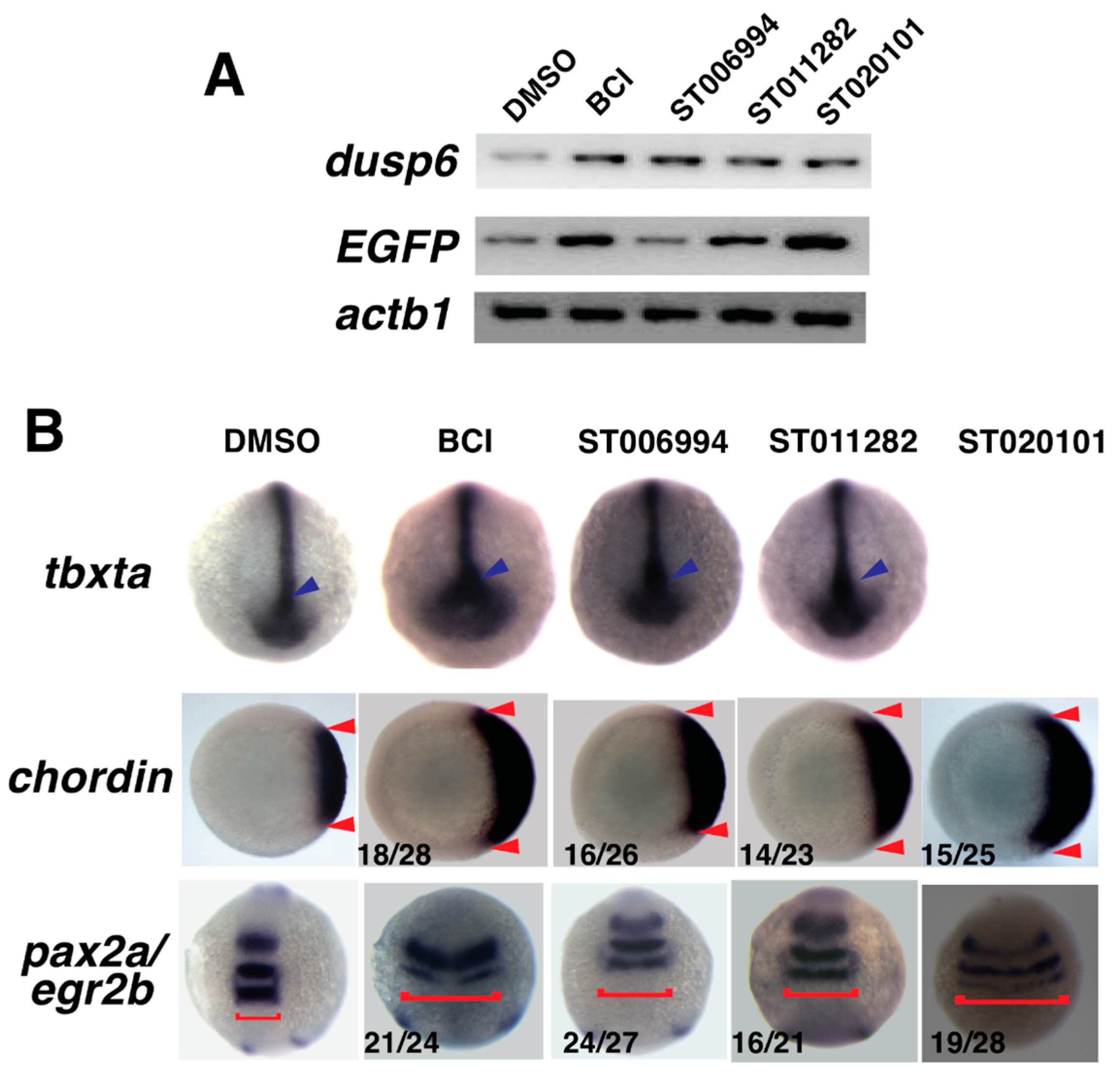
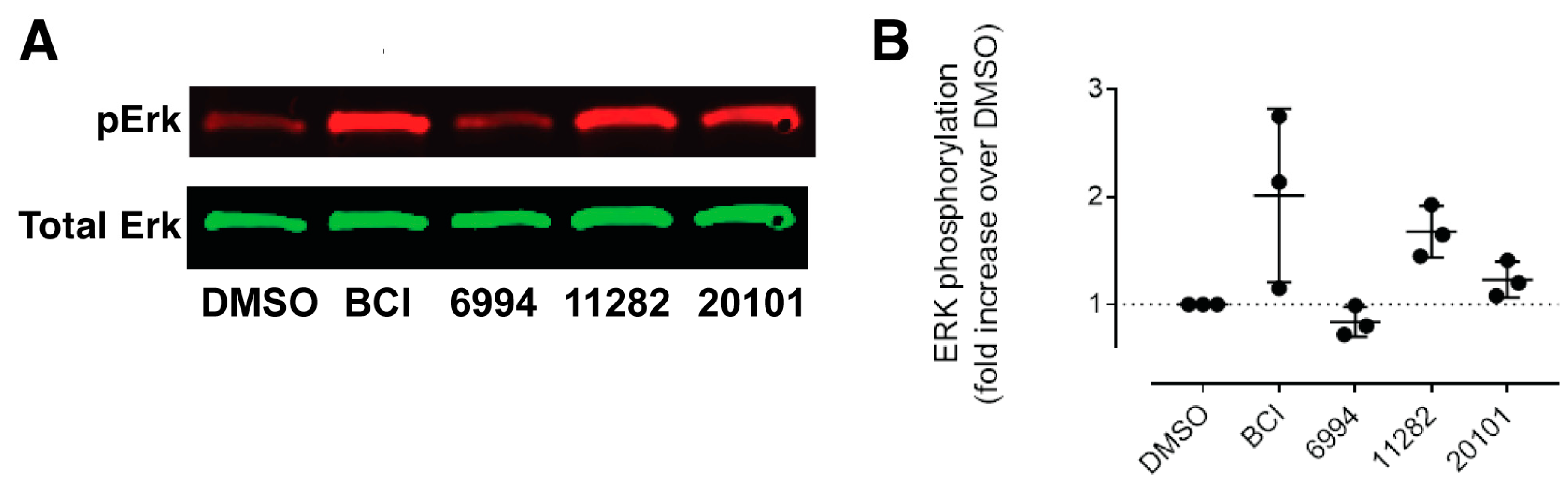
2.4. Confirmation of Fgf Hyperactivation by Orthogonal Assays
2.5. Fgf Hyperactivators Influence Cardiac Chamber Development
2.6. ST006994 Activates Fgf Signaling without Hyperactivating Erk
3. Discussion
3.1. Unique Features of the TimTec ActiProbe Library
3.2. High-Content Screening in Transgenic Fluorescent Zebrafish
3.3. Hypotheses Derived from Cheminformatics
3.4. Zebrafish Chemical Screening Identified a Non-Canonical Hyperactivator of Fgf Signaling
4. Materials and Methods
4.1. Zebrafish Handling and Maintenance
4.2. Plate Preparation and Processing
4.3. Automated Embryo Imaging and Analysis
4.4. Cheminformatics Analysis
4.5. Dose-Response Confirmation and Analysis of Compound Identity and Purity
4.6. Whole Mount In Situ Hybridization
4.7. Cardiomyocyte Imaging
4.8. Western Blotting
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCI | (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one |
| Dusp | dual specificity phosphatase |
| Erk | extracellular signal related kinase |
| Fgf | fibroblast growth factor |
| Mapk | mitogen activated protein kinase |
| SAR | structure-activity relationship |
References
- Den Hertog, J. Chemical genetics: Drug screens in zebrafish. Biosci. Rep. 2005, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.T.; Link, B.A.; Dowling, J.E.; Schreiber, S.L. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc. Natl. Acad. Sci. USA 2000, 97, 12965–12969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, M. Zebrafish: A tool for chemical screens. Birth Defects Res. Part C Embryo Today Rev. 2010, 90, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.V.; Zon, L.I. Swimming into the future of drug discovery: In vivo chemical screens in zebrafish. ACS Chem. Biol. 2010, 5, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Delvecchio, C.; Tiefenbach, J.; Krause, H.M. The zebrafish: A powerful platform for in vivo, HTS drug discovery. Assay Drug Dev. Technol. 2011, 9, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Rajpurohit, S.K.; Delaspre, F.; Walker, S.L.; White, D.T.; Ceasrine, A.; Kuruvilla, R.; Li, R.J.; Shim, J.S.; Liu, J.O.; et al. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic beta-cell mass. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, E.; Scheetz, S.D.; Xie, W.; Burton, E.A. Modulation of the zebrafish optokinetic reflex by pharmacologic agents targeting gabaa receptors. Neurosci. Lett. 2018, 671, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, V.E.; Varshney, G.K.; Lee, M.; Bupp, S.; Xu, L.; Shinn, P.; Crawford, N.P.; Inglese, J.; Burgess, S.M. Phenotype-driven chemical screening in zebrafish for compounds that inhibit collective cell migration identifies multiple pathways potentially involved in metastatic invasion. Dis. Model Mech. 2015, 8, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hukriede, N.; Vogt, A.; de Caestecker, M. Drug discovery to halt the progression of acute kidney injury to chronic kidney disease: A case for phenotypic drug discovery in acute kidney injury. Nephron 2017, 137, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.; Maegawa, S.; Kiang, A.; Habas, R.; Weinberg, E.; Dawid, I.B. A role for mkp3 in axial patterning of the zebrafish embryo. Development 2004, 131, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- De Pater, E.; Clijsters, L.; Marques, S.R.; Lin, Y.F.; Garavito-Aguilar, Z.V.; Yelon, D.; Bakkers, J. Distinct phases of cardiomyocyte differentiation regulate growth of the zebrafish heart. Development 2009, 136, 1633–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, S.R.; Lee, Y.; Poss, K.D.; Yelon, D. Reiterative roles for Fgf signaling in the establishment of size and proportion of the zebrafish heart. Dev. Biol. 2008, 321, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Zeng, X.I.; Sidhwani, P.; Marques, S.R.; George, V.; Targoff, K.L.; Chi, N.C.; Yelon, D. Fgf signaling enforces cardiac chamber identity in the developing ventricle. Development 2017, 144, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Simoes, F.C.; Peterkin, T.; Patient, R. Fgf differentially controls cross-antagonism between cardiac and haemangioblast regulators. Development 2011, 138, 3235–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrell, M.R.J.; Waxman, J.S. Restraint of Fgf8 signaling by retinoic acid signaling is required for proper heart and forelimb formation. Dev. Biol. 2011, 358, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Reifers, F.; Walsh, E.C.; Leger, S.; Stainier, D.Y.; Brand, M. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (Fgf8/acerebellar). Development 2000, 127, 225–235. [Google Scholar] [PubMed]
- Molina, G.; Vogt, A.; Bakan, A.; Dai, W.X.; de Oliveira, P.Q.; Znosko, W.; Smithgall, T.E.; Bahar, I.; Lazo, J.S.; Day, B.W.; et al. Zebrafish chemical screening reveals an inhibitor of dusp6 that expands cardiac cell lineages. Nat. Chem. Biol. 2009, 5, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.A.; Watkins, S.C.; Tsang, M. Generation of Fgf reporter transgenic zebrafish and their utility in chemical screens. BMC Dev. Biol. 2007, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhou, X.H.; Chang, N.; Xiao, C.L.; Yan, S.; Ren, H.; Yang, X.Z.; Zhang, M.L.; Wu, Q.; Tang, B.; et al. Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res. 2014, 24, 1091–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missinato, M.A.; Saydmohammed, M.; Zuppo, D.A.; Rao, K.S.; Opie, G.W.; Kuhn, B.; Tsang, M. Dusp6 attenuates ras/mapk signaling to limit zebrafish heart regeneration. Development 2018, 145, 157206. [Google Scholar] [CrossRef] [PubMed]
- Kaltenmeier, C.T.; Vollmer, L.L.; Vernetti, L.A.; Caprio, L.; Davis, K.; Korotchenko, V.N.; Day, B.W.; Tsang, M.; Hulkower, K.I.; Lotze, M.T.; et al. A tumor cell-selective inhibitor of mitogen-activated protein kinase phosphatases sensitizes breast cancer cells to lymphokine-activated killer cell activity. J. Pharmacol. Exp. Ther. 2017, 361, 39–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saydmohammed, M.; Vollmer, L.L.; Onuoha, E.O.; Vogt, A.; Tsang, M. A high-content screening assay in transgenic zebrafish identifies two novel activators of Fgf signaling. Birth Defects Res. C Embryo Today 2011, 93, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, K.A.; Haskins, J.R.; Taylor, D.L. Advances in high content screening for drug discovery. Assay Drug Dev. Technol. 2003, 1, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Codore, H.; Day, B.W.; Hukriede, N.A.; Tsang, M. Development of automated imaging and analysis for zebrafish chemical screens. J. Vis. Exp. 2010, 40, 1900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D. A pair of new statistical parameters for quality control in RNA interference high-throughput screening assays. Genomics 2007, 89, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.; Walters, M.A. Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Ursu, O.; Lipinski, C.A.; Sklar, L.A.; Oprea, T.I.; Bologa, C.G. Badapple: Promiscuity patterns from noisy evidence. J. Cheminform. 2016, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Jasial, S.; Hu, Y.; Bajorath, J. How frequently are pan-assay interference compounds active? Large-scale analysis of screening data reveals diverse activity profiles, low global hit frequency, and many consistently inactive compounds. J. Med. Chem. 2017, 60, 3879–3886. [Google Scholar] [CrossRef] [PubMed]
- Capuzzi, S.J.; Muratov, E.N.; Tropsha, A. Phantom pains: Problems with the utility of alerts for pan-assay interference compounds. J. Chem. Inf. Model. 2017, 57, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.L.; Walters, M.A. How to triage pains-full research. Assay Drug Dev. Technol. 2016, 14, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Korotchenko, V.N.; Saydmohammed, M.; Vollmer, L.L.; Bakan, A.; Sheetz, K.; Debiec, K.T.; Greene, K.A.; Agliori, C.S.; Bahar, I.; Day, B.W.; et al. In vivo structure-activity relationship studies support allosteric targeting of a dual specificity phosphatase. ChemBioChem 2014, 15, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Rodriguez-Leon, J.; Koth, C.M.; Buscher, D.; Itoh, T.; Raya, A.; Ng, J.K.; Esteban, C.R.; Takahashi, S.; Henrique, D.; et al. Mkp3 mediates the cellular response to Fgf8 signalling in the vertebrate limb. Nat. Cell Biol. 2003, 5, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Latinkic, B.V.; Umbhauer, M.; Neal, K.A.; Lerchner, W.; Smith, J.C.; Cunliffe, V. The xenopus brachyury promoter is activated by Fgf and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev. 1997, 11, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Furthauer, M.; Van Celst, J.; Thisse, C.; Thisse, B. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development 2004, 131, 2853–2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mably, J.D.; Mohideen, M.A.; Burns, C.G.; Chen, J.N.; Fishman, M.C. Heart of glass regulates the concentric growth of the heart in zebrafish. Curr. Biol. 2003, 13, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Schoenebeck, J.J.; Keegan, B.R.; Yelon, D. Vessel and blood specification override cardiac potential in anterior mesoderm. Dev. Cell 2007, 13, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.; Dawid, I.B. Promotion and attenuation of Fgf signaling through the ras-mapk pathway. Sci. STKE 2004, 2004, pe17. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Lazo, J.S. Chemical complementation: A definitive phenotypic strategy for identifying small molecule inhibitors of elusive cellular targets. Pharmacol. Ther. 2005, 107, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Znosko, W.A.; Yu, S.; Thomas, K.; Molina, G.A.; Li, C.; Tsang, W.; Dawid, I.B.; Moon, A.M.; Tsang, M. Overlapping functions of pea3 ets transcription factors in Fgf signaling during zebrafish development. Dev. Biol. 2010, 342, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, R.A.; Patrick, E.A. Clustering using a similarity measure based on shared near neighbors. IEEE Trans. Comput. 1973, 22, 1025–1034. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (pains) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Petter, R.C.; Baillie, T.A.; Whitty, A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011, 10, 307–317. [Google Scholar] [CrossRef] [PubMed]
- De Cesco, S.; Kurian, J.; Dufresne, C.; Mittermaier, A.K.; Moitessier, N. Covalent inhibitors design and discovery. Eur. J. Med. Chem. 2017, 138, 96–114. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.A. Covalent inhibitors in drug discovery: From accidental discoveries to avoided liabilities and designed therapies. Drug Discov. Today 2015, 20, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Znosko, W.; Tsang, M. The characterization of a zebrafish mid-hindbrain mutant, mid-hindbrain gone (MGO). Dev. Dyn. 2009, 238, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Merker, S.; Lee, K.J.; McMahon, A.P.; Hammerschmidt, M. The zebrafish organizer requires chordino. Nature 1997, 387, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, T.; Tsang, M.; Hukriede, N.A.; Chen, X.F.; Dedekian, M.; Clarke, C.J.; Kiang, A.; Schultz, S.; Epstein, J.A.; Toyama, R.; et al. A gene expression screen in zebrafish embryogenesis. Genome Res. 2001, 11, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Johansen, T.; Korzh, V.; Fjose, A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development 1991, 113, 1193–1206. [Google Scholar] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic-development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds ST006994, ST011282 and ST20101 are available from the TimTec (http://www.timtec.net). |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saydmohammed, M.; Vollmer, L.L.; Onuoha, E.O.; Maskrey, T.S.; Gibson, G.; Watkins, S.C.; Wipf, P.; Vogt, A.; Tsang, M. A High-Content Screen Reveals New Small-Molecule Enhancers of Ras/Mapk Signaling as Probes for Zebrafish Heart Development. Molecules 2018, 23, 1691. https://doi.org/10.3390/molecules23071691
Saydmohammed M, Vollmer LL, Onuoha EO, Maskrey TS, Gibson G, Watkins SC, Wipf P, Vogt A, Tsang M. A High-Content Screen Reveals New Small-Molecule Enhancers of Ras/Mapk Signaling as Probes for Zebrafish Heart Development. Molecules. 2018; 23(7):1691. https://doi.org/10.3390/molecules23071691
Chicago/Turabian StyleSaydmohammed, Manush, Laura L. Vollmer, Ezenwa O. Onuoha, Taber S. Maskrey, Gregory Gibson, Simon C. Watkins, Peter Wipf, Andreas Vogt, and Michael Tsang. 2018. "A High-Content Screen Reveals New Small-Molecule Enhancers of Ras/Mapk Signaling as Probes for Zebrafish Heart Development" Molecules 23, no. 7: 1691. https://doi.org/10.3390/molecules23071691




