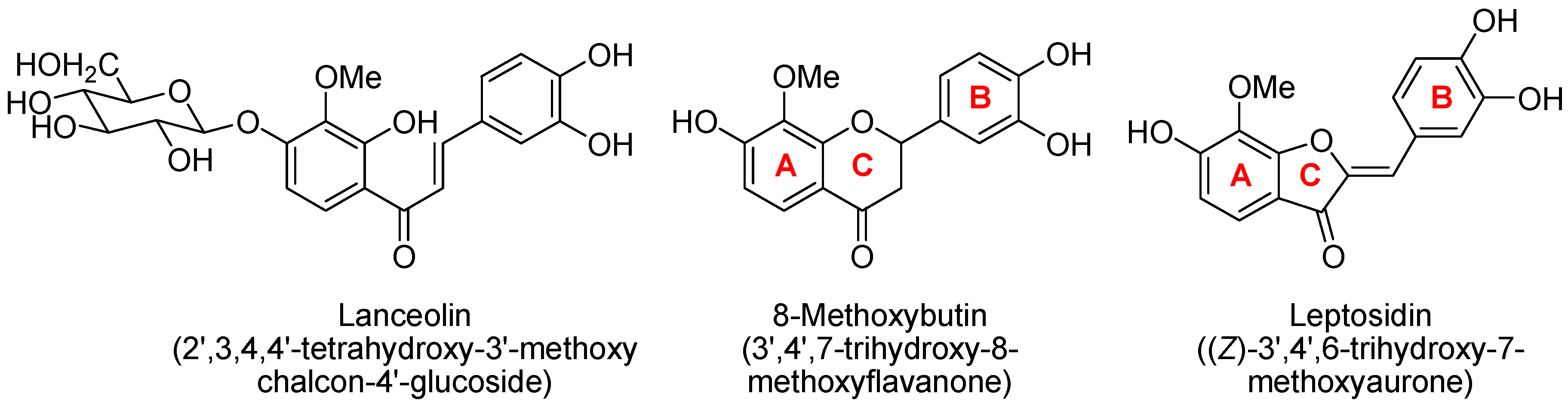
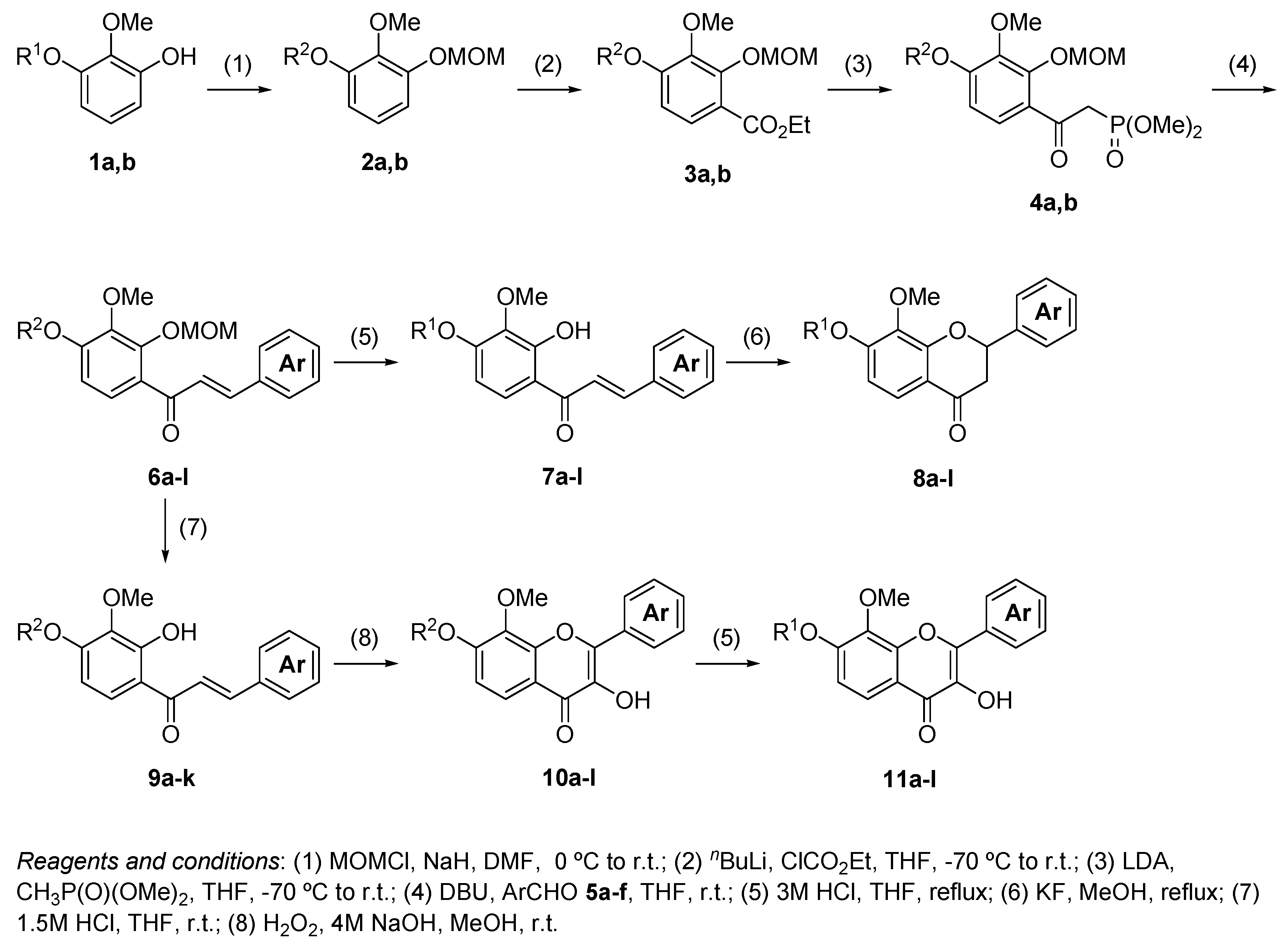
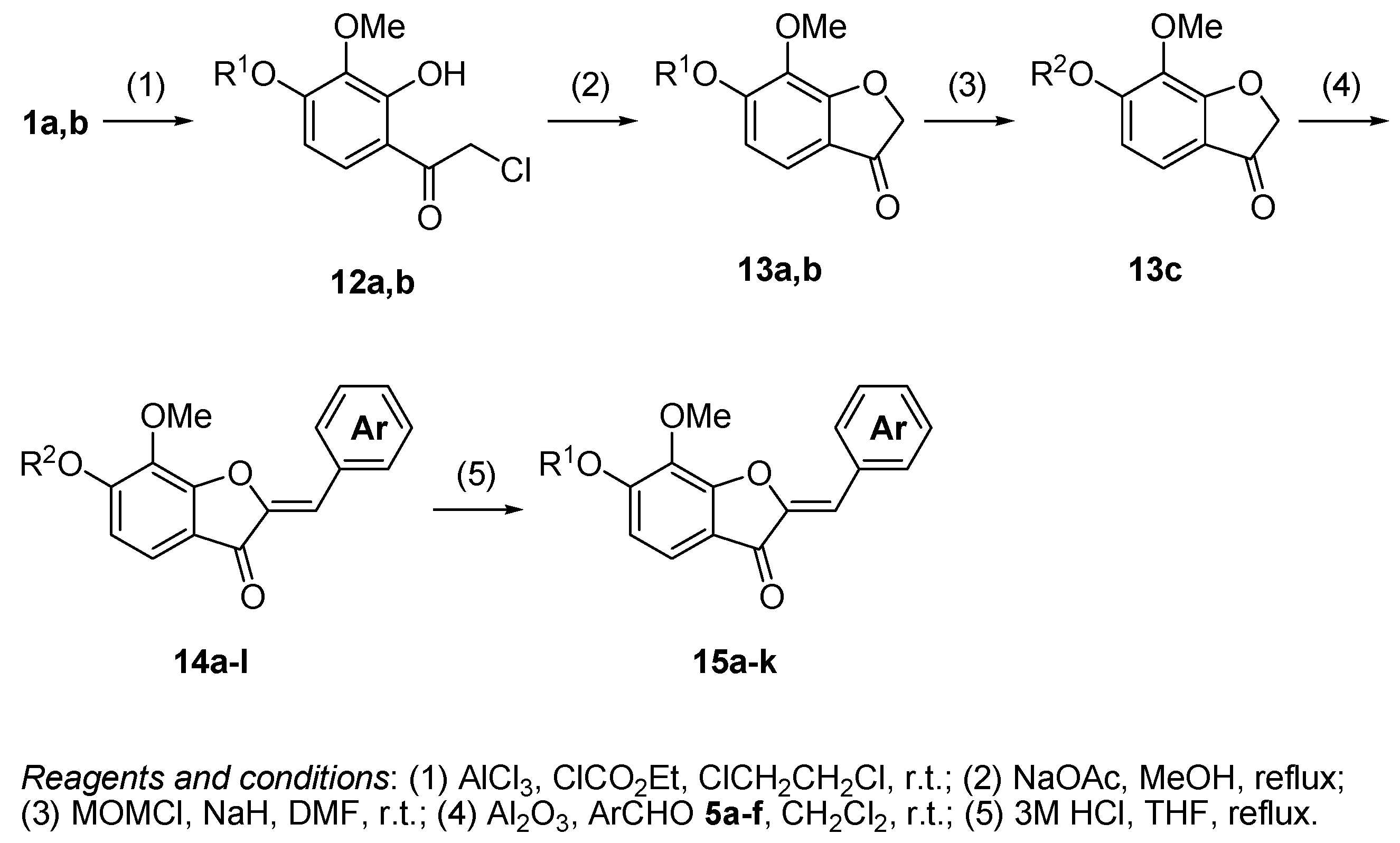
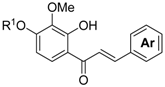
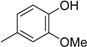
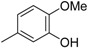
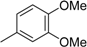
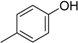
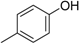
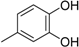
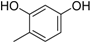
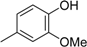
3. Materials and Methods
3.1. General Methods
1H-NMR and 13C-NMR spectra were obtained on a JEOL JNM-EX400 (Tokyo, Japan) spectrometer in CDCl3, CD3OD, or dimethyl sulfoxide (DMSO)-d6 operating at 400 MHz and 100 MHz, respectively, with Me4Si as the internal standard. The absorbance was measured with a microplate reader Corona MTP-300 (Tokyo, Japan). The absorbance was recorded in the 200–600 nm range at room temperature with Jasco V630 (Tokyo, Japan). The mass spectra were obtained on a Shimadzu gas chromatograph mass spectrometer (GCMS)-QP5000 (Kyoto, Japan) with a column temperature of 240 °C, injection temperature of 200 °C, and interface temperature of 230 °C, with He as the carrier gas at a flow rate of 1.3 mL/min. Tetrahydrofuran (THF) was purified by distillation over benzophenone ketyl under an argon atmosphere before use. The melting points were measured in open capillary tubes and were uncorrected.
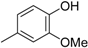
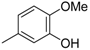
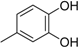
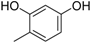
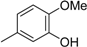
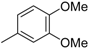
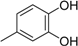
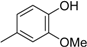
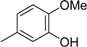
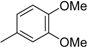
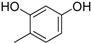
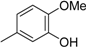
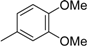
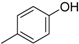
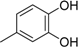
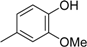
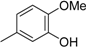
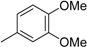
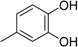
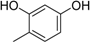
3.2. The General Procedure for the Protection of 2-O-Methylpyrogallol 1a,b with Chloromethyl Methyl Ether
A solution of 1a,b (100.0 mmol) in N,N-dimethylformamide (DMF) (50 mL) was added to a suspension of sodium hydride (60% in mineral oil, 9.60 g, 240.0 mmol or 4.80 g, 120.0 mmol) in DMF (150 mL) at 0 °C. After being stirred at room temperature for 30 min, chloromethyl methyl ether (15.2 mL, 200.0 mmol or 11.4 mL, 150.0 mmol) was added to the mixture at 0 °C. After being stirred at room temperature for 6 h, 100 mL Et2O was added to the mixture. The reaction mixture was poured into ice water (400 mL). The mixture was extracted with Et2O. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on silica gel with CHCl3-Et2O (9:1) to produce 2a,b.
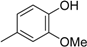
2-Methoxy-1,3-di(methoxymethoxy)benzene (2a): (22.14 g, 97.0 mmol, 97% yield); 1H-NMR (CDCl3) 3.52 (s, 6H, OCH3), 3.89 (s, 3H, OCH3), 5.22 (s, 4H, OCH2), 6.85 (d, J = 8.1 Hz, 2H, H-4 and H-6), 6.95 (t, J = 8.3 Hz, 1H, H-5).
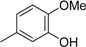
1,2-Dimethoxy-3-(methoxymethoxy)benzene (2b): (18.63 g, 94.0 mmol, 94% yield); 1H-NMR (CDCl3) 3.52 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 5.23 (s, 2H, OCH2), 6.63 (d, J = 8.1 Hz, 1H, H-6), 6.80 (d, J = 7.6 Hz, 1H, H-4), 6.98 (t, J = 8.3 Hz, 1H, H-5).
3.3. The General Procedure for the Preparation of Ethyl Benzoates 3a,b
n-BuLi (1.55 M hexane solution, 23.2 mL, 36.0 mmol) was added to a solution of 2a,b (30.0 mmol) in THF (150 mL) at −70 °C. The reaction mixture was warmed to 0 °C and stirred for 90 min at the same temperature. The mixture was cooled to −70 °C and a solution of ethyl chloroformate (14.3 mL, 150.0 mmol) in THF (15 mL) was added dropwise to the mixture. After being stirred for 30 min at −70 °C, the mixture was stirred at room temperature for 2 h. The mixture was poured into an ice-saturated ammonium chloride aqueous solution. The mixture was extracted with Et2O. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on silica gel with CHCl3-Et2O (9:1) to produce 3a,b.
Ethyl 3-methoxy-2,4-di(methoxymethoxy)benzoate (3a): (7.75 g, 25.8 mmol, 86% yield); 1H-NMR (CDCl3) δ 1.38 (t, J = 7.1 Hz, 3H, CH3), 3.52 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 4.37 (q, J = 7.1 Hz, 1H, OCH2CH3), 5.18 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 6.97 (d, J = 9.0 Hz, 1H, H-5), 7.60 (d, J = 8.8 Hz, 1H, H-6); 13C-NMR (CDCl3) δ 14.3, 56.4, 57.4, 60.8, 61.0, 94.6, 100.1, 110.6, 119.3, 126.7, 143.2, 151.3, 154.4, 165.1.
Ethyl 3,4-dimethoxy-2-(methoxymethoxy)benzoate (3b): (5.92 g, 21.9 mmol, 73% yield); 1H-NMR (CDCl3) δ 1.38 (t, J = 7.1 Hz, 3H, CH3), 3.61 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 4.35 (q, J = 7.1 Hz, 1H, OCH2CH3), 5.17 (s, 2H, OCH2), 6.72 (d, J = 9.0 Hz, 1H, H-5), 7.65 (d, J = 8.8 Hz, 1H, H-6); 13C-NMR (CDCl3) δ 14.3, 56.0, 57.4, 60.7, 60.9, 100.0, 106.9, 118.2, 127.0, 142.5, 151.2, 156.8, 165.2.
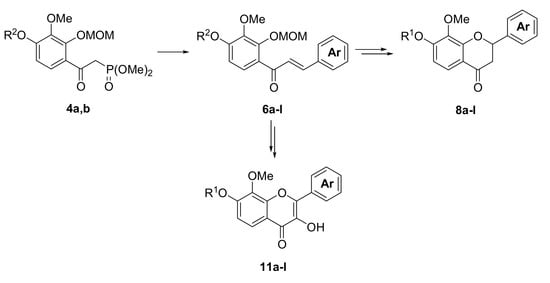
3.4. The General Procedure for the Synthesis of α-(Dimethylphosphono)acetylbenzenes 4a,b
n-BuLi (1.55 M hexane solution, 42.6 mL, 66 mmol) was added to a solution of diisopropylamine (8.4 mL, 60.0 mmol) in THF (100 mL) at −70 °C. After being stirred for 30 min at the same temperature, a solution of dimethyl methylphosphonate (4.47 g, 36.0 mmol) in THF (15 mL) was added dropwise to the mixture. The reaction mixture was stirred for 15 min at −70 °C and a solution of 3a,b (30.0 mmol) in THF (15 mL) was added to the mixture. The mixture was stirred for 1 h at −70 °C and for 12 h at room temperature. The mixture was poured into ice and a 2 M hydrochloric acid aqueous solution. The mixture was extracted with EtOAc. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on silica gel with CHCl3-Et2O-MeOH (8:2:0.05) to produce 4a,b.
α-(Dimethylphosphono)-3-methoxy-2,4-di(methoxymethoxy)acetophenone (4a): (9.87 g, 26.1 mmol, 87% yield); reddish brown viscous oil; 1H-NMR (CDCl3) δ 3.52 (s, 6H, OCH3), 3.77 (d, J = 11.2 Hz, 6H, P(O)OCH3), 3.86 (d, J = 21.5 Hz, 2H, P(O)CH2), 3.88 (s, 3H, OCH3), 5.23 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 6.98 (d, J = 8.8 Hz, 1H, H-5), 7.48 (d, J = 9.0 Hz, 1H, H-6); 13C-NMR (CDCl3) δ 40.3 (d, J = 131.0 Hz, P(O)CH2), 52.9 (d, J = 5.8 Hz, P(O)OCH3), 56.5, 58.0, 61.0, 94.7, 100.2, 110.9, 126.0, 126.9 (d, J = 3.3 Hz, Ar), 142.0, 150.6, 155.1, 191.9 (d, J = 6.6 Hz, CO).
α-(Dimethylphosphono)-3,4-dimethoxy-2-(methoxymethoxy)acetophenone (4b): (7.94 g, 22.8 mmol, 76% yield); reddish brown viscous oil; 1H-NMR (CDCl3) δ 3.51 (s, 3H, OCH3), 3.77 (d, J = 11.0 Hz, 6H, OCH3), 3.86 (s, 3H, OCH3), 3.87 (d, J = 21.7 Hz, 2H, P(O)CH2), 3.92 (s, 3H, OCH3), 5.23 (s, 2H, OCH2), 6.76 (d, J = 8.8 Hz, 1H, H-5), 7.54 (d, J = 8.8 Hz, 1H, H-6); 13C-NMR (CDCl3) δ 40.9 (d, J = 131.0 Hz, P(O)CH2), 52.8 (d, J = 6.6 Hz, P(O)OCH3), 56.1, 58.0, 60.8, 100.2, 107.3, 125.9 (d, J = 3.3 Hz, Ar), 126.2, 141.4, 150.6, 157.5, 191.7 (d, J = 6.6 Hz, CO).
3.5. The General Procedure for the Synthesis of Chalcones 6a–l
A solution of benzaldehydes 5a–f (1.2 mmol) in THF (1 mL) was added to a solution of 4a,b (1.0 mmol) and DBU (0.30 g, 2.0 mmol) in THF (4 mL) at room temperature. The reaction mixture was poured into an ice-saturated ammonium chloride aqueous solution and extracted with Et2O. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on a preparative thin layer chromatography (hexane:EtOAc = 3:2) to produce chalcones 6a–l.
3′-Methoxy-4,2′,4′-tri(methoxymethoxy)chalcone (6a): (0.35 g, 0.78 mmol, 78% yield); yellow viscous oil; 1H-NMR (CDCl3) δ 3.45 (s, 3H, OCH3), 3.49 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.15 (s, 2H, OCH2), 5.21 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 7.00 (d, J = 8.8 Hz, 1H, H-5′), 7.06 (d, J = 8.5 Hz, 2H, H-3 and H-5), 7.34 (d, J = 15.9 Hz, 1H, H-α), 7.37 (d, J = 8.8 Hz, 1H, H-6′), 7.57 (d, J = 8.8 Hz, 2H, H-2 and H-6), 7.64 (d, J = 15.6 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 56.0, 56.3, 57.5, 60.9, 94.0, 94.7, 99.9, 111.1, 116.2, 124.7, 125.0, 128.5, 128.8, 129.8, 142.4, 142.9, 149.8, 153.7, 158.7, 190.9.
3′-Methoxy-3,4,2′,4′-tetra(methoxymethoxy)chalcone (6b): (0.44 g, 0.91 mmol, 91% yield); yellow viscous oil; 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.52 (s, 3H, OCH3), 3.54 (s, 6H, OCH3), 3.93 (s, 3H, OCH3), 5.16 (s, 2H, OCH2), 5.27 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 7.00 (d, J = 8.8 Hz, 1H, H-5′), 7.17 (d, J = 8.5 Hz, 1H, H-5), 7.26 (dd, J = 2.0 and 8.3 Hz, 1H, H-6), 7.31 (d, J = 15.9 Hz, 1H, H-α), 7.36 (d, J = 8.8 Hz, 1H, H-6′), 7.43 (d, J = 2.0 Hz, H-2), 7.58 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 56.1, 56.2, 56.3, 57.5, 60.9, 94.7, 94.9, 95.3, 99.8, 111.1, 116.0, 116.0, 123.5, 124.9, 125.2, 128.6, 129.2, 142.4, 143.1, 147.0, 149.0, 149.7, 153.7, 190.9.
3′-Methoxy-2,4,2′,4′-tetra(methoxymethoxy)chalcone (6c): (0.43 g, 0.89 mmol, 89% yield); yellow viscous oil; 1H-NMR (CDCl3) δ 3.47 (s, 3H, OCH3), 3.49 (s, 3H, OCH3), 3.50 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.14 (s, 2H, OCH2), 5.20 (s, 2H, OCH2), 5.25 (s, 2H, OCH2), 5.29 (s, 2H, OCH2), 6.73 (dd, J = 2.2 Hz and 8.5 Hz, 1H, H-5), 6.84 (d, J = 2.2 Hz, 1H, H-3), 7.00 (d, J = 8.8 Hz, 1H, H-5′), 7.38 (d, J = 8.3 Hz, 1H, H-6′), 7.41 (d, J = 14.6 Hz, 1H, H-α), 7.60 (d, J = 8.5 Hz, 1H, H-6), 8.02 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 56.3, 56.4, 56.5, 57.7, 61.1, 94.1, 94.5, 94.8, 100.0, 103.1, 109.2, 111.1, 118.4, 124.9, 125.2, 129.2, 129.3, 138.3, 142.5, 149.9, 153.7, 157.4, 160.0, 191.3.
3,3′-Dimethoxy-4,2′,4′-tri(methoxymethoxy)chalcone (6d): (0.37 g, 0.82 mmol, 82% yield); 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.52 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 3.93 (s, 6H, OCH3), 5.16 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 5.29 (s, 2H, OCH2), 7.00 (d, J = 8.8 Hz, 1H, H-5′), 7.16–7.17 (m, 3H, H-2, H-5 and H-6), 7.36 (d, J = 15.9 Hz, 1H, H-α), 7.37 (d, J = 8.5 Hz, 1H, H-6′), 7.61 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 55.8, 56.2, 56.3, 57.5, 60.9, 94.7, 95.0, 99.8, 110.6, 111.1, 115.6, 122.3, 124.9, 125.0, 128.6, 129.1, 142.4, 143.2, 148.3, 149.5, 149.7, 153.7, 190.9.
4,3′-Dimethoxy-3,2′,4′-tri(methoxymethoxy)chalcone (6e): (0.36 g, 0.80 mmol, 80% yield); 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.53 (s, 6H, OCH3), 3.92 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.16 (s, 2H, OCH2), 5.26 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 6.91 (d, J = 8.3 Hz, 1H, H-5), 7.00 (d, J = 8.8 Hz, 1H, H-5′), 7.26 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6), 7.29 (d, J = 15.6 Hz, 1H, H-α), 7.35 (d, J = 8.8 Hz, 1H, H-6′), 7.43 (d, J = 1.7 Hz, 1H, H-2), 7.59 (d, J = 15.6 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 55.9, 56.3, 56.4, 57.6, 61.0, 94.9, 95.5, 99.9, 111.2, 111.5, 115.6, 124.1, 124.9, 125.1, 128.0, 128.9, 142.6, 143.5, 146.6, 149.9, 151.7, 153.8, 191.3.
3,4,3′-Trimethoxy-2′,4′-di(methoxymethoxy)chalcone (6f): (0.35 g, 0.83 mmol, 83% yield); 1H-NMR (CDCl3) δ 3.47 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.93 (s, 6H, OCH3), 5.17 (s, 2H, OCH2), 5.29 (s, 2H, OCH2), 6.88 (d, J = 8.3 Hz, 1H, H-5), 7.00 (d, J = 8.8 Hz, H-5′), 7.15 (d, J = 2.0 Hz, 1H, H-2), 7.20 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6), 7.32 (d, J = 15.9 Hz, 1H, H-α), 7.37 (d, J = 8.5 Hz, 1H, H-6′), 7.62 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 55.9, 56.0, 56.5, 57.7, 61.1, 94.9, 100.0, 110.0, 111.0, 111.3, 123.1, 124.7, 125.2, 127.9, 128.9, 143.7, 149.1, 150.0, 151.2, 153.9, 191.2.
3′,4′-Dimethoxy-4,2′-di(methoxymethoxy)chalcone (6g): (0.35 g, 0.89 mmol, 89% yield); yellow viscous oil; 1H-NMR (CDCl3) δ 3.45 (s, 3H, OCH3), 3.49 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.15 (s, 2H, OCH2), 5.22 (s, 2H, OCH2), 6.78 (d, J = 8.8 Hz, 1H, H-5′), 7.06 (d, J = 8.5 Hz, 2H, H-3 and H-5), 7.39 (d, J = 15.9 Hz, 1H, H-α), 7.45 (d, J = 8.5 Hz, 1H, H-6′), 7.58 (d, J = 8.8 Hz, 2H, H-2 and H-6), 7.66 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 56.1, 56.2, 57.7, 61.0, 94.1, 100.1, 107.4, 116.3, 124.8, 125.5, 127.8, 128.7, 129.9, 141.7, 142.8, 150.0, 156.4, 158.8, 190.8.
3′,4′-Dimethoxy-3,4,2′-tri(methoxymethoxy)chalcone (6h): (0.35 g, 0.79 mmol, 79% yield); 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.52 (s, 3H, OCH3), 3.54 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.16 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 5.29 (s, 2H, OCH2), 6.78 (d, J = 8.8 Hz, 1H, H-5′), 7.18 (d, J = 8.5 Hz, 1H, H-5), 7.26 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6), 7.36 (d, J = 15.9 Hz, 1H, H-α), 7.44 (d, J = 8.5 Hz, 1H, H-6′), 7.44 (d, J = 2.0 Hz, 1H, H-2), 7.60 (d, J = 15.6 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 56.0, 56.2, 56.3, 57.6, 60.9, 94.9, 95.3, 99.9, 107.3, 116.0, 116.0, 123.6, 125.3, 125.4, 127.6, 129.3, 141.7, 143.0, 147.1, 149.0, 150.0, 156.4, 191.0.
3′,4′-Dimethoxy-2,4,2′-tri(methoxymethoxy)chalcone (6i): (0.37 g, 0.83 mmol, 83% yield); yellow viscous oil; 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.49 (s, 3H, OCH3), 3.50 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.14 (s, 2H, OCH2), 5.20 (s, 2H, OCH2), 5.25 (s, 2H, OCH2), 6.73 (dd, J = 2.2 and 8.5 Hz, 1H, H-5), 6.77 (d, J = 8.8 Hz, 1H, H-5′), 6.84 (d, J = 2.2 Hz, 1H, H-3), 7.45 (d, J = 15.9 Hz, 1H, H-α), 7.45 (d, J = 8.8 Hz, 1H, H-6′), 7.61 (d, J = 8.5 Hz, 1H, H-6), 8.03 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 55.9, 56.1, 56.2, 57.5, 60.8, 94.0, 94.4, 99.8, 103.0, 107.2, 109.1, 118.4, 124.8, 125.3, 127.9, 129.1, 137.9, 141.6, 149.7, 156.1, 157.3, 159.8, 191.0.
3,3′,4′-Trimethoxy-4,2′-di(methoxymethoxy)chalcone (6j): (0.33 g, 0.78 mmol, 78% yield); yellow viscous oil; 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.52 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.93 (s, 6H, OCH3), 5.17 (s, 2H, OCH2), 5.28 (s, 2H, OCH2), 6.78 (d, J = 8.8 Hz, 1H, H-5′), 7.17 (br s, 3H, H-2, H-5, and H-6), 7.39 (d, J = 15.6 Hz, 1H, H-α), 7.45 (d, J = 8.8 Hz, 1H, H-6′), 7.63 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 55.9, 56.0, 56.3, 57.6, 60.9, 95.1, 100.0, 107.4, 110.7, 115.6, 122.5, 125.1, 125.5, 127.6, 129.3, 141.7, 143.1, 148.4, 149.6, 150.0, 156.5, 190.8.
4,3′,4′-Trimethoxy-3,2′-di(methoxymethoxy)chalcone (6k): (0.37 g, 0.89 mmol, 89% yield); yellow viscous oil; 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.92 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.16 (s, 2H, OCH2), 5.26 (s, 2H, OCH2), 6.77 (d, J = 8.8 Hz, 1H, H-5′), 6.91 (d, J = 8.5 Hz, 1H, H-5), 7.27 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6), 7.34 (d, J = 15.9 Hz, 1H, H-α), 7.43 (d, J = 8.5 Hz, 1H, H-6′), 7.44 (d, J = 1.7 Hz, 1H, H-2), 7.60 (d, J = 15.6 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 55.9, 56.0, 56.2, 57.5, 60.8, 95.3, 99.8, 107.2, 111.4, 115.5, 123.9, 124.7, 125.2, 127.6, 127.9, 141.6, 143.0, 146.3, 149.7, 151.5, 156.2, 190.7.
3,4,3′,4′-Tetramethoxy-2′-(methoxymethoxy)chalcone (6l): (0.36 g, 0.92 mmol, 92% yield); 1H-NMR (CDCl3) δ 3.46 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.17 (s, 2H, OCH2), 6.78 (d, J = 8.8 Hz, 1H, H-5′), 6.89 (d, J = 8.3 Hz, 1H, H-5), 7.16 (d, J = 2.0 Hz, 1H, H-2), 7.20 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6), 7.37 (d, J = 15.9 Hz, 1H, H-α), 7.44 (d, J = 8.5 Hz, 1H, H-6′), 7.63 (d, J = 15.9 Hz, 1H, H-β); 13C-NMR (CDCl3) δ 55.8, 55.9, 56.0, 57.6, 60.9, 100.0, 107.4, 109.9, 110.9, 123.0, 124.6, 125.4, 127.7, 127.9, 141.7, 143.3, 149.0, 149.9, 151.0, 156.4, 190.9.
3.6. The General Procedure for the Deprotection of 6a–l
A solution of 6a–l (1.0 mmol) in methanol (5 mL) and 3 M hydrochloric acid (5 mL) was refluxed for 1 h. The mixture was extracted with EtOAc. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on preparative thin layer chromatography (hexane:EtOAc = 2:3) to produce aurones 7a–l.
4,2′,4′-Trihydroxy-3′-methoxychalcone (7a): (0.22 g, 0.76 mmol, 76% yield); yellowish brown solid, 204–209 °C; 1H-NMR (CDCl3:DMSO-d6 = 9:1) δ 3.93 (s, 3H, OCH3), 6.53 (d, J = 8.8 Hz, 1H, H-5′), 6.90 (d, J = 7.8 Hz, 2H, H-3 and H-5), 7.43 (d, J = 15.9 Hz, 1H, H-α), 7.54 (d, J = 8.1 Hz, 2H, H-2 and H-6), 7.61 (d, J = 8.8 Hz, 1H, H-6′), 7.83 (d, J = 15.4 Hz, 1H, H-β), 9.25 (s, 1H, OH), 9.57 (s, 1H, OH), 13.76 (s, 1H, OH); 13C-NMR (CDCl3:DMSO-d6 = 9:1) δ 60.3, 107.4, 114.0, 115.9, 116.4, 125.7, 125.7, 130.2, 134.6, 144.3, 156.2, 158.3, 159.9, 191.7; UV/Vis (3.0 × 10−5 M, DMSO); λ = 380.0 nm (ε, 3.0 × 104).
3,4,2′,4′-Tetrahydroxy-3′-methoxychalcone (7b): (0.25 g, 0.84 mmol, 84% yield); yellow solid, 191–195 °C; 1H-NMR (DMSO-d6) δ 3.74 (s, 3H, OCH3), 6.48 (d, J = 9.3 Hz, 1H, H-5′), 6.82 (d, J = 8.3 Hz, 1H, H-5), 7.22 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6), 7.29 (d, J = 2.0 Hz, 1H, H-2), 7.65 (d, J = 15.1 Hz, 1H, H-α), 7.69 (d, J = 15.1 Hz, 1H, H-β), 7.93 (d, J = 9.3 Hz, 1H, H-6′), 9.12 (s, 1H, OH), 9.71 (s, 1H, OH), 10.40 (s, 1H, OH), 13.74 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 59.7, 107.9, 113.7, 115.7, 115.9, 117.2, 122.4, 126.2, 126.8, 134.7, 144.9, 145.6, 149.0, 157.1, 158.5, 192.0; UV/Vis (2.6 × 10−5 M, DMSO); λ = 400.5 nm (ε, 2.1 × 104).
2,4,2′,4′-Tetrahydroxy-3′-methoxychalcone (7c): (0.14 g, 0.47 mmol, 47% yield); reddish orange solid, 189–193 °C; 1H-NMR (CD3OD) δ 3.85 (s, 3H, OCH3), 6.34 (d, J = 2.2 Hz, 1H, H-3), 6.36 (dd, J = 2.2 Hz and 9.5 Hz, 1H, H-5), 6.46 (d, J = 8.8 Hz, 1H, H-5′), 7.51 (d, J = 8.3 Hz, 1H, H-6), 7.68 (d, J = 9.5 Hz, 1H, H-6′), 7.69 (d, J = 15.1 Hz, 1H, H-α), 8.10 (d, J = 15.4 Hz, 1H, H-β); 13C-NMR (CD3OD) δ 60.8, 103.4, 108.6, 109.0, 115.4, 115.5, 117.4, 127.2, 132.3, 136.0, 142.2, 157.6, 159.3, 160.6, 162.6, 194.3; UV/Vis (2.8 × 10−5 M, DMSO); λ = 399.0 nm (ε, 3.0 × 104).
4,2′,4′-Trihydroxy-3,3′-dimethoxychalcone (7d): (0.30 g, 0.95 mmol, 95% yield); orange solid, 158–163 °C; 1H-NMR (DMSO-d6) δ 3.76 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 6.51 (d, J = 9.0 Hz, 1H, H-5′), 6.86 (d, J = 8.1 Hz, 1H, H-5), 7.30 (d, J = 8.3 Hz, 1H, H-6), 7.56 (br s, 1H, H-2), 7.76 (d, J = 15.1 Hz, 1H, H-α), 7.81 (d, J = 15.4 Hz, 1H, H-β), 8.01 (d, J = 8.8 Hz, 1H, H-6′), 9.77 (s, 1H, OH), 10.46 (s, 1H, OH), 13.83 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.8, 59.7, 107.7, 111.5, 113.5, 115.4, 117.2, 124.4, 125.9, 126.7, 134.5, 144.6, 147.7, 149.7, 156.9, 158.2, 191.7; UV/Vis (2.5 × 10−5 M, DMSO); λ = 397.5 nm (ε, 2.8 × 104).
3,2′,4′-Trihydroxy-4,3′-dimethoxychalcone (7e): (0.29 g, 0.90 mmol, 90% yield); yellowish brown solid, 180–185 °C; 1H-NMR (DMSO-d6) δ 3.77 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.52 (d, J = 8.8 Hz, 1H, H-5′), 7.02 (d, J = 8.5 Hz, 1H, H-5), 7.34 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6), 7.39 (d, J = 1.5 Hz, 1H, H-2), 7.73 (d, J = 15.4 Hz, 1H, H-α), 7.78 (d, J = 15.4 Hz, 1H, H-β), 8.00 (d, J = 9.0 Hz, 1H, H-6), 9.24 (s, 1H, OH), 10.54 (s, 1H, OH), 13.76 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.7, 59.8, 107.9, 111.7, 113.6, 114.9, 118.1, 122.2, 126.8, 127.3, 134.5, 144.3, 146.4, 150.2, 157.0, 158.3, 191.7; UV/Vis (2.7 × 10−5 M, DMSO); λ = 400.0 nm (ε, 2.2 × 104).
2′,4′-Dihydroxy-3,4,3′-trimethoxychalcone (7f): (0.32 g, 0.98 mmol, 98% yield); orange-yellow solid, 140–144 °C; 1H-NMR (DMSO-d6) δ 3.75 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.88 (s, 3H, OCH3), 6.51 (d, J = 9.0 Hz, 1H, H-5′), 7.04 (d, J = 8.3 Hz, 1H, H-5), 7.57 (d, J = 2.0 Hz, 1H, H-2), 7.41 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6), 7.78 (d, J = 15.4 Hz, 1H, H-α), 7.86 (d, J = 15.4 Hz, 1H, H-β), 8.02 (d, J = 9.0 Hz, 1H, H-6′), 10.48 (s, 1H, OH), 13.76 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.6, 55.7, 59.7, 107.7, 110.6, 111.3, 113.5, 118.2, 124.2, 126.8, 127.1, 134.5, 144.2, 148.7, 151.2, 157.0, 158.2, 191.7; UV/Vis (3.3 × 10−5 M, DMSO); λ = 394.0 nm (ε, 2.3 × 104).
4,2′-Dihydroxy-3′,4′-dimethoxychalcone (7g): (0.25 g, 0.84 mmol, 84% yield); yellow solid, 152–156 °C; 1H-NMR (CDCl3) δ 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.75 (s, 1H, OH), 6.54 (d, J = 9.0 Hz, 1H, H-5′), 6.90 (d, J = 8.5 Hz, 2H, H-3 and H-5), 7.45 (d, J = 15.4 Hz, 1H, H-α), 7.57 (d, J = 8.5 Hz, 2H, H-2 and H-6), 7.69 (d, J = 9.0 Hz, 1H, H-6′), 7.86 (d, J = 15.4 Hz, 1H, H-β), 13.35 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.0, 60.6, 102.6, 115.4, 115.8, 117.4, 125.6, 127.2, 130.3, 136.3, 144.3, 157.8, 157.9, 158.0, 192.1.
3,4,2′-Trihydroxy-3′,4′-dimethoxychalcone (7h): (0.16 g, 0.51 mmol, 51% yield); green-yellow solid, 147–151 °C; 1H-NMR (CDCl3) δ 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 5.91 (s, 1H, OH), 6.01 (s, 1H, OH), 6.54 (d, J = 9.0 Hz, 1H, H-5′), 6.92 (d, J = 8.1 Hz, 1H, H-5), 7.14 (d, J = 8.3 Hz, 1H, H-6), 7.18 (s, 1H, H-2), 7.39 (d, J = 15.1 Hz, 1H, H-α), 7.67 (d, J = 9.0 Hz, 1H, H-6′), 7.77 (d, J = 15.4 Hz, 1H, H-β), 13.39 (s, 1H, OH); 13C-NMR (CD3OD) δ 56.5, 60.8, 104.2, 115.8, 116.5, 116.7, 117.9, 123.7, 127.6, 128.1, 137.3, 146.6, 146.7, 150.0, 158.5, 159.6, 193.9.
2,4,2′-Trihydroxy-3′,4′-methoxychalcone (7i): (0.13 g, 0.41 mmol, 41% yield); reddish orange solid, 120–125 °C; 1H-NMR (DMSO-d6) δ 3.69 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 6.33 (dd, J = 1.7 Hz and 8.8 Hz, 1H, H-5), 6.38 (d, J = 1.7 Hz, 1H, H-3), 6.68 (d, J = 8.8 Hz, 1H, H-5′), 7.70 (d, J = 14.9 Hz, 1H, H-α), 7.73 (d, J = 7.6 Hz, 1H, H-6′), 7.95 (d, J = 9.0 Hz, 1H, H-6), 8.09 (d, J = 15.4 Hz, 1H, H-β), 10.33 (br s, 2H, OH), 13.57 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 56.2, 59.9, 102.4, 103.5, 108.2, 113.2, 115.2, 115.4, 126.5, 130.6, 135.7, 140.5, 157.1, 157.9, 159.3, 161.8, 192.4.
4,2′-Dihydroxy-3,3′,4′-trimethoxychalcone (7j): (0.25 g, 0.77 mmol, 77% yield); yellowish brown solid, 117–124 °C; 1H-NMR (CDCl3) δ 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 5.96 (s, 1H, OH), 6.54 (d, J = 9.0 Hz, 1H, H-5′), 6.97 (d, J = 8.3 Hz, 1H, H-5), 7.13 (d, J = 1.7 Hz, 1H, H-2), 7.25 (dd, J = 1.7 Hz and 8.5 Hz, 1H, H-6), 7.43 (d, J = 15.4 Hz, 1H, H-α), 7.70 (d, J = 9.0 Hz, 1H, H-6′), 7.85 (d, J = 15.4 Hz, 1H, H-β), 13.32 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.0, 56.1, 60.6, 102.6, 110.3, 114.8, 115.6, 117.6, 123.2, 125.7, 127.2, 136.6, 144.9, 146.6, 148.3, 158.1, 158.2, 192.2.
3,2′-Dihydroxy-4,3′,4′-trimethoxychalcone (7k): (0.24 g, 0.73 mmol, 73% yield); yellowish brown solid, 107–110 °C; 1H-NMR (CDCl3) δ 3.92 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.73 (s, 1H, OH), 6.54 (d, J = 9.0 Hz, 1H, H-5′), 6.89 (d, J = 8.3 Hz, 1H, H-5), 7.14 (dd, J = 2.2 Hz and 8.3 Hz 1H, H-6), 7.30 (d, J = 2.2 Hz, 1H, H-2), 7.44 (d, J = 15.4 Hz, 1H, H-α), 7.68 (d, J = 9.0 Hz, 1H, H-6′), 7.82 (d, J = 15.4 Hz, 1H, H-β), 13.32 (s, 1H, OH); 13C-NMR (CDCl3) δ 55.9, 56.0, 60.5, 102.6, 110.3, 112.6, 115.4, 118.0, 122.9, 125.6, 128.1, 136.4, 144.3, 145.6, 148.7, 157.9, 158.0, 192.0.
2′-Hydroxy-3,4,3′,4′-tetramethoxychalcone (7l): (0.28 g, 0.80 mmol, 80% yield); orange-yellow solid, 117–120 °C; 1H-NMR (CDCl3) δ 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 6.55 (d, J = 9.3 Hz, 1H, H-5′), 6.92 (d, J = 8.3 Hz, 1H, H-5), 7.17 (d, J = 2.0 Hz, 1H, H-2), 7.27 (dd, J = 2.0 Hz and 8.3 Hz 1H, H-6), 7.45 (d, J = 15.4 Hz, 1H, H-α), 7.72 (d, J = 9.3 Hz, 1H, H-6′), 7.87 (d, J = 15.4 Hz, 1H, H-β), 13.34 (s, 1H, OH); 13C-NMR (CDCl3) δ 55.9, 55.9, 56.0, 60.5, 102.4, 109.9, 110.8, 115.3, 117.6, 123.1, 125.6, 127.3, 136.4, 144.5, 148.9, 151.2, 157.9, 158.0, 191.9.
3.7. The General Procedure for the Synthesis of Flavanones 8a–l
A solution of 7a–l (1.0 mmol) and potassium fluoride (0.29 g, 5.0 mmol) in methanol (5 mL) was refluxed for 24 h. The water was added to a mixture solution and the mixture was extracted with EtOAc. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on a preparative thin layer chromatography (hexane:toluene:EtOAc = 1:1:1) to produce flavanones 8a–l.
7,4′-Dihydroxy-8-methoxyflavanone (8a): (0.28 g, 0.98 mmol, 98% yield); pale yellow solid, 195–200 °C; 1H-NMR (CD3OD) δ 2.72 (dd, J = 2.9 Hz and 17.1 Hz, 1H, H-3ax), 3.07 (dd, J = 12.9 Hz and 17.1 Hz, 1H, H-3eq), 3.79 (s, 3H, OCH3), 5.42 (dd, J = 2.9 Hz and 12.9 Hz, 1H, H-2), 6.55 (d, J = 8.8 Hz, 1H, H-6), 6.82 (td, J = 2.0 Hz and 8.5 Hz, 2H, H-3′ and H-5′), 7.35 (td, J = 1.7 Hz and 8.3 Hz, 2H, H-2′ and H-6′), 7.51 (d, J = 8.8 Hz, 1H, H-5); 13C-NMR (CD3OD) δ 44.8, 61.2, 81.3, 111.4, 115.9, 116.2, 123.7, 128.8, 131.0, 136.4, 157.4, 158.5, 158.7, 193.2; UV/Vis (1.5 × 10−3 M, DMSO); λ = 376.4 nm (ε, 1.2 × 102).
7,3′,4′-Trihydroxy-8-methoxyflavanone (8b): (0.20 g, 0.65 mmol, 65% yield); yellowish brown solid, 188–193 °C; 1H-NMR (CD3OD) δ 2.72 (dd, J = 2.9 Hz and 17.1 Hz, 1H, H-3ax), 3.01 (dd, J = 12.9 Hz and 17.1 Hz, 1H, H-3eq), 3.81 (s, 3H, OCH3), 5.35 (dd, J = 2.9 Hz and 12.9 Hz, 1H, H-2), 6.54 (d, J = 8.8 Hz, 1H, H-6), 6.78 (d, J = 8.1 Hz, 1H, H-5′), 6.82 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6′), 6.97 (d, J = 1.7 Hz, 1H, H-2′), 7.50 (d, J = 8.8 Hz, 1H, H-5); 13C-NMR (CD3OD) δ 44.9, 61.3, 81.3, 111.3, 114.5, 115.9, 116.1, 119.0, 123.6, 131.7, 136.4, 146.2, 146.6, 157.3, 158.4, 193.2; UV/Vis (2.6 × 10−5 M, CH3OH); λ = 392.0 nm (ε, 2.7 × 103).
7,4′-Dihydroxy-8,3′-dimethoxyflavanone (8d): (0.28 g, 0.90 mmol, 90% yield); yellow solid, 193–195 °C; 1H-NMR (CD3OD) δ 2.67 (dd, J = 2.7 Hz and 16.8 Hz, 1H, H-3ax), 3.16 (dd, J = 12.7 Hz and 16.6 Hz, 1H, H-3eq), 3.69 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 5.46 (dd, J = 2.4 Hz and 12.9 Hz, 1H, H-2), 6.57 (d, J = 8.8 Hz, 1H, H-6), 6.79 (d, J = 8.8 Hz, 1H, H-6′), 6.93 (d, J = 8.8 Hz, 1H, H-5′), 7.11 (s, 1H, H-2′), 7.40 (d, J = 8.8 Hz, 1H, H-5); 13C-NMR (CD3OD) δ 43.2, 55.7, 60.2, 79.4, 110.4, 111.0, 114.4, 115.1, 119.3, 122.0, 129.9, 135.1, 146.7, 147.4, 155.7, 156.9, 190.2; UV/Vis (2.7 × 10−5 M, CH3OH); λ = 383.8 nm (ε, 1.7 × 104).
7,3′-Dihydroxy-8,4′-dimethoxyflavanone (8e): (0.27 g, 0.85 mmol, 85% yield); yellowish brown solid, 180–185 °C; 1H-NMR (DMSO-d6) δ 2.69 (dd, J = 2.9 Hz and 16.8 Hz, 1H, H-3ax), 3.06 (dd, J = 12.5 Hz and 16.8 Hz, 1H, H-3eq), 3.71 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 5.48 (dd, J = 2.9 Hz Hz and 12.5 Hz, 1H, H-2), 6.58 (d, J = 8.8 Hz, 1H, H-6), 6.91 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6′), 6.95 (d, J = 8.5 Hz, 1H, H-5′), 6.97 (d, J = 2.4 Hz, 1H, H-2′), 7.41 (d, J = 8.8 Hz, 1H, H-5); 13C-NMR (DMSO-d6) δ 43.1, 55.6, 60.1, 78.9, 110.2, 111.8, 113.8, 114.3, 117.3, 121.8, 131.4, 134.9, 146.2, 147.5, 155.4, 156.6, 189.8; UV/Vis (2.9 × 10−5 M, DMSO); λ = 363.6 nm (ε, 2.2 × 103).
7-Hydroxy-8,3′,4′-trimethoxyflavanone (8f): (0.30 g, 0.90 mmol, 90% yield); yellow solid, 143–145 °C; 1H-NMR (CDCl3) δ 2.87 (dd, J = 2.9 Hz and 16.8 Hz, 1H, H-3ax), 3.06 (dd, J = 12.9 Hz and 16.8 Hz, 1H, H-3eq), 3.91 (s, 6H, OCH3), 3.95 (s, 3H, OCH3), 5.46 (dd, J = 2.7 Hz and 12.9 Hz, 1H, H-2), 6.59 (s, 1H, OH), 6.68 (d, J = 8.8 Hz, 1H, H-6), 6.91 (d, J = 8.8 Hz, 1H, H-5′), 7.02–7.03 (m, 2H, H-2′ and H-6′), 7.65 (d, J = 8.5 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 44.2, 55.8, 61.1, 80.0, 109.1, 109.3, 110.9, 115.4, 118.4, 122.9, 130.9, 134.1, 148.8, 149.0, 154.2, 155.0, 190.2; UV/Vis (2.3 × 10−5 M, CHCl3); λ = 392.6 nm (ε, 1.4 × 103).
4′-Hydroxy-7,8-dimethoxyflavanone (8g): (0.26 g, 0.87 mmol, 87% yield); yellow solid, 165–170 °C; 1H-NMR (CDCl3) δ 2.88 (dd, J = 2.9 Hz and 16.8 Hz, 1H, H-3ax), 3.07 (dd, J = 12.2 Hz and 16.8 Hz, 1H, H-eq), 3.88 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.45 (dd, J = 2.9 Hz and 12.2 Hz, 1H, H-2), 6.39 (s, 1H, OH), 6.67 (d, J = 9.0 Hz, 1H, H-6), 6.87 (d, J = 8.5 Hz, 2H, H-3′ and H-5′), 7.31 (d, J = 8.8 Hz, 2H, H-2′ and H-6′), 7.73 (d, J = 9.0 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 44.0, 56.3, 61.1, 79.7, 105.7, 115.5, 116.0, 123.1, 127.8, 130.3, 136.7, 155.4, 156.2, 158.8, 191.6; UV/Vis (3.1 × 10−5 M, CHCl3); λ = 391.2 nm (ε, 1.2 × 103).
3′,4′-Dihydroxy-7,8-dimethoxyflavanone (8h) (0.20 g, 0.63 mmol, 63% yield); yellowish brown solid, 175–177 °C; 1H-NMR (DMSO-d6) δ 2.71 (dd, J = 2.9 Hz and 16.8 Hz, 1H, H-3ax), 3.11 (dd, J = 12.5 Hz and 16.8 Hz, 1H, H-3eq), 3.69 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 5.45 (dd, J = 2.9 Hz and 12.5 Hz, 1H, H-2), 6.76–6.77 (m, 2H, H-6 and H-6′), 6.84 (d, J = 8.8 Hz, 1H, H-5′), 6.92 (d, J = 1.7 Hz, 1H, H-2′), 7.55 (d, J = 8.8 Hz, 1H, H-5); 13C-NMR (DMSO-d6) δ 43.2, 56.1, 60.2, 79.1, 105.8, 114.1, 115.1, 115.6, 117.6, 121.9, 129.6, 136.2, 144.9, 145.4, 154.7, 158.0, 190.3.
4′-Hydroxy-7,8,3′-trimethoxyflavanone (8j): (0.30 g, 0.91 mmol, 91% yield); yellow solid, 145–146 °C; 1H-NMR (CDCl3) δ 2.88 (dd, J = 2.9 Hz and 16.8 Hz, 1H, H-3ax), 3.06 (dd, J = 12.5 Hz and 16.8 Hz, 1H, H-3eq), 3.87 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.44 (dd, J = 2.9 Hz and 12.5 Hz, 1H, H-2), 5.78 (s, 1H, OH), 6.67 (d, J = 8.8 Hz, 1H, H-6), 6.92–6.99 (m, 2H, H-5′ and H-6′), 7.02 (d, J = 1.5 Hz, 1H, H-2′), 7.71 (d, J = 9.0 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 44.1, 55.8, 56.1, 60.9, 79.7, 105.4, 108.6, 114.2, 115.9, 119.1, 122.6, 130.4, 136.7, 145.6, 146.3, 155.0, 158.4, 190.6; UV/Vis (6.1 × 10−4 M, CHCl3); λ = 370.0 nm (ε, 1.9 × 103).
3′-Hydroxy-7,8,4′-trimethoxyflavanone (8k): (0.30 g, 0.90 mmol, 90% yield); 1H-NMR (CDCl3) δ 2.85 (dd, J = 2.9 Hz and 16.8 Hz, 1H, H-3ax), 3.02 (dd, J = 12.5 Hz and 16.8 Hz, 1H, H-3eq), 3.87 (s, 3H, OCH3), 3.89 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 5.41 (dd, J = 2.9 Hz and 12.5 Hz, 1H, H-2), 5.94 (s, 1H, OH), 6.66 (d, J = 9.0 Hz, 1H, H-6), 6.87 (d, J = 8.3 Hz, 1H, H-5′), 6.95 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6′), 7.08 (d, J = 2.0 Hz, 1H, H-2′), 7.70 (d, J = 9.0 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 44.1, 55.9, 56.1, 60.9, 79.4, 105.4, 110.4, 112.4, 115.9, 117.7, 122.6, 131.6, 136.7, 145.5, 146.5, 155.0, 158.4, 190.7.
3′,4′,7,8-Tetramethoxyflavanone (8l): (0.16 g, 0.46 mmol, 46% yield); yellow solid, 141–143 °C; 1H-NMR (CDCl3) δ 2.90 (dd, J = 3.2 Hz and 16.8 Hz, 1H, H-3ax), 3.07 (dd, J = 12.2 Hz and 16.8 Hz, 1H, H-eq), 3.88 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.91 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.48 (dd, J = 2.9 Hz and 12.2 Hz, 1H, H-2), 6.67 (d, J = 9.0 Hz, 1H, H-6), 6.90 (d, J = 8.1 Hz, 1H, H-5′), 7.01–7.04 (m, 2H, H-2′ and H-6′), 7.71 (d, J = 8.8 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 44.1, 55.8, 55.8, 56.1, 60.9, 79.5, 105.4, 109.2, 110.8, 116.0, 118.4, 122.6, 131.0, 136.7, 148.8, 148.9, 154.9, 158.4, 190.5; GC-MS 344 (M+, 44), 180 (35), 164 (100).
3.8. The General Procedure for the Synthesis of Chalcones 9a–k by the Selective Deprotection of the 2′-Methoxymethyl Group
A solution of 6a–k (1.0 mmol) and 1.5 M hydrochloric acid aqueous solution (5 mL) in THF (5 mL) was stirred at room temperature for 45 min. The mixture was extracted with Et2O. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on a preparative thin layer chromatography (hexane:EtOAc = 3:2) to produce chalcones 9a–k.
2′-Hydroxy-3′-methoxy-4,4′-di(methoxymethoxy)chalcone (9a): (0.29 g, 0.77 mmol, 77% yield); 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.23 (s, 2H, OCH2), 5.32 (s, 2H, OCH2), 6.75 (d, J = 9.0 Hz, 1H, H-5′), 7.09 (d, J = 8.8 Hz, 2H, H-3 and H-5), 7.47 (d, J = 15.4 Hz, 1H, H-α), 7.61 (d, J = 8.8 Hz, 2H, H-2 and H-6), 7.64 (d, J = 9.0 Hz, 1H, H-6′), 7.88 (d, J = 15.4 Hz, 1H, H-β), 13.33 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.1, 56.4, 60.6, 94.0, 94.5, 106.0, 116.0, 116.3, 117.9, 125.2, 128.1, 130.0, 144.3, 155.6, 158.2, 159.1, 192.1.
2′-Hydroxy-3′-methoxy-3,4,4′-tri(methoxymethoxy)chalcone (9b): (0.38 g, 0.87 mmol, 87% yield); orange solid, 84–88 °C; 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 5.30 (s, 2H, OCH2), 5.30 (s, 2H, OCH2), 5.32 (s, 2H, OCH2), 6.76 (d, J = 9.0 Hz, 1H, H-5′), 7.21 (d, J = 8.5 Hz, 1H, H-5), 7.29 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6), 7.44 (d, J = 15.4 Hz, 1H, H-α), 7.48 (d, J = 2.0 Hz, 1H, H-2), 7.66 (d, J = 9.0 Hz, H-6′), 7.84 (d, J = 15.4 Hz, 1H, H-β), 13.31 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.3, 56.3, 56.4, 60.7, 94.6, 95.0, 95.5, 106.2, 116.1, 116.1, 118.6, 124.1, 125.5, 129.0, 137.4, 144.3, 144.5, 147.3, 149.6, 155.8, 158.4, 192.3.
2′-Hydroxy-3′-methoxy-2,4,4′-tri(methoxymethoxy)chalcone (9c): (0.32 g, 0.73 mmol, 73% yield); 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 5.21 (s, 2H, OCH2), 5.29 (s, 2H, OCH2), 5.32 (s, 2H, OCH2), 6.75 (d, J = 9.0 Hz, 1H, H-5′), 6.76 (dd, J = 2.2 Hz and 8.5 Hz, 1H, H-5), 6.87 (d, J = 2.2 Hz, 1H, H-3), 7.59 (d, J = 15.6 Hz, 1H, H-α), 7.61 (d, J = 8.5 Hz, 1H, H-6), 7.64 (d, J = 9.3 Hz, 1H, H-6′), 8.21 (d, J = 15.4 Hz, 1H, H-β), 13.46 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.2, 56.3, 56.4, 60.6, 94.1, 94.4, 94.5, 103.1, 105.9, 109.2, 116.1, 118.1, 118.3, 125.2, 129.8, 137.2, 139.8, 155.4, 157.6, 158.2, 160.3, 192.6.
2′-Hydroxy-3,3′-dimethoxy-4,4′-di(methoxymethoxy)chalcone (9d): (0.35 g, 0.87 mmol, 87% yield); 1H-NMR (CDCl3) δ 3.53 (s, 6H, OCH3), 3.95 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.30 (s, 2H, OCH2), 5.33 (s, 2H, OCH2), 6.77 (d, J = 9.3 Hz, 1H, H-5′), 7.17 (d, J = 1.7 Hz, 1H, H-2), 7.20 (d, J = 8.3 Hz, 1H, H-5), 7.25 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6), 7.46 (d, J = 15.4 Hz, 1H, H-α), 7.67 (d, J = 9.3 Hz, 1H, H-6′), 7.86 (d, J = 15.4 Hz, 1H, H-β), 13.34 (s, 1H, OH); 13C-NMR (CDCl3) δ 55.9, 56.3, 56.4, 60.6, 94.4, 94.9, 105.9, 110.8, 115.5, 115.9, 118.1, 122.5, 125.2, 128.6, 137.2, 144.5, 148.7, 149.4, 155.6, 158.2, 192.0.
2′-Hydroxy-4,3′-dimethoxy-3,4′-di(methoxymethoxy)chalcone (9e): (0.26 g, 0.65 mmol, 65% yield); 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.95 (s, 6H, OCH3), 5.30 (s, 2H, OCH2), 5.33 (s, 2H, OCH2), 6.76 (d, J = 9.0 Hz, 1H, H-5′), 6.94 (d, J = 8.3 Hz, 1H, H-5), 7.31 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6), 7.43 (d, J = 15.4 Hz, 1H, H-α), 7.49 (d, J = 2.2 Hz, 1H, H-2), 7.67 (d, J = 9.3 Hz, 1H, H-6′), 7.85 (d, J = 15.4 Hz, 1H, H-β), 13.36 (s, 1H, OH); 13C-NMR (CDCl3) δ 55.9, 56.2, 56.4, 60.6, 94.4, 95.3, 105.9, 111.3, 115.0, 115.9, 117.8, 124.5, 125.3, 127.4, 137.1, 144.5, 146.5, 151.9, 155.5, 158.2, 192.0.
2′-Hydroxy-3,4,3′-trimethoxy-4′-(methoxymethoxy)chalcone (9f): (0.36 g, 0.95 mmol, 95% yield); 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 3.95 (s, 6H, OCH3), 3.97 (s, 3H, OCH3), 5.33 (s, 2H, OCH2), 6.76 (d, J = 9.0 Hz, 1H, H-5′), 6.92 (d, J = 8.5 Hz, H-5), 7.16 (d, J = 1.7 Hz, 1H, H-2), 7.26 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6), 7.44 (d, J = 15.4 Hz, 1H, H-α), 7.67 (d, J = 9.0 Hz, 1H, H-6′), 7.87 (d, J = 15.4 Hz, 1H, H-β), 13.37 (s, 1H, OH); 13C-NMR (CDCl3) δ 55.8, 55.9, 56.4, 60.6, 94.4, 105.9, 109.9, 110.8, 115.9, 117.5, 123.1, 125.2, 127.3, 137.2, 144.7, 148.9, 151.3, 155.5, 158.2, 192.0.
2′-Hydroxy-3′,4′-dimethoxy-4-(methoxymethoxy)chalcone (9g): (0.32 g, 0.93 mmol, 93% yield); yellow solid, 92–95 °C; 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.23 (s, 2H, OCH2), 6.54 (d, J = 9.0 Hz, 1H, H-5′), 7.09 (d, J = 8.8 Hz, 2H, H-3 and H-5), 7.48 (d, J = 15.4 Hz, 1H, H-α), 7.61 (d, J = 8.8 Hz, 2H, H-2 and H-6), 7.69 (d, J = 9.0 Hz, 1H, H-6′), 7.87 (d, J = 15.4 Hz, 1H, H-β), 13.31 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.0, 56.1, 60.5, 94.0, 102.5, 115.4, 116.3, 117.9, 125.6, 128.1, 130.0, 136.4, 144.1, 157.9, 158.0, 159.0, 192.0.
2′-Hydroxy-3′,4′-dimethoxy-3,4-di(methoxymethoxy)chalcone (9h): (0.38 g, 0.93 mmol, 93% yield); 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.30 (s, 2H, OCH2), 5.31 (s, 2H, OCH2), 6.55 (d, J = 9.0 Hz, 1H, H-5′), 7.21 (d, J = 8.3 Hz, 1H, H-5), 7.29 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6), 7.45 (d, J = 15.4 Hz, 1H, H-α), 7.48 (d, J = 2.0 Hz, 1H, H-2), 7.70 (d, J = 9.0 Hz, 1H, H-6′), 7.84 (d, J = 15.4 Hz, 1H, H-β), 13.28 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.0, 56.2, 60.5, 94.9, 95.3, 102.5, 115.4, 115.8, 115.9, 118.4, 123.9, 125.7, 128.8, 136.4, 144.1, 147.1, 149.3, 157.9, 158.1, 191.9.
2′-Hydroxy-3′,4′-dimethoxy-2,4-di(methoxymethoxy)chalcone (9i): (0.38 g, 0.93 mmol, 93% yield); light yellow solid, 93–95 °C; 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.53 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.22 (s, 2H, OCH2), 5.29 (s, 2H, OCH2), 6.54 (d, J = 9.0 Hz, 1H, H-5′), 6.76 (dd, J = 2.4 Hz and 8.8 Hz, 1H, H-5), 6.87 (d, J = 2.2 Hz, 1H, H-3), 7.59 (d, J = 15.6 Hz, 1H, H-α), 7.62 (d, J = 8.8 Hz, 1H, H-6), 7.69 (d, J = 9.0 Hz, 1H, H-6′), 8.22 (d, J = 15.4 Hz, 1H, H-β), 13.43 (s, 1H, OH); 13C-NMR (CDCl3) δ 56.0, 56.2, 56.3, 60.5, 94.0, 94.4, 102.4, 103.0, 109.1, 115.5, 118.1, 118.3, 125.5, 129.6, 136.4, 139.5, 157.6, 157.9, 157.9, 160.2, 192.4.
2′-Hydroxy-3,3′,4′-trimethoxy-4-(methoxymethoxy)chalcone (9j): (0.34 g, 0.90 mmol, 90% yield); 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 5.30 (s, 2H, OCH2), 6.54 (d, J = 9.0 Hz, 1H, H-5′), 7.17 (d, J = 2.0 Hz, 1H, H-2), 7.20 (d, J = 8.5 Hz, 1H, H-5), 7.24 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6), 7.46 (d, J = 15.4 Hz, 1H, H-α), 7.70 (d, J = 9.0 Hz, 1H, H-6′), 7.85 (d, J = 15.4 Hz, 1H, H-β), 13.30 (s, 1H, OH); 13C-NMR (CDCl3) δ 55.9, 56.0, 56.3, 60.5, 94.9, 102.5, 111.0, 115.4, 115.6, 118.2, 122.4, 125.6, 128.7, 136.4, 144.3, 148.7, 149.5, 157.9, 158.1, 191.9.
2′-Hydroxy-4,3′,4′-trimethoxy-3-(methoxymethoxy)chalcone (9k): (0.36 g, 0.95 mmol, 95% yield); 1H-NMR (CDCl3) δ 3.57 (s, 3H, OCH3), 3.93 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 5.30 (s, 2H, OCH2), 6.55 (d, J = 9.3 Hz, 1H, H-5′), 6.94 (d, J = 8.3 Hz, 1H, H-5), 7.31 (dd, J = 2.2 Hz and 8.3 Hz, 1H, H-6), 7.44 (d, J = 15.4 Hz, 1H, H-α), 7.49(d, J = 2.2 Hz, 1H, H-2), 7.71 (d, J = 9.0 Hz, 1H, H-6′), 7.85 (d, J = 15.4 Hz, 1H, H-β), 13.32 (s, 1H, OH); 13C-NMR (CDCl3) δ 55.9, 56.0, 56.2, 60.5, 95.4, 102.5, 111.4, 115.0, 115.4, 117.8, 124.5, 125.7, 127.5, 136.4, 144.3, 146.5, 151.9, 157.9, 158.0, 191.9.
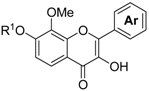
3.9. The General Procedure for the Synthesis of Flavonols 10a–l
To a solution of 7l and 9a–k (1.0 mmol) in methanol (25 mL), a 4 M sodium hydroxide aqueous solution (0.3 mL, 1.2 mmol) and a 30% hydrogen peroxide solution (0.5 mL, 5.0 mmol) were added at room temperature. After being stirred at room temperature for 12 h, the mixture was poured into ice and a 2 M hydrochloric acid aqueous solution. The precipitate was filtered, washed with water, and dried in vacuo to produce flavonols 10a–l.
8-Methoxy-7,4′-di(methoxymethoxy)flavonol (10a): (0.20 g, 0.51 mmol, 51% yield); 1H-NMR (CDCl3) δ 3.51 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 4.07 (s, 3H, OCH3), 5.26 (s, 2H, OCH2), 5.36 (s, 2H, OCH2), 7.20 (d, J = 9.0 Hz, 2H, H-3′ and H-5′), 7.26 (d, J = 9.0 Hz, 1H, H-6), 7.93 (d, J = 9.0 Hz, 1H, H-5), 8.26 (d, J = 9.0 Hz, 2H, H-2′ and H-6′); 13C-NMR (CDCl3) δ 56.1, 56.5, 61.6, 94.1, 95.1, 113.6, 116.0, 116.1, 116.5, 120.5, 124.7, 129.2, 129.2, 137.2, 137.7, 144.8, 149.7, 153.9, 158.4, 172.7.
8-Methoxy-7,3′,4′-tri(methoxymethoxy)flavonol (10b): (0.16 g, 0.36 mmol, 36% yield); 1H-NMR (CDCl3) δ 3.55 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.58 (s, 3H, OCH3), 4.08 (s, 3H, OCH3), 5.33 (s, 2H, OCH2), 5.34 (s, 2H, OCH2), 5.36 (s, 2H, OCH2), 7.09 (s, 1H, OH), 7.26 (d, J = 9.0 Hz, 1H, H-6), 7.32 (d, J = 8.8 Hz, 1H, H-5′), 7.92 (d, J = 9.3 Hz, 1H, H-5), 7.99 (dd, J = 2.0 Hz and 8.8 Hz, 1H, H-6′), 8.18 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 56.2, 56.2, 56.4, 61.5, 94.9, 95.0, 95.5, 109.7, 113.5, 115.8, 116.2, 120.3, 122.5, 125.1, 137.1, 137.5, 143.9, 146.7, 148.5, 149.6, 153.7, 172.4.
8-Methoxy-7,2′,4′-tri(methoxymethoxy)flavonol (10c): (0.15 g, 0.33 mmol, 33% yield); 1H-NMR (CDCl3) δ 3.48 (s, 3H, OCH3), 3.51 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 5.20 (s, 2H, OCH2), 5.23 (s, 2H, OCH2), 5.34 (s, 2H, OCH2), 6.43 (s, 1H, OH), 6.85 (dd, J = 2.2 Hz and 8.5 Hz, 1H, H-6′), 7.00 (d, J = 2.2 Hz, 1H, H-2′), 7.27 (d, J = 9.0 Hz, 1H, H-6), 7.54 (d, J = 8.5 Hz, 1H, H-5′), 7.96 (d, J = 9.0 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 56.1, 56.4, 61.4, 94.1, 94.9, 95.0, 104.0, 108.8, 113.4, 114.0, 117.0, 120.3, 131.4, 137.5, 137.8, 145.1, 150.2, 153.4, 156.2, 159.8, 172.4.
8,3′-Dimethoxy-7,4′-di(methoxymethoxy)flavonol (10d): (0.11 g, 0.26 mmol, 26% yield); 1H-NMR (CDCl3) δ 3.55 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 4.07 (s, 3H, OCH3), 5.33 (s, 2H, OCH2), 5.36 (s, 2H, OCH2), 7.00 (s, 1H, OH), 7.27 (d, J = 9.0 Hz, 1H, H-6), 7.32 (d, J = 8.5 Hz, 1H, H-5′), 7.90 (dd, J = 2.0 Hz and 8.8 Hz, 1H, H-6′), 7.93 (d, J = 9.0 Hz, 1H, H-5), 7.95 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.8, 56.2, 56.5, 61.5, 95.0, 110.8, 113.5, 115.5, 116.2, 120.4, 120.8, 125.1, 137.1, 137.4, 144.1, 147.7, 149.1, 149.5, 153.7, 172.4.
8,4′-Dimethoxy-7,3′-di(methoxymethoxy)flavonol (10e): (0.27 g, 0.65 mmol, 65% yield); 1H-NMR (CDCl3) δ 3.56 (s, 3H, OCH3), 3.58 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.08 (s, 3H, OCH3), 5.34 (s, 2H, OCH2), 5.36 (s, 2H, OCH2), 6.98 (s, 1H, OH), 7.06 (d, J = 8.8 Hz, 1H, H-5′), 7.26 (d, J = 9.0 Hz, 1H, H-6), 7.92 (d, J = 9.0 Hz, 1H, H-5), 8.04 (dd, J = 2.2 Hz and 8.5 Hz, 1H, H-6′), 8.17 (d, J = 2.2 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.9, 56.2, 56.4, 61.5, 95.0, 95.5, 111.3, 113.4, 115.3, 116.3, 120.3, 122.7, 123.8, 137.0, 137.5, 144.0, 146.1, 149.5, 151.0, 153.7, 172.4.
8,3′,4′-Trimethoxy-7-(methoxymethoxy)flavonol (10f): (0.15 g, 0.38 mmol, 38% yield); light yellow solid, 163–166 °C; 1H-NMR (CDCl3) δ 3.56 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 4.08 (s, 3H, OCH3), 5.37 (s, 2H, OCH2), 7.00 (s, 1H, OH), 7.05 (d, J = 8.5 Hz, 1H, H-6), 7.28 (d, J = 7.6 Hz, 1H, H-5′), 7.93 (s, 1H, H-2′), 7.94 (d, J = 8.8 Hz, 1H, H-5), 7.96 (dd, J = 2.0 Hz and 8.1Hz, 1H, H-6′); 13C-NMR (CDCl3) δ 55.7, 55.8, 56.5, 61.5, 94.9, 110.1, 110.7, 113.3, 116.2, 120.4, 121.0, 123.6, 136.9, 137.3, 144.2, 148.4, 149.5, 150.2, 153.6, 172.3.
7,8-Dimethoxy-4′-(methoxymethoxy)flavonol (10g): (0.16 g, 0.45 mmol, 45% yield); 1H-NMR (CDCl3) δ 3.51 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.04 (s, 3H, OCH3), 5.27 (s, 2H, OCH2), 6.94 (s, 1H, OH), 7.07 (d, J = 9.0 Hz, 1H, H-6), 7.20 (d, J = 9.0 Hz, 2H, H-3′ and H-5′), 7.97 (d, J = 9.0 Hz, 1H, H-5), 8.26 (d, J = 9.0 Hz, 2H, H-2′ and H-6′); 13C-NMR (CDCl3) δ 56.0, 56.4, 61.4, 94.0, 109.8, 115.4, 115.9, 120.5, 124.6, 129.0, 136.7, 144.3, 149.4, 156.0, 158.1, 172.5.
7,8-Dimethoxy-3′,4′-di(methoxymethoxy)flavonol (10h): (0.12 g, 0.28 mmol, 28% yield); 1H-NMR (CDCl3) δ 3.55 (s, 3H, OCH3), 3.58 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.05 (s, 3H, OCH3), 5.33 (s, 2H, OCH2), 5.34 (s, 2H, OCH2), 6.97 (s, 1H, OH), 7.07 (d, J = 9.3 Hz, 1H, H-6), 7.33 (d, J = 8.8 Hz, 1H, H-5′), 7.96 (d, J = 9.0 Hz, 1H, H-5), 7.98 (dd, J = 2.2 Hz and 8.8 Hz, 1H, H-6′), 8.18 (d, J = 2.2 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 56.2, 56.2, 56.4, 61.4, 94.9, 95.5, 109.8, 115.3, 115.7, 115.8, 120.5, 122.5, 125.2, 136.5, 136.9, 143.8, 146.7, 148.4, 149.4, 156.1, 172.5.
7,8-Dimethoxy-2′,4′-di(methoxymethoxy)flavonol (10i): (0.07 g, 0.17 mmol, 17% yield); 1H-NMR (CDCl3) δ 3.48 (s, 3H, OCH3), 3.51 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 5.20 (s, 2H, OCH2), 5.23 (s, 2H, OCH2), 6.42 (s, 1H, OH), 6.85 (dd, J = 2.2 Hz and 8.8 Hz, 1H, H-6′), 7.00 (d, J = 2.2 Hz, 1H, H-2′), 7.08 (d, J = 9.0 Hz, 1H, H-6), 7.54 (d, J = 8.8 Hz, 1H, H-5′), 8.00 (d, J = 9.0 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 56.1, 56.4, 61.3, 94.1, 94.8, 104.0, 108.8, 109.6, 114.0, 116.1, 120.5, 131.4, 136.5, 137.6, 145.0, 150.0, 155.7, 156.2, 159.8, 172.5.
7,8,3′-Trimethoxy-4′-(methoxymethoxy)flavonol (10j): (0.22 g, 0.56 mmol, 56% yield); 1H-NMR (CDCl3) δ 3.55 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.05 (s, 3H, OCH3), 5.33 (s, 2H, OCH2), 7.00 (s, 1H, OH), 7.08 (d, J = 9.0 Hz, 1H, H-6), 7.32 (d, J = 8.8 Hz, 1H, H-5′), 7.90 (dd, J = 2.0 Hz and 8.8 Hz, 1H, H-6′), 7.95 (d, J = 2.0 Hz, 1H, H-2′), 7.97 (d, J = 9.0 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 55.8, 56.2, 56.4, 61.3, 94.9, 109.8, 110.8, 115.3, 115.4, 120.6, 120.8, 125.1, 136.4, 136.9, 144.0, 147.7, 149.0, 149.4, 156.1, 172.5.
7,8,4′-Trimethoxy-3′-(methoxymethoxy)flavonol (10k): (0.24 g, 0.61 mmol, 61% yield); 1H-NMR (CDCl3) δ 3.58 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.06 (s, 3H, OCH3), 5.34 (s, 2H, OCH2), 7.00 (s, 1H, OH), 7.06 (d, J = 8.5 Hz, 1H, H-5′), 7.07 (d, J = 9.0 Hz, 1H, H-6), 7.96 (d, J = 9.0 Hz, 1H, H-5), 8.04 (dd, J = 2.2 Hz and 8.8 Hz, 1H, H-6′), 8.18 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 56.0, 56.4, 56.6, 61.6, 95.7, 109.9, 111.4, 115.5, 116.0, 120.7, 122.9, 124.0, 136.6, 137.0, 144.2, 146.2, 149.5, 151.1, 156.2, 172.6.
7,8,3′,4′-Tetramethoxyflavonol (10l): (0.12 g, 0.33 mmol, 33% yield); pale yellow solid, 215–217 °C; 1H-NMR (CDCl3) δ 3.98 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.05 (s, 3H, OCH3), 7.01 (s, 1H, OH), 7.04 (d, J = 8.5 Hz, 1H, H-5′), 7.08 (d, J = 9.0 Hz, 1H, H-6), 7.92 (d, J = 2.0 Hz, 1H, H-2′), 7.95 (dd, J = 2.0 Hz and 8.1 Hz, 1H, H-6′), 7.97 (d, J = 8.8 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 55.7, 55.8, 56.4, 61.3, 109.7, 110.2, 110.7, 115.3, 120.5, 121.0, 123.7, 136.4, 136.7, 144.2, 148.5, 149.3, 150.2, 156.0, 172.4; UV/Vis (2.1 × 10−5 M, CHCl3); λ = 363.6 nm (ε, 2.8 × 104).
3.10. The General Procedure for the Deprotection of Flavonols 10a–k
A solution of 10a–k (1.0 mmol) in methanol (5 mL) and 3 M hydrochloric acid (5 mL) was refluxed for 1 h. The mixture was extracted with EtOAc. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on a preparative thin layer chromatography (hexane:EtOAc = 2:3) to produce flavonols 11a–k.
7,4′-Dihydroxy-8-methoxyflavonol (11a): (0.18 g, 0.45 mmol, 80% yield); pale yellowish brown solid, 263–267 °C; 1H-NMR (DMSO-d6) δ 3.93 (s, 3H, OCH3), 6.96 (d, J = 9.0 Hz, 2H, H-3′ and H-5′), 6.98 (d, J = 8.8 Hz, 1H, H-6), 7.68 (d, J = 8.8 Hz, 1H, H-5), 8.06 (d, J = 8.8 Hz, 2H, H-2′ and H-6′), 9.14 (s, 1H, OH), 10.07 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 60.8, 114.6, 114.9, 115.3, 119.8, 122.1, 128.8, 134.4, 136.8, 144.7, 149.1, 154.1, 158.5, 171.8; UV/Vis (2.8 × 10−5 M, CH3OH); λ = 358.5 nm (ε, 1.8 × 104).
7,3′,4′-Trihydroxy-8-methoxyflavonol (11b): (0.16 g, 0.49 mmol, 98% yield); yellow solid, 257–258 °C; 1H-NMR (DMSO-d6) δ 3.94 (s, 3H, OCH3), 6.91 (d, J = 8.5 Hz, 1H, H-5′), 6.98 (d, J = 9.0 Hz, 1H, H-6), 7.59 (dd, J = 2.2 and 8.5 Hz, 1H, H-6′), 7.67 (d, J = 8.8 Hz, 1H, H-5), 7.72 (d, J = 2.2 Hz, 1H, H-2′), 9.10 (s, 1H, OH), 9.35 (s, 1H, OH), 9.52 (s, 1H, OH), 10.51 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 60.9, 114.5, 114.6, 114.9, 115.4, 119.4, 119.8, 122.4, 134.4, 136.8, 144.7, 144.8, 147.0, 149.1, 154.0, 171.7; UV/Vis (2.5 × 10−5 M, CH3OH); λ = 366.0 nm (ε, 2.1 × 104).
7,2′,4′-Trihydroxy-8-methoxyflavonol (11c): (0.06 g, 0.18 mmol, 36% yield); pale green-yellow solid, 265 °C (decomp.); 1H-NMR (DMSO-d6) δ 3.90 (s, 3H, OCH3), 6.17 (d, J = 1.7 Hz, 1H, H-3′), 6.31 (dd, J = 2.0 Hz and 8.8 Hz, 1H, H-5′), 6.95 (d, J = 8.8 Hz, 1H, H-6), 7.52 (d, J = 8.3 Hz, 1H, H-6′), 7.68 (d, J = 8.8 Hz, 1H, H-5), 9.46 (s, 1H, OH), 10.32 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 60.8, 104.8, 106.1, 113.2, 114.1, 115.0, 119.7, 127.9, 134.4, 142.9, 146.8, 149.4, 153.1, 159.8, 160.6, 175.9; UV/Vis (2.7 × 10−5 M, CH3OH); λ = 395.0 nm (ε, 1.5 × 104).
7,4′-Dihydroxy-8,3′-dimethoxyflavonol (11d): (0.14 g, 0.43 mmol, 85% yield); pale yellow solid, 281–282 °C; 1H-NMR (DMSO-d6) δ 3.85 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 6.97 (d, J = 8.5 Hz, 1H, H-5′), 6.99 (d, J = 8.8 Hz, 1H, H-6), 7.68 (d, J = 8.8 Hz, 1H, H-5), 7.74 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.78 (d, J = 2.0 Hz, 1H, H-2′), 9.25 (s, 1H, OH), 9.75 (s, 1H, OH), 10.56 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.6, 60.9, 111.0, 114.7, 114.9, 115.5, 119.9, 121.2, 122.5, 134.4, 137.0, 144.6, 147.2, 148.2, 149.2, 154.2, 171.9; UV/Vis (2.7 × 10−5 M, acetone); λ = 353.0 nm (ε, 1.8 × 104).
7,3′-Dihydroxy-8,4′-dimethoxyflavonol (11e): (0.14 g, 0.43 mmol, 86% yield); yellow solid, 241–244 °C; 1H-NMR (DMSO-d6) δ 3.86 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 7.00 (d, J = 8.8 Hz, 1H, H-6), 7.13 (d, J = 8.5 Hz, 1H, H-5′), 7.69 (d, J = 8.8 Hz, 1H, H-5), 7.71 (dd, J = 2.2 Hz and 8.5 Hz, 1H, H-6′), 7.74 (d, J = 2.2 Hz, 1H, H-2′), 9.21 (s, 1H, OH), 9.39 (s, 1H, OH), 10.54 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.5, 60.9, 111.7, 114.2, 114.7, 114.9, 119.1, 119.8, 123.9, 134.4, 137.2, 144.2, 146.0, 148.7, 149.1, 154.1, 171.8; UV/Vis (2.8 × 10−5 M, CH3OH); λ = 361.5 nm (ε, 2.2 × 104).
7-Hydroxy-8,3′,4′-trimethoxyflavonol (11f): (0.07 g, 0.19 mmol, 38% yield); ocher solid, 202–206 °C; 1H-NMR (DMSO-d6) δ 3.85 (s, 3H, OCH3), 3.86 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 7.00 (d, J = 8.8 Hz, 1H, H-6), 7.18 (d, J = 8.5 Hz, 1H, H-5′), 7.69 (d, J = 8.8 Hz, 1H, H-5), 7.80 (d, J = 2.0 Hz, 1H, H-2′), 7.85 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 9.29 (s, 1H, OH), 10.56 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.4, 55.6, 60.8, 110.3, 111.5, 114.8, 114.9, 119.9, 120.8, 123.8, 134.4, 137.4, 144.1, 148.2, 149.2, 149.8, 154.3, 171.9; UV/Vis (2.4 × 10−5 M, CHCl3); λ = 359.0 nm (ε, 1.9 × 104).
4′-Hydroxy-7,8,dimethoxyflavonol (11g): (0.09 g, 0.29 mmol, 57% yield); pale yellow solid, 235–236 °C; 1H-NMR (DMSO-d6) δ 3.93 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 6.97 (d, J = 8.8 Hz, 2H, H-3′ and H-5′), 7.27 (d, J = 9.0 Hz, 1H, H-6), 7.83 (d, J = 9.0 Hz, 1H, H-5), 8.08 (d, J = 8.8 Hz, 2H, H-2′ and H-6′), 9.26 (s, 1H, OH), 10.12 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 56.5, 61.0, 110.4, 115.3, 116.1, 119.9, 122.0, 129.0, 135.9, 136.9 145.3, 148.4, 155.5, 158.7, 171.9; UV/Vis (2.7 × 10−5 M, CH3OH); λ = 361.5 nm (ε, 2.5 × 104).
3′,4′-Dihydroxy-7,8-dimethoxyflavonol (11h): (0.13 g, 0.40 mmol, 79% yield); yellow ocher solid, 261–263 °C; 1H-NMR (DMSO-d6) δ 3.84 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 6.37 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-5′), 6.42 (d, J = 2.0 Hz, 1H, H-3′), 7.25 (d, J = 9.0 Hz, 1H, H-6), 7.26 (d, J = 8.3 Hz, 1H, H-6′), 7.83 (d, J = 9.0 Hz, 1H, H-5), 9.68 (s, 1H, OH), 9.78 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 56.4, 60.9, 102.9, 106.6, 109.5, 110.3, 116.8, 119.9, 131.6, 136.0, 137.4, 147.5, 149.2, 155.3, 156.7, 160.1, 172.1; UV/Vis (2.5 × 10−5 M, CH3OH); λ = 369.0 nm (ε, 2.4 × 104).
2′,4′-Dihydroxy-7,8,3′-trimethoxyflavonol (11i): (0.07 g, 0.22 mmol, 43% yield); red-clay solid, 192–196 °C; 1H-NMR (DMSO-d6) δ 3.95 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 6.92 (d, J = 8.5 Hz, 1H, H-5′), 7.26 (d, J = 9.0 Hz, 1H, H-6), 7.62 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.75 (d, J = 2.0 Hz, 1H, H-2′), 7.82 (d, J = 9.0 Hz, 1H, H-5), 9.22 (s, 1H, OH), 9.40 (s, 1H, OH), 9.56 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 56.4, 61.1, 110.3, 114.6, 115.4, 116.0, 119.6, 119.9, 122.3, 135.8, 136.9, 144.8, 145.2, 147.2, 148.3, 155.4, 171.8.
4′-Hydroxy-7,8,3′-trimethoxyflavonol (11j): (0.08 g, 0.24 mmol, 48% yield); ocher solid, 177–181 °C; 1H-NMR (CDCl3) δ 4.01 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 4.05 (s, 3H, OCH3), 6.01 (s, 1H, OH), 7.00 (s, 1H, OH), 7.07 (d, J = 9.0 Hz, 1H, H-6), 7.09 (d, J = 8.3 Hz, 1H, H-5′), 7.88 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.93 (d, J = 2.0 Hz, 1H, H-2′), 7.97 (d, J = 9.0 Hz, 1H, H-5); 13C-NMR (CDCl3) δ 55.8, 56.4, 61.3, 109.7, 109.9, 114.4, 115.3, 120.5, 121.4, 123.2, 136.4, 136.6, 144.3, 146.1, 147.1, 149.3, 156.0, 172.4; UV/Vis (2.8 × 10−5 M, CHCl3); λ = 362.5 nm (ε, 2.3 × 104).
3′-Hydroxy-7,8,4′-trimethoxyflavonol (11k): (0.09 g, 0.27 mmol, 53% yield); pale yellow solid, 231–234 °C; 1H-NMR (DMSO-d6) δ 3.86 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 3.97 (s, 3H, OCH3), 7.13 (d, J = 8.5 Hz, 1H, H-5′), 7.27 (d, J = 9.0 Hz, 1H, H-6), 7.72 (dd, J = 2.2 Hz and 8.5 Hz, 1H, H-6′), 7.75 (d, J = 2.2 Hz, 1H, H-2′), 7.83 (d, J = 9.0 Hz, 1H, H-5), 9.33 (s, 1H, OH), 9.42 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.5, 56.4, 61.0, 110.3, 111.7, 114.2, 116.0, 119.3, 119.9, 123.8, 135.8, 137.3, 144.7, 146.0, 148.4, 148.8, 155.5, 171.9; UV/Vis (2.3 × 10−5 M, CHCl3); λ = 360.5 nm (ε, 2.6 × 104).
3.11. The General Procedure for the Preparation of 4-Chloroacetylpyrogallols 12a,b
Chloroacetyl chloride (4.3 mL, 54.0 mmol) was added to a suspension of anhydrous aluminum chloride (8.00 g, 60.0 mmol) and 1,2-dichloroethane (100 mL) was added under an argon atmosphere at room temperature. A solution of 1a,b (30.0 mmol) in 1,2-dichloroethane (30 mL) was added to the mixture and the reaction mixture was stirred at room temperature for 12 h. The mixture was poured into ice and a 2 M HCl solution and extracted with CHCl3. The organic layer was washed with water and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on silica gel with CHCl3-Et2O (9:1) to produce 12a,b.
1-Chloroacetyl-2,4-dihydroxy-3-methoxybenzene (12a): (3.77 g, 17.4 mmol, 58% yield); 1H-NMR (CDCl3) δ 4.00 (s, 3H, OCH3), 4.64 (s, 2H, CH2), 6.56 (d, J = 9.0 Hz, 1H, H-5), 6.57 (s, 1H, OH), 7.41 (d, J = 9.0 Hz, 1H, H-6), 12.24 (s, 1H, OH); 13C-NMR (CDCl3) δ 44.8, 60.9, 107.2, 112.2, 126.3, 134.3, 155.9, 156.8, 195.2.
1-Chloroacetyl-2-hydroxy-3,4-dimethoxybenzene (12b): (3.74 g, 16.2 mmol, 54% yield); colorless solid, 155–158 °C; 1H-NMR (CDCl3) δ 3.89 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.65 (s, 2H, CH2), 6.54 (d, J = 9.0 Hz, 1H, H-5), 7.49 (d, J = 9.0 Hz, 1H, H-6), 11.85 (s, 1H, OH); 13C-NMR (CDCl3) δ 45.0, 56.2, 60.7, 103.5, 112.7, 126.1, 136.6, 157.2, 159.1, 195.0.
3.12. The General Procedure for the Preparation of Benzofuranones 13a,b
A solution of 12a,b (20.0 mmol) and sodium acetate (6.56 g, 80.0 mmol or 3.28 g, 40.0 mmol) in methanol (100 mL) was refluxed for 2 h. Water was added to the mixture and extracted with Et2O. The organic layer was washed with brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on silica gel with CHCl3-Et2O (9:1) to produce 13a,b.
6-Hydroxy-7-methoxy-3(2H)-benzofuranone (13a): (2.77 g, 15.4 mmol, 77% yield); 1H-NMR (DMSO-d6) δ 3.89 (s, 3H, OCH3), 4.76 (s, 2H, CH2), 6.85 (d, J = 8.5 Hz, 1H, H-5), 7.12 (d, J = 8.5 Hz, 1H, H-4), 9.37 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 56.5, 75.2, 107.4, 113.7, 115.5, 131.7, 154.6, 162.1, 198.0.
6,7-Dimethoxy-3(2H)-benzofuranone (13b): (3.22 g, 16.6 mmol, 83% yield); reddish yellow solid, 119–123 °C; 1H-NMR (CDCl3) δ 3.97 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 4.67 (s, 2H, CH2), 6.72 (d, J = 8.5 Hz, 1H, H-5), 7.41 (d, J = 8.5 Hz, 1H, H-4); 13C-NMR (CDCl3) δ 56.7, 61.0, 75.5, 107.3, 116.3, 119.1, 134.2, 159.4, 166.0, 197.8.
3.13. The General Procedure for the Protection of 13a with a Chloromethyl Methyl Ether
A solution of 13a (0.90 g, 5.0 mmol) in DMF (5 mL) was added to a suspension of sodium hydride (60% in mineral oil, 0.24 g, 6.0 mmol) in DMF (15 mL) at 0 °C. After being stirred at room temperature for 30 min, a chloromethyl methyl ether (0.57 mL, 7.5 mmol) was added to the mixture at 0 °C. After being stirred at room temperature for 6 h, Et2O (20 mL) was added to the mixture. The reaction mixture was poured into ice water (200 mL). The mixture was extracted with Et2O. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on silica gel with CHCl3-Et2O (9:1) to produce 13c.
7-Methoxy-6-(methoxymethoxy)-3(2H)-benzofuranone (13c): (0.81 g, 3.6 mmol, 72% yield); dark brown solid, 203–210 °C; 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 4.03 (s, 3H, OCH3), 4.68 (s, 2H, OCH2), 5.32 (s, 2H, OCH2), 6.94 (d, J = 8.5 Hz, 1H, H-5), 7.37 (d, J = 8.5 Hz, 1H, H-4); 13C-NMR (CDCl3) δ 56.6, 61.0, 75.4, 95.0, 110.8, 117.0, 118.8, 156.9, 166.3, 197.9.
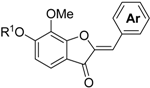
3.14. The General Procedure for the Synthesis of Aurones 14a–l
Aluminum oxide (basic, 2.00 g, 19.6 mmol) was added to a solution of benzofuranones 13b,c (1.0 mmol) and benzaldehydes 5a–f (1.2 mmol) in dichloromethane (5 mL). The mixture was thoroughly stirred for 2 days at room temperature. The suspension was filtered off and the residue was washed with CHCl3. The filtrate was concentrated in vacuo and the residue was chromatographed on a preparative thin layer chromatography (CHCl3:Et2O = 9:1) to produce (Z)-aurones 14a–l.
(Z)-7-Methoxy-6,4′-di(methoxymethoxy)aurone (14a): (0.32 g, 0.86 mmol, 86% yield); reddish yellow solid, 92–94 °C; 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 4.19 (s, 3H, OCH3), 5.24 (s, 2H, OCH2), 5.33 (s, 2H, OCH2), 6.84 (s, 1H, H-10), 7.03 (d, J = 8.5 Hz, 1H, H-5), 7.12 (d, J = 9.0 Hz, 2H, H-3′ and H-5′), 7.48 (d, J = 8.3 Hz, 1H, H-4), 7.87 (d, J = 8.8 Hz, 2H, H-2′ and H-6′); 13C-NMR δ (CDCl3) 56.1, 56.5, 61.0, 93.9, 95.1, 111.7, 112.2, 116.3, 117.6, 119.0, 125.7, 132.7, 134.6, 146.1, 155.9, 157.4, 158.1, 182.7.
(Z)-7-Methoxy-6,3′,4′-tri(methoxymethoxy)aurone (14b): (0.23 g, 0.54 mmol, 54% yield); light yellow solid, 85–86 °C; 1H-NMR (CDCl3) δ 3.54 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 4.21 (s, 3H, OCH3), 5.32 (s, 4H, OCH2), 5.34 (s, 2H, OCH2), 6.82 (s, 1H, H-10), 7.04 (d, J = 8.5 Hz, 1H, H-5), 7.24 (d, J = 8.5 Hz, 1H, H-5′), 7.47 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6′), 7.48 (d, J = 8.5 Hz, 1H, H-4), 7.88 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 56.1, 56.2, 56.5, 61.0, 94.7, 95.0, 95.1, 111.5, 112.2, 115.7, 117.5, 118.4, 118.9, 126.2, 126.5, 134.5, 146.1, 146.8, 148.3, 155.8, 157.3, 182.8.
(Z)-7-Methoxy-6,2′,4′-tri(methoxymethoxy)aurone (14c): (0.29 g, 0.67 mmol, 67% yield); yellow solid, 105–110 °C; 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.52 (s, 3H, OCH3), 3.67 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 5.22 (s, 2H, OCH2), 5.27 (s, 2H, OCH2), 5.33 (s, 2H, OCH2), 6.78 (dd, J = 2.2 Hz and 9.0 Hz, 1H, H-5′) 6.81, (d, J = 8.8 Hz, 1H, H-5), 6.87 (d, J = 2.2 Hz, 1H, H-3′), 7.38 (s, 1H, H-10), 7.58 (d, J = 8.5 Hz, 1H, H-4), 8.27 (d, J = 8.8 Hz, 1H, H-6′); 13C-NMR (CDCl3) δ 56.2, 56.3, 56.5, 61.0, 93.9, 94.4, 95.1, 102.7, 106.5, 109.1, 111.4, 115.5, 117.8, 118.9, 132.4, 133.6, 146.2, 155.6, 157.2, 157.6, 159.5, 182.8.
(Z)-7,3′-Dimethoxy-6,4′-di(methoxymethoxy)aurone (14d): (0.28 g, 0.69 mmol, 69% yield); reddish yellow solid, 95–100 °C; 1H-NMR (CDCl3) δ 3.54 (s, 3H, OCH3), 3.55 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.18 (s, 3H, OCH3), 5.32 (s, 2H, OCH2), 5.34 (s, 2H, OCH2), 6.84 (s, 1H, H-10), 7.04 (d, J = 8.5 Hz, 1H, H-5), 7.24 (d, J = 8.5 Hz, 1H, H-5′), 7.44 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.50 (d, J = 8.5 Hz, 1H, H-4), 7.60 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.6, 56.2, 56.5, 60.8, 94.8, 95.1, 111.6, 112.4, 113.5, 115.4, 117.5, 119.2, 125.2, 126.2, 134.4, 146.2, 147.7, 149.2, 155.9, 157.5, 182.7.
(Z)-7,4′-Dimethoxy-6,3′-di(methoxymethoxy)aurone (14e): (0.34 g, 0.85 mmol, 85% yield); light yellow solid, 130–133 °C; 1H-NMR (CDCl3) δ 3.55 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 4.20 (s, 3H, OCH3), 5.30 (s, 2H, OCH2), 5.33 (s, 2H, OCH2), 6.82 (s, 1H, H-10), 6.97 (d, J = 8.5 Hz, 1H, H-5′), 7.03 (d, J = 8.5 Hz, 1H, H-5), 7.48 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.48 (d, J = 8.5 Hz, 1H, H-4), 7.89 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.8, 56.1, 56.5, 61.0, 95.2, 95.3, 111.4, 111.7, 112.5, 117.7, 118.1, 118.9, 125.0, 126.9, 134.6, 146.0, 146.4, 151.0, 155.8, 157.3, 182.7.
(Z)-7,3′,4′-Trimethoxy-6-(methoxymethoxy)aurone (14f): (0.12 g, 0.31 mmol, 31% yield); light yellow solid, 157–161 °C; 1H-NMR (CDCl3) δ 3.55 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.18 (s, 3H, OCH3), 5.34 (s, 2H, OCH2), 6.84 (s, 1H, H-10), 6.95 (d, J = 8.3 Hz, 1H, H-5′), 7.04 (d, J = 8.5 Hz, 1H, H-5), 7.44 (dd, J = 1.7 Hz and 8.5 Hz, 1H, H-6′), 7.49 (d, J = 8.3 Hz, 1H, H-4), 7.60 (d, J = 1.7 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.7, 55.9, 56.6, 60.9, 95.2, 111.0, 111.7, 112.9, 113.1, 117.7, 119.3, 125.1, 125.7, 134.6, 146.1, 148.8, 150.5, 156.1, 157.6, 182.9.
(Z)-6,7-Dimethoxy-4′-(methoxymethoxy)aurone (14g): (0.31 g, 0.89 mmol, 89% yield); 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.18 (s, 3H, OCH3), 5.24 (s, 2H, OCH2), 6.80 (d, J = 8.5 Hz, 1H, H-5), 6.83 (s, 1H, H-10), 7.12 (d, J = 8.5 Hz, 2H, H-3′ and H-5′), 7.52 (d, J = 8.5 Hz, 1H, H-4), 7.87 (d, J = 8.8 Hz, 2H, H-2′ and H-6′); 13C-NMR (CDCl3) δ 56.2, 56.8, 61.1, 94.2, 108.0, 112.1, 116.6, 117.1, 119.5, 126.0, 132.9, 133.9, 146.6, 157.4, 158.4, 158.6, 183.0.
(Z)-6,7-Dimethoxy-3′,4′-di(methoxymethoxy)aurone (14h): (0.31 g, 0.78 mmol, 78% yield); yellow solid, 133–135 °C; 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 3.56 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.19 (s, 3H, OCH3), 5.31 (s, 4H, OCH2), 6.80 (s, 1H, H-10), 6.80 (d, J = 8.5 Hz, 1H, H-5), 7.24 (d, J = 8.3 Hz, 1H, H-5′), 7.47 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6′), 7.52 (d, J = 8.5 Hz, 1H, H-4), 7.87 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 56.1, 56.2, 56.7, 61.0, 94.8, 95.2, 107.7, 111.9, 115.8, 116.7, 118.6, 119.2, 126.4, 126.5, 133.6, 146.3, 146.9, 148.4, 157.0, 158.3, 182.7.
(Z)-6,7-Dimethoxy-2′,4′-di(methoxymethoxy)aurone (14i): (0.36 g, 0.89 mmol, 89% yield); light yellow solid, 115–120 °C; 1H-NMR (CDCl3) δ 3.50 (s, 3H, OCH3), 3.52 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.16 (s, 3H, OCH3), 5.22 (s, 2H, OCH2), 5.27 (s, 2H, OCH2), 6.80 (d, J = 8.5 Hz, 1H, H-5), 6.83 (dd, J = 2.2 Hz and 8.8 Hz, 1H, H-5′), 6.88 (d, J = 2.2 Hz, 1H, H-3′), 7.38 (s, 1H, H-10), 7.52 (d, J = 8.3 Hz, 1H, H-4), 8.26 (d, J = 8.5 Hz, 1H, H-6′); 13C-NMR (CDCl3) δ 56.1, 56.3, 56.6, 60.9, 94.0, 94.5, 102.8, 106.2, 107.6, 109.2, 115.7, 117.0, 119.2, 132.4, 133.6, 146.4, 156.9, 157.6, 158.1, 159.5, 182.7.
(Z)-6,7,3′-Trimethoxy-4′-(methoxymethoxy)aurone (14j): (0.30 g, 0.81 mmol, 81% yield); yellow solid, 158–162 °C; 1H-NMR (CDCl3) δ 3.53 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 4.16 (s, 3H, OCH3), 5.31 (s, 2H, OCH2), 6.81 (d, J = 8.1 Hz, 1H, H-5), 6.82 (s, 1H, H-10), 7.23 (d, J = 8.5 Hz, 1H, H-5′), 7.43 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.53 (d, J = 8.5 Hz, 1H, H-4), 7.59 (d, J = 1.7 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.7, 56.2, 56.7, 60.8, 94.9, 107.8 112.2, 113.6, 115.5, 116.7, 119.4, 125.2, 126.3, 133.5, 146.4, 147.7, 149.2, 157.2, 158.4, 182.6.
(Z)-6,7,4′-Trimethoxy-3′-(methoxymethoxy)aurone (14k): (0.29 g, 0.78 mmol, 78% yield); yellow solid, 162–167 °C; 1H-NMR (CDCl3) δ 3.55 (s, 3H, OCH3), 3.94 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 4.19 (s, 3H, OCH3), 5.30 (s, 2H, OCH2), 6.80 (d, J = 8.3 Hz, 1H, H-5), 6.80 (s, 1H, H-10), 6.97 (d, J = 8.3 Hz, 1H, H-5′), 7.48 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.51 (d, J = 8.5 Hz, 1H, H-4), 7.88 (d, J = 2.2 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 56.0, 56.3, 56.8, 61.1, 95.4, 107.9 111.6, 112.3, 117.0, 118.3, 119.3, 125.2, 126.9, 133.8, 146.3, 146.5, 151.1, 157.1, 158.4, 182.8.
(Z)-6,7,3′4′-Tetramethoxyaurone (14l): (0.27 g, 0.80 mmol, 80% yield); yellow solid, 156–157 °C; 1H-NMR (CDCl3) δ 3.95 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.16 (s, 3H, OCH3), 6.80 (d, J = 8.5 Hz, 1H, H-5), 6.82 (s, 1H, H-10), 6.95 (d, J = 8.3 Hz, 1H, H-5′), 7.44 (dd, J = 2.0 Hz and 8.5 Hz, 1H, H-6′), 7.53 (d, J = 8.3 Hz, 1H, H-4), 7.60 (d, J = 2.0 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.6, 55.8, 56.6, 60.8, 107.8, 110.9, 112.4, 113.0, 116.8, 119.4, 125.0, 125.5, 133.5, 146.2, 148.7, 150.3, 157.1, 158.4, 182.6; UV/Vis (2.2 × 10−5 M, CHCl3); λ = 406.8 nm (ε, 2.3 × 104).
3.15. The General Procedure for the Deprotection of 14a–k
A solution of 14a–k (1.0 mmol) in methanol (5 mL) and 3 M hydrochloric acid (5 mL) was refluxed for 1 h. The mixture was extracted with EtOAc. The organic layer was washed with water and brine and dried over anhydrous MgSO4. The solvent was evaporated in vacuo and the residue was chromatographed on a preparative thin layer chromatography (hexane:EtOAc = 2:3) to produce aurones 15a–k.
(Z)-6,4′-Dihydroxy-7-methoxyaurone (15a): (0.18 g, 0.63 mmol, 63% yield); yellow brown solid, 240–241 °C; 1H-NMR (CDCl3:CD3OD = 1:1) δ 4.15 (s, 3H, OCH3), 6.75 (d, J = 8.5 Hz, 1H, H-5), 6.80 (s, 1H, H-10), 6.90 (d, J = 8.5 Hz, 2H, H-3′ and H-5′), 7.37 (d, J = 8.3 Hz, 1H, H-4), 7.81 (d, J = 8.8 Hz, 2H, H-2′ and H-6); 13C-NMR (CDCl3:CD3OD = 1:1) δ 61.1, 113.8, 114.2, 116.0, 116.5, 120.1, 124.2, 132.8, 133.9, 146.7, 158.5, 158.8, 160.0, 183.8; UV/Vis (3.0 × 10−5 M, CH3OH); λ = 394.6 nm (ε, 2.6 × 104).
(Z)-6,3′,4′-Trihydroxy-7-methoxyaurone (15b): (0.27 g, 0.91 mmol, 91% yield); reddish ocher solid, 235–238 °C; 1H-NMR (DMSO-d6) δ 4.04 (s, 3H, OCH3), 6.70 (s, 1H, H-10), 6.79 (d, J = 8.3 Hz, 1H, H-5), 6.87 (d, J = 8.3 Hz, 1H, H-5′), 7.27 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6′), 7.35 (d, J = 8.3 Hz, 1H, H-4), 7.46 (d, J = 1.7 Hz, 1H, H-2′), 9.45 (s, 1H, OH), 9.84 (s, 1H, OH), 10.96 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 60.8, 112.3, 113.3, 114.7, 115.9, 117.8, 119.3, 123.2, 124.6, 132.1, 145.3, 145.4, 148.0, 157.6, 157.8, 181.1; UV/Vis (2.9 × 10−5 M, DMSO); λ = 411.2 nm (ε, 2.1 × 104).
(Z)-6,2′,4′-Trihydroxy-7-methoxyaurone (15c): (0.25 g, 0.84 mmol, 84% yield); ocher solid, 288–290 °C; 1H-NMR (DMSO-d6) δ 3.94 (s, 3H, OCH3), 6.43 (d, J = 2.0 Hz, 1H, H-3′), 6.45 (dd, J = 2.2 Hz and 9.3 Hz, 1H, H-5′), 6.96 (d, J = 8.5 Hz, 1H, H-5), 7.12 (s, 1H, H-10), 7.26 (d, J = 8.5 Hz, 1H, H-4), 8.16 (d, J = 8.3 Hz, 1H, H-6′), 9.82 (s, 1H, OH), 10.16 (s, 1H, OH), 10.42 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 56.6, 102.1, 106.3, 108.3, 108.3, 110.6, 114.6, 116.1, 131.3, 132.8, 145.0, 153.3, 154.5, 159.0, 160.8, 181.9; UV/Vis (2.8 × 10−5 M, DMSO); λ = 428.2 nm (ε, 3.3 × 104).
(Z)-6,4′-Dihydroxy-7,3′-dimethoxyaurone (15d): (0.24 g, 0.75 mmol, 75% yield); yellow solid, 218–220 °C; 1H-NMR (DMSO-d6) δ 3.87 (s, 3H, OCH3), 4.04 (s, 3H, OCH3), 6.79 (d, J = 8.5 Hz, 1H, H-5), 6.79 (s, 1H, H-10), 6.92 (d, J = 8.3 Hz, 1H, H-5′), 7.36 (d, J = 8.5 Hz, 1H, H-4), 7.42 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6′), 7.63 (d, J = 1.7 Hz, 1H, H-2′); 13C-NMR (DMSO-d6) δ 55.6, 60.7, 112.3, 113.5, 114.3, 114.7, 116.0, 119.5, 123.4, 125.9, 132.1, 145.6, 147.7, 148.9, 157.8, 157.9, 181.2; UV/Vis (2.4 × 10−5 M, DMSO); λ = 407.2 nm (ε, 2.9 × 104).
(Z)-6,3′-Dihydroxy-7,4′-dimethoxyaurone (15e): (0.31 g, 0.97 mmol, 97% yield); yellow brown solid, 241–243 °C; 1H-NMR (DMSO-d6) δ 3.82 (s, 3H, OCH3), 4.01 (s, 3H, OCH3), 6.70 (s, 1H, H-10), 6.77 (d, J = 8.5 Hz, 1H, H-5), 7.05 (d, J = 8.5 Hz, 1H, H-5′), 7.33 (d, J = 8.3 Hz, 1H, H-4), 7.37 (dd, J = 1.5 Hz and 8.5 Hz, 1H, H-6′), 7.45 (d, J = 1.5 Hz, 1H, H-2′), 9.44 (s, 1H, OH), 10.97 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 55.7, 60.8, 111.7, 112.1, 113.4, 114.5, 117.3, 119.4, 124.2, 124.5, 132.1, 145.7, 146.4, 149.5, 157.8, 157.9, 181.2; UV/Vis (2.7 × 10−5 M, DMSO); λ = 405.4 nm (ε, 2.1 × 104).
(Z)-6-Hydroxy-7,3′,4′-trimethoxyaurone (15f): (0.21 g, 0.65 mmol, 65% yield); yellow solid, 204–205 °C; 1H-NMR (CDCl3) δ 3.95 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.27 (s, 3H, OCH3), 6.65 (s, 1H, OH), 6.82 (d, J = 8.5 Hz, 1H, H-5), 6.83 (s, 1H, H-10), 6.94 (d, J = 8.5 Hz, 1H, H-5′), 7.42 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6′), 7.46 (d, J = 8.3 Hz, 1H, H-4), 7.51 (d, J = 2.0 Hz, H-2′); 13C-NMR (CDCl3) δ 55.7, 55.8, 60.6, 111.0, 111.7, 112.6, 113.0, 116.3, 119.7, 124.9, 125.4, 130.9, 146.0, 148.7, 150.4, 154.9, 115.9, 182.1; UV/Vis (2.4 × 10−5 M, CHCl3); λ = 400.0 nm (ε, 2.6 × 104).
(Z)-4′-Hydroxy-6,7-dimethoxyaurone (15g): (0.27 g, 0.89 mmol, 89% yield); dark yellow solid, 230–231 °C; 1H-NMR (DMSO-d6) δ 4.00 (s, 3H, OCH3), 4.17 (s, 3H, OCH3), 5.76 (s, 1H, OH), 6.81 (d, J = 8.3 Hz, 1H, H-5), 6.84 (s, 1H, H-10), 6.95 (d, J = 8.3 Hz, 2H, H-3′ and H-5′), 7.54 (d, J = 8.5 Hz, 1H, H-4), 7.83 (d, J = 8.3 Hz, 2H, H-2′ and H-6′); 13C-NMR (DMSO-d6) δ 56.6, 60.6, 108.7, 112.0, 114.9, 116.0, 119.0, 119.1, 122.7, 133.1, 154.1, 156.5, 158.3, 159.2, 181.3; UV/Vis (3.0 × 10−5 M, DMSO); λ = 404.2 nm (ε, 2.5 × 104).
(Z)-3′,4′-Dihydroxy-6,7-dimethoxyaurone (15h): (0.16 g, 0.51 mmol, 51% yield); dark yellow solid, 219–220 °C; 1H-NMR (CD3OD) δ 3.97 (s, 3H, OCH3), 4.08 (s, 3H, OCH3), 6.71 (s, 1H, H-10), 6.84 (d, J = 8.3 Hz, 1H, H-5), 6.94 (d, J = 8.5 Hz, 1H, H-5′), 7.26 (dd, J = 2.2 Hz and 8.3 Hz, 1H, H-6′), 7.46 (d, J = 8.5 Hz, 1H, H-4), 7.46 (d, J = 2.2 Hz, 1H, H-2′); 13C-NMR (CD3OD) δ 57.2, 61.5, 109.7, 115.0, 116.4, 117.6, 118.8, 120.3, 125.1, 126.3, 135.0, 146.4, 147.0, 149.3, 158.7, 160.5, 184.3; UV/Vis (2.5 × 10−5 M, CH3OH); λ = 408.2 nm (ε, 2.6 × 104).
(Z)-2′,4′-Dihydroxy-6,7-dimethoxyaurone (15i): (0.25 g, 0.78 mmol, 78% yield); reddish clay solid, 245 °C (decomp.); 1H-NMR (DMSO-d6) δ 3.94 (s, 3H, OCH3), 4.02 (s, 3H, OCH3), 6.43 (s, 1H, H-10), 6.44 (d, J = 7.1 Hz, 1H, H-5), 7.01 (d, J = 8.3 Hz, 1H, H-5′), 7.14 (s, 1H, H-3′), 7.48 (d, J = 8.3 Hz, 1H, H-4), 7.98 (d, J = 8.5 Hz, 1H, H-6′), 10.17 (s, 1H, OH), 10.36 (s, 1H, OH); 13C-NMR (DMSO-d6) δ 56.8, 60.7, 102.3, 106.8, 108.5, 108.9, 110.4, 116.4, 119.0, 132.2, 133.3, 144.7, 156.5, 158.2, 159.2, 161.0, 181.2; UV/Vis (2.4 × 10−5 M, DMSO); λ = 424.6 nm (ε, 2.9 × 104).
(Z)-4′-Hydroxy-6,7,3′-trimethoxyaurone (15j): (0.27 g, 0.81 mmol, 81% yield); dark yellow solid, 171–176 °C; 1H-NMR (CDCl3) δ 3.97 (s, 3H, OCH3), 3.99 (s, 3H, OCH3), 4.16 (s, 3H, OCH3), 6.13 (s, 1H, OH), 6.80 (d, J = 8.3 Hz, 1H, H-5), 6.81 (s, 1H, H-10), 6.99 (d, J = 8.3 Hz, 1H, H-5′), 7.39 (dd, J = 1.7 Hz and 8.3 Hz, 1H, H-6′), 7.52 (d, J = 8.5 Hz, 1H, H-4), 7.56 (d, J = 1.7 Hz, 1H, H-2′); 13C-NMR (CDCl3) δ 55.7, 56.6, 60.7, 107.8, 112.7, 112.7, 114.7, 116.8, 119.3, 124.5, 126.1, 133.5, 146.0, 146.3, 147.3, 157.0, 158.3, 182.5; UV/Vis (2.8 × 10−5 M, CHCl3); λ = 405.4 nm (ε, 1.7 × 104).
(Z)-3′-Hydroxy-6,7,4′-trimethoxyaurone (15k): (0.30 g, 0.91 mmol, 91% yield); dark yellow solid, 196–200 °C; 1H-NMR (CDCl3) δ 3.94 (s, 3H, OCH3), 3.98 (s, 3H, OCH3), 4.20 (s, 3H, OCH3), 5.86 (s, 1H, OH), 6.78 (s, 1H, H-10), 6.79 (d, J = 9.3 Hz, 1H, H-5), 6.92 (d, J = 8.3 Hz, 1H, H-5′), 7.39 (dd, J = 2.0 Hz and 8.3 Hz, 1H, H-6′), 7.51 (d, J = 8.5 Hz, 1H, H-4), 7.57 (d, J = 2.2 Hz, 1H, H-2′); 13C-NMR (CDCl3) δδ 56.0, 56.8, 61.1, 107.9, 110.6, 112.4, 116.7, 116.9, 119.3, 124.7, 125.6, 133.8, 145.6, 146.4, 148.0, 157.1, 158.4, 182.9; UV/Vis (2.6 × 10−5 M, CHCl3); λ = 405.0 nm (ε, 1.8 × 104).
3.16. The DPPH Radical Scavenging Assay
The measurement of the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging effect was performed according to the established procedure [
8]. Sample compounds were dissolved in ethanol to obtain a 0.1 mM concentration. The DPPH free radical was dissolved in ethanol to obtain a concentration of 0.2 mM. The ethanol (100 μL) and DPPH solutions (50 μL) were added to a sample solution (100 μL) on a 96-well transparent microplate. The mix solution was mixed on a plate-mixer for 1 min. The mix solution was allowed to stand at 25 °C for 30 min in the dark, followed by measuring the absorbance with a microplate reader at 517 nm. The sample blank test (B) was performed with ethanol instead of the sample solution using a similar procedure. The blank test of the sample (C) was performed similarly, with ethanol instead of the DPPH solution. The blank test of the sample blank (D) was performed similarly, but with ethanol instead of the sample and DPPH solution. The DPPH radical scavenging rate was calculated as follows:
where A is the absorbance of the sample, B is the absorbance of the sample blank, C is the absorbance of the blank of the sample, and D is the absorbance of the blank of the sample blank.
3.17. Tyrosinase Activity Inhibition Assay
The Tyrosinase activity was determined using the dopachrome method with
l-3-(3,4-dihydroxyphenyl)alanine (
l-DOPA) as the substrate [
9]. Sample compounds were dissolved in DMSO to obtain a concentration of 3.0 mM.
l-DOPA was dissolved in a 0.2 M phosphate buffer solution (PBS, pH 6.8) to obtain a concentration of 1.66 mM. The enzyme tyrosinase from mushrooms was dissolved in PBS to obtain a concentration of 600 units/mL. The sample solution (10 μL) was added to a
l-DOPA solution (280 μL) on a 96-well transparent microplate. The mix solution was mixed on a plate-mixer for 1 min. The mix solution was left to stand at 25 °C for 5 min. The tyrosinase solution (10 μL) was added to the mixture and the mixture was incubated at 25 °C for 10 min, followed by measuring the absorbance with a microplate reader at 475 nm. The sample blank test (B) was performed with DMSO instead of the sample solution with similar procedure. The blank test of sample (C) was similarly performed with PBS instead of the enzyme solution. The blank test of the sample blank (D) was similarly performed with the DMSO and PBS instead of the sample and enzyme solutions, respectively. The percentage inhibition of tyrosinase activity was calculated as follows.
where A is the absorbance of the sample, B is the absorbance of the sample blank, C is the absorbance of the blank of the sample, and D is the absorbance of the blank of the sample blank.