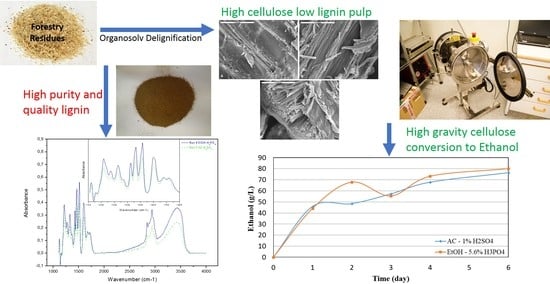
Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of the Type of Organic Solvent
2.2. Effect of Catalyst Type and Concentration
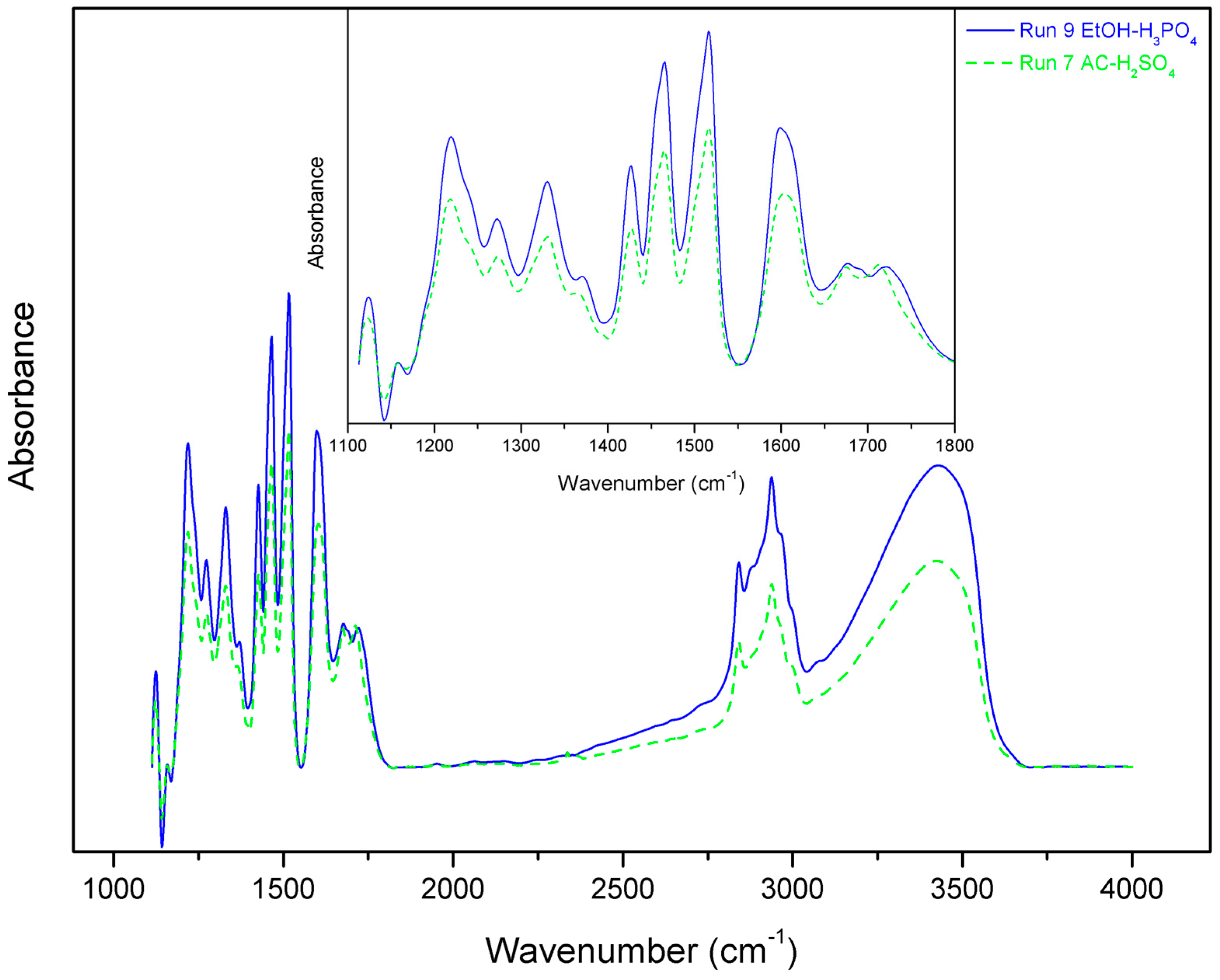
2.3. Pulp and Lignin Quality
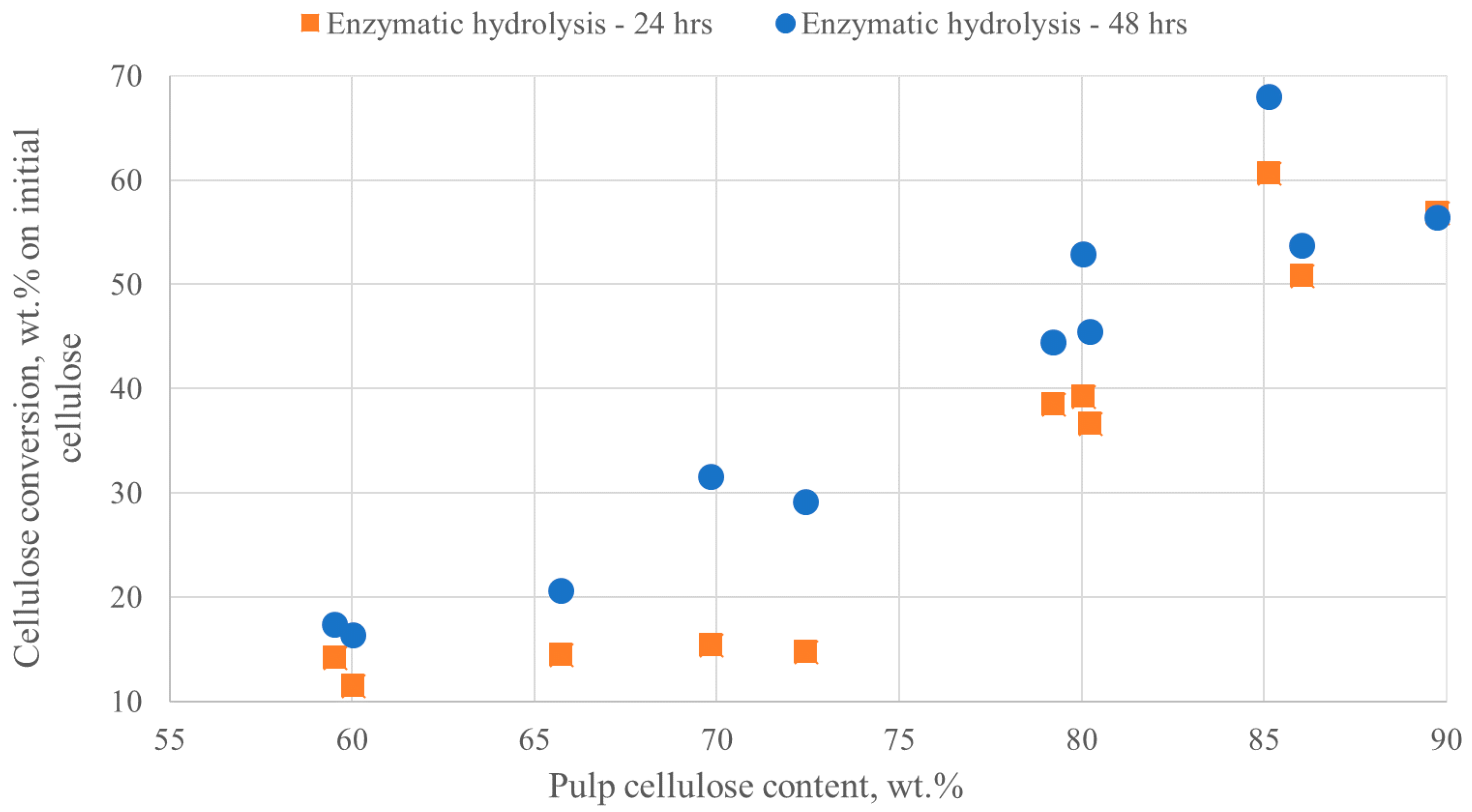
2.4. Enzymatic Saccharification of Pretreated Pulps
2.5. High Solids Hydrolysis and Fermentation
3. Materials and Methods
3.1. Raw Materials
3.2. Strains and Enzymes
3.3. Organosolv Pretreatment
3.4. Enzymatic Saccharification Trials
3.5. High Gravity Saccharification and Fermentation
3.6. Analytical Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Studer, M.H.; DeMartini, J.D.; Davis, M.F.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.A.; Wyman, C.E. Lignin content in natural Populus variants affects sugar release. Proc. Natl. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shahbazi, A. Recent developments in pretreatment technologies for production of lignocellulosic biofuels. J. Pet. Environ. Biotechnol. 2011, 2. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, M.; Viikari, L. Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour. Technol. 2012, 121, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jorgensen, H.; Elder, T. Cell wall structural changes in wheat straw pretreated for bioethanol production. Biotechnol. Biofuels 2008, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Smit, A.T.; de Wild, P.J.; den Uil, H. Fractionation of wheat straw by prehydrolysis, organosolv delignification and enzymatic hydrolysis for production of sugars and lignin. Bioresour. Technol. 2012, 114, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, M.E.; Zambon, M.D.; Area, M.C.; da Silva Curvelo, A.A. Low liquid-solid ratio fractionation of sugarcane bagasse by hot water autohydrolysis and organosolv delignification. Ind. Crops Prod. 2015, 65, 349–353. [Google Scholar] [CrossRef]
- Oliet, M.; García, J.; Rodríguez, F.; Gilarrranz, M. Solvent effects in autocatalyzed alcohol–water pulping. Chem. Eng. J. 2002, 87, 157–162. [Google Scholar] [CrossRef]
- Oliet, M.; Rodríguez, F.; García, J.; Gilarranz, M.A. The effect of autocatalyzed ethanol pulping on lignin characteristics. J. Wood Chem. Technol. 2001, 21, 81–95. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Polysaccharide degradation during ozone-based TCF bleaching of non-wood organosolv pulps. Carbohydr. Polym. 2007, 67, 275–281. [Google Scholar] [CrossRef]
- Garrote, G.; Falqué, E.; Domínguez, H.; Parajó, J.C. Autohydrolysis of agricultural residues: Study of reaction byproducts. Bioresour. Technol. 2007, 98, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Amendola, D.; De Faveri, D.M.; Egües, I.; Serrano, L.; Labidi, J.; Spigno, G. Autohydrolysis and organosolv process for recovery of hemicelluloses, phenolic compounds and lignin from grape stalks. Bioresour. Technol. 2012, 107, 267–274. [Google Scholar] [CrossRef] [PubMed]
- García, A.; González, M.; Llano-Ponte, R.; Labidi, J. Energy and economic assessment of soda and organosolv biorefinery processes. Biomass Bioenergy 2011, 35, 516–525. [Google Scholar] [CrossRef] [Green Version]
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 2014, 152, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Cuilty, K.; Ballinas-Casarrubias, L.; Rodríguez de San Miguel, E.; de Gyves, J.; Robles-Venzor, J.C.; González-Sánchez, G. Cellulose recovery from Quercus sp. sawdust using Ethanosolv pretreatment. Biomass Bioenergy 2018, 111, 114–124. [Google Scholar] [CrossRef]
- Katsimpouras, C.; Kalogiannis, K.G.; Kalogianni, A.; Lappas, A.A.; Topakas, E. Production of high concentrated cellulosic ethanol by acetone/water oxidized pretreated beech wood. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Zacchi, G.; Axelsson, A. Economic evaluation of preconcentration in production of ethanol from dilute sugar solutions. Biotechnol. Bioeng. 1989, 34, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Cardona, M.J.; Tozzi, E.J.; Karuna, N.; Jeoh, T.; Powell, R.L.; McCarthy, M.J. A process for energy-efficient high-solids fed-batch enzymatic liquefaction of cellulosic biomass. Bioresour. Technol. 2015, 198, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Xu, J.; Yuan, Z.; Zhang, Y.; Liu, Y.; Liang, C. Optimization of fed-batch enzymatic hydrolysis from alkali-pretreated sugarcane bagasse for high-concentration sugar production. Bioresour. Technol. 2014, 167, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Christakopoulos, P. Fermentation of liquefacted hydrothermally pretreated sweet sorghum bagasse to ethanol at high-solids content. Bioresour. Technol. 2013, 127, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Kekos, D.; Loizidou, M.; Christakopoulos, P. Utilization of household food waste for the production of ethanol at high dry material content. Biotechnol. Biofuels 2014, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Katsimpouras, C.; Zacharopoulou, M.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Topakas, E. Sequential high gravity ethanol fermentation and anaerobic digestion of steam explosion and organosolv pretreated corn stover. Bioresour. Technol. 2017, 244, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Paschos, T.; Xiros, C.; Christakopoulos, P. Simultaneous saccharification and fermentation by co-cultures of Fusarium oxysporum and Saccharomyces cerevisiae enhances ethanol production from liquefied wheat straw at high solid content. Ind. Crops Prod. 2015, 76, 793–802. [Google Scholar] [CrossRef]
- Hoyer, K.; Galbe, M.; Zacchi, G. The effect of prehydrolysis and improved mixing on high-solids batch simultaneous saccharification and fermentation of spruce to ethanol. Process. Biochem. 2013, 48, 289–293. [Google Scholar] [CrossRef]
- Tippkötter, N.; Duwe, A.-M.; Wiesen, S.; Sieker, T.; Ulber, R. Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour. Technol. 2014, 167, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Abu-Omar, M.M. Advances in 5-hydroxymethylfurfural production from biomass in biphasic solvents. Green Chem. 2014, 16, 24–38. [Google Scholar] [CrossRef]
- Bozell, J.J.; Black, S.K.; Myers, M.; Cahill, D.; Miller, W.P.; Park, S. Solvent fractionation of renewable woody feedstocks: Organosolv generation of biorefinery process streams for the production of biobased chemicals. Biomass Bioenergy 2011, 35, 4197–4208. [Google Scholar] [CrossRef]
- Katahira, R.; Mittal, A.; McKinney, K.; Ciesielski, P.N.; Donohoe, B.S.; Black, S.K.; Johnson, D.K.; Biddy, M.J.; Beckham, G.T. Evaluation of clean fractionation pretreatment for the production of renewable fuels and chemicals from corn stover. ACS Sustain. Chem. Eng. 2014, 2, 1364–1376. [Google Scholar] [CrossRef]
- Brudecki, G.; Cybulska, I.; Rosentrater, K. Optimization of clean fractionation process applied to switchgrass to produce pulp for enzymatic hydrolysis. Bioresour. Technol. 2013, 131, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kohn, B.; Holmes, L.; Faulkner, R.; Davis, M.; Maciel, G.E. Molecular-level consequences of biomass pretreatment by dilute sulfuric acid at various temperatures. Energy Fuels 2011, 25, 1790–1797. [Google Scholar] [CrossRef]
- Sturgeon, M.R.; Kim, S.; Lawrence, K.; Paton, R.S.; Chmely, S.C.; Nimlos, M.; Foust, T.D.; Beckham, G.T. A mechanistic investigation of acid-catalyzed cleavage of aryl-ether linkages: Implications for lignin depolymerization in acidic environments. ACS Sustain. Chem. Eng. 2014, 2, 472–485. [Google Scholar] [CrossRef]
- Teng, J.; Ma, H.; Wang, F.; Wang, L.; Li, X. Catalytic fractionation of raw biomass to biochemicals and organosolv lignin in a methyl isobutyl ketone/H2O biphasic system. ACS Sustain. Chem. Eng. 2016, 4, 2020–2026. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Philipp, B.; Heinze, T.; Heinze, U.; Wagenknecht, W. Comprehensive Cellulose Chemistry. Volume 1. Fundamentals and Analytical Methods; Wiley: Weinheim, Germany, 1998; 260p. [Google Scholar]
- Demain, A.L.; Adrio, J.L. Contributions of microorganisms to industrial biology. Mol. Biotechnol. 2008, 38, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Vom Stein, T.; Grande, P.M.; Kayser, H.; Sibilla, F.; Leitner, W.; Domínguez de María, P. From biomass to feedstock: One-step fractionation of lignocellulose components by the selective organic acid-catalyzed depolymerization of hemicellulose in a biphasic system. Green Chem. 2011, 13, 1772. [Google Scholar] [CrossRef]
- Lee, J.-W.; Rodrigues, R.C.L.B.; Kim, H.J.; Choi, I.-G.; Jeffries, T.W. The roles of xylan and lignin in oxalic acid pretreated corncob during separate enzymatic hydrolysis and ethanol fermentation. Bioresour. Technol. 2010, 101, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- Arantes, V.; Saddler, J.N. Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol. Biofuels 2011, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, L.G.; Hidayat, B.J.; Johansen, K.S.; Felby, C. Role of supramolecular cellulose structures in enzymatic hydrolysis of plant cell walls. J. Ind. Microbiol. Biotechnol. 2011, 38, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Kalogiannis, K.G.; Stefanidis, S.; Marianou, A.; Michailof, C.; Kalogianni, A.; Lappas, A. Lignocellulosic biomass fractionation as a pretreatment step for production of fuels and green chemicals. Waste Biomass Valorization 2015, 6. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Michailof, C.M.; Lappas, A.A.; Sjöholm, E. Pyrolysis of lignin with 2DGC quantification of lignin oil: Effect of lignin type, process temperature and ZSM-5 in situ upgrading. J. Anal. Appl. Pyrolysis 2015, 115, 410–418. [Google Scholar] [CrossRef]
- Takata, E.; Tsutsumi, K.; Tsutsumi, Y.; Tabata, K. Production of monosaccharides from napier grass by hydrothermal process with phosphoric acid. Bioresour. Technol. 2013, 143, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Zheng, Z.; Qin, W.; Dong, C.; Yang, Y. Efficient fractionation of Chinese white poplar biomass with enhanced enzymatic digestibility and modified acetone-soluble lignin. BioResources 2011, 6, 4705–4720. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Zhu, Z.; Wi, S.; Percival Zhang, Y.-H. Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass. Biotechnol. Bioeng. 2011, 108, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Yang, C.; Shi, R.; Jameel, H.; Chiang, V.; Chang, H. The elucidation of the lignin structure effect on the cellulase-mediated saccharification by genetic engineering poplars (Populus nigra L. × Populus maximowiczii A.). Biomass Bioenergy 2013, 58, 52–57. [Google Scholar] [CrossRef]
- De Souza, A.P.; Kamei, C.L.A.; Torres, A.F.; Pattathil, S.; Hahn, M.G.; Trindade, L.M.; Buckeridge, M.S. How cell wall complexity influences saccharification efficiency in Miscanthus sinensis. J. Exp. Bot. 2015, 66, 4351–4365. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kim, N.J.; Jiang, M.; Kang, J.W.; Chang, H.N. Simultaneous saccharification and fermentation of lignocellulosic residues pretreated with phosphoric acid-acetone for bioethanol production. Bioresour. Technol. 2009, 100, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Nitsos, C.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. A novel hybrid organosolv: Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol. Biofuels 2018, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Koppram, R.; Olsson, L.; Franzén, C.J. Kinetic modeling of multi-feed simultaneous saccharification and co-fermentation of pretreated birch to ethanol. Bioresour. Technol. 2014, 172, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Qin, L.; Zhu, J.Q.; Li, B.Z.; Yuan, Y.J. Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnol. Biofuels 2014, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Ramachandriya, K.D.; Wilkins, M.; Atiyeh, H.K.; Dunford, N.T.; Hiziroglu, S. Effect of high dry solids loading on enzymatic hydrolysis of acid bisulfite pretreated Eastern redcedar. Bioresour. Technol. 2013, 147, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-S, M.; Rojas, J.; Castro, J.; Ragauskas, A.; Baeza, J.; Freer, J. Fuel ethanol production from Eucalyptus globulus wood by autocatalized organosolv pretreatment ethanol-water and SSF. J. Chem. Technol. Biotechnol. 2013, 88, 39–48. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Cara, C.; Ruiz, E.; Moya, M.; Castro, E. Bioethanol production from rapeseed straw at high solids loading with different process configurations. Fuel 2014, 122, 112–118. [Google Scholar] [CrossRef]
- Dong, C.; Wang, Y.; Zhang, H.; Leu, S.Y. Feasibility of high-concentration cellulosic bioethanol production from undetoxified whole Monterey pine slurry. Bioresour. Technol. 2018, 250, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Koppram, R.; Olsson, L. Combined substrate, enzyme and yeast feed in simultaneous saccharification and fermentation allow bioethanol production from pretreated spruce biomass at high solids loadings. Biotechnol. Biofuels 2014, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertilsson, M.; Olofsson, K.; Lidén, G. Prefermentation improves xylose utilization in simultaneous saccharification and co-fermentation of pretreated spruce. Biotechnol. Biofuels 2009, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankó, B.; Galbe, M.; Wallberg, O. Influence of bark on fuel ethanol production from steam-pretreated spruce. Biotechnol. Biofuels 2015, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Moreno, A.D.; Ibarra, D.; Sáez, F.; Ballesteros, M. Improving the fermentation performance of saccharomyces cerevisiae by laccase during ethanol production from steam-exploded wheat straw at high-substrate loadings. Biotechnol. Prog. 2013, 29, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, D.C. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
Sample Availability: Samples of the materials produced in the current work are available from the authors at a reasonable request. |



| Run No. * | Solvent | Solvent, vol.% | Catalyst, wt.% on Dry Basis |
|---|---|---|---|
| 1 | Ethanol | 60 | - |
| 2 | Ethanol | 60 | H2SO4, 1.0% |
| 3 | MIBK | 60 | - |
| 4 | MIBK | 60 | H2SO4, 1.0% |
| 5 | Acetone | 25 | - |
| 6 | Acetone | 60 | - |
| 7 | Acetone | 60 | H2SO4, 1.0% |
| 8 | Ethanol | 60 | H3PO4, 1.0% |
| 9 | Ethanol | 60 | H3PO4, 5.6% |
| 10 | Ethanol | 60 | C2H2O4, 1.0% |
| 11 | Ethanol | 60 | C2H2O4, 2.6% |
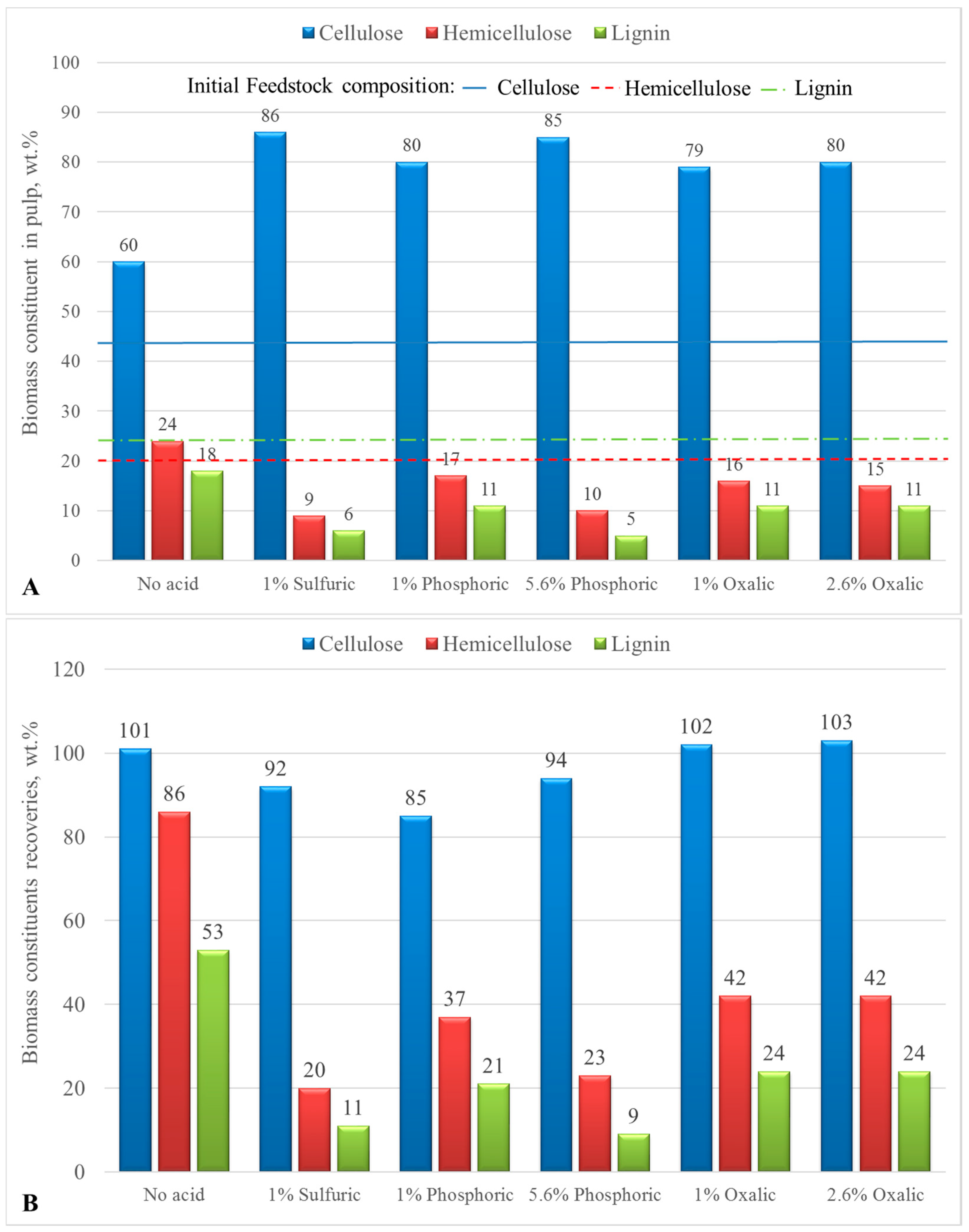
| Run No. | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Cellulose Retrieved (%) | Hemicellulose Retrieved (%) | Lignin Retrieved (%) |
|---|---|---|---|---|---|---|
| Initial Biomass * | 43.1 | 20.2 | 24.2 | - | - | - |
| 1 | 60.0 | 23.8 | 17.8 | 101.5 | 85.8 | 53.5 |
| 2 | 86.0 | 9.0 | 5.7 | 92.5 | 20.5 | 11.0 |
| 3 | 65.7 | 6.4 | 25.2 | 92.1 | 19.0 | 62.8 |
| 4 | 72.4 | 1.4 | 24.5 | 83.8 | 3.4 | 50.6 |
| 5 | 69.8 | 9.1 | 19.9 | 94.4 | 26.2 | 47.9 |
| 6 | 59.5 | 19.1 | 17.2 | 102.3 | 70.0 | 52.7 |
| 7 | 89.7 | 6.3 | 4.2 | 91.7 | 13.6 | 7.6 |
| 8 | 80.2 | 16.6 | 10.9 | 85.0 | 37.5 | 20.6 |
| 9 | 85.1 | 10.0 | 4.6 | 93.6 | 23.3 | 9.1 |
| 10 | 79.2 | 15.5 | 10.6 | 102.0 | 42.4 | 24.2 |
| 11 | 80.0 | 15.2 | 10.7 | 103.2 | 41.7 | 24.5 |
| Run No. | Crystallinity Index CrI (%) * |
|---|---|
| 1 | 68.8 |
| 2 | 77.5 |
| 3 | 74.1 |
| 4 | 78.2 |
| 5 | 74.4 |
| 6 | 69.0 |
| 7 | 78.1 |
| 8 | 75.1 |
| 9 | 77.3 |
| 10 | 73.2 |
| 11 | 72.9 |
| Run No. | 24 h * (wt.% on Feed Cellulose) | 48 h (wt.% on Feed Cellulose) |
|---|---|---|
| 1 | 11.6 (70.0) | 16.5 |
| 2 | 50.9 (94.6) | 53.8 |
| 3 | 14.6 (70.5) | 20.7 |
| 4 | 14.9 (50.8) | 29.3 |
| 5 | 15.5 (48.9) | 31.7 |
| 6 | 14.4 (82.3) | 17.5 |
| 7 | 57.0 (100.9) | 56.5 |
| 8 | 36.8 (80.6) | 45.6 |
| 9 | 60.8 (89.3) | 68.1 |
| 10 | 38.7 (86.8) | 44.6 |
| 11 | 39.3 (74.2) | 53.0 |
| WIS (%) | Material | Pre-Treatment | Enzyme Loading | Ethanol (g/L) | Time (h) | Reference |
|---|---|---|---|---|---|---|
| 20 | Beechwood | Organosolv with acetone and sulphuric acid | 8.4 mg/g | 76.3 | 144 | Current work |
| 20 | Beechwood | Organosolv with ethanol and phosphoric acid | 8.4 mg/g | 80 | 144 | Current work |
| 20 | Beechwood | Acetone/water oxidation | 8.4 FPU/g | 75.9 | 120 | [20] |
| 36 | Bermudagrass | Phosphoric acid-acetone | 25 FPU/g cellulose | 56.1 | 96 | [52] |
| 20 | Birch | Hybrid organosolv–steam explosion | 18.5 FPU/g | 80 | 192 | [53] |
| 20 | Birch | Steam pre-treated | 20 FPU/g | 14.4 | 144 | [54] |
| 20 | Corn stover | Steam explosion | 17.7 FPU/g | 59.8 | 192 | [55] |
| 20 | Eastern redcedar | Acid bisulfite | 46 FPU/g glucan | 52 | 42 | [56] |
| 15 | Eucalyptus | Organosolv | 20 FPU/g | 42 | 72 | [57] |
| 20 | Rapeseed straw | Dilute acid | 15 FPU/g | 39.9 | 24 | [58] |
| 25 | Pine | Sulfite | 15 FPU/g | 82 | 24 | [59] |
| 20 | Spruce | Steam pre-treated | 22.5 FPU/g | 40 | 96 | [60] |
| 10 | Spruce | Steam pre-treated | 30 FPU/g glucan | 45 | 100 | [61] |
| 10 | Spruce | Steam pre-treated | 20 FPU/g | 45.8 | 96 | [62] |
| 25 | Wheat straw | Steam explosion | 15 FPU/g | 58.6 | 80 | [63] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogiannis, K.G.; Matsakas, L.; Aspden, J.; Lappas, A.A.; Rova, U.; Christakopoulos, P. Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation. Molecules 2018, 23, 1647. https://doi.org/10.3390/molecules23071647
Kalogiannis KG, Matsakas L, Aspden J, Lappas AA, Rova U, Christakopoulos P. Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation. Molecules. 2018; 23(7):1647. https://doi.org/10.3390/molecules23071647
Chicago/Turabian StyleKalogiannis, Konstantinos G., Leonidas Matsakas, James Aspden, Angelos A. Lappas, Ulrika Rova, and Paul Christakopoulos. 2018. "Acid Assisted Organosolv Delignification of Beechwood and Pulp Conversion towards High Concentrated Cellulosic Ethanol via High Gravity Enzymatic Hydrolysis and Fermentation" Molecules 23, no. 7: 1647. https://doi.org/10.3390/molecules23071647