Ferrocenes as Building Blocks in Molecular Rectifiers and Diodes
Abstract
:1. Introduction
2. Discussion of Prior Work
2.1. Computational/Theoretical Work on Ferrocene-Containing Rectifiers
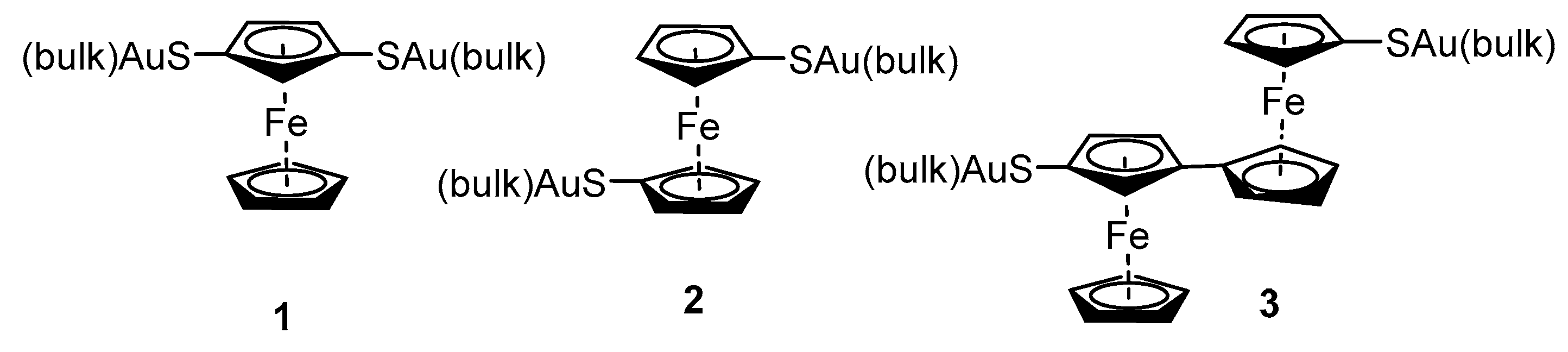
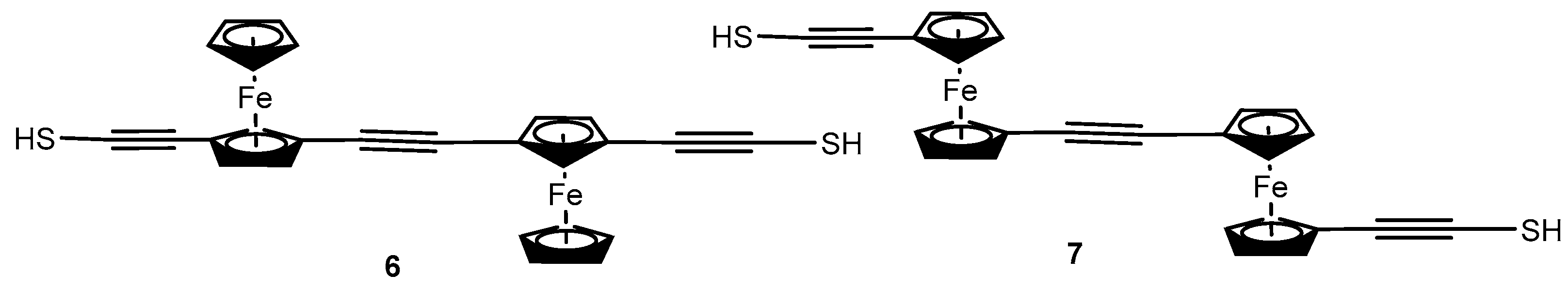
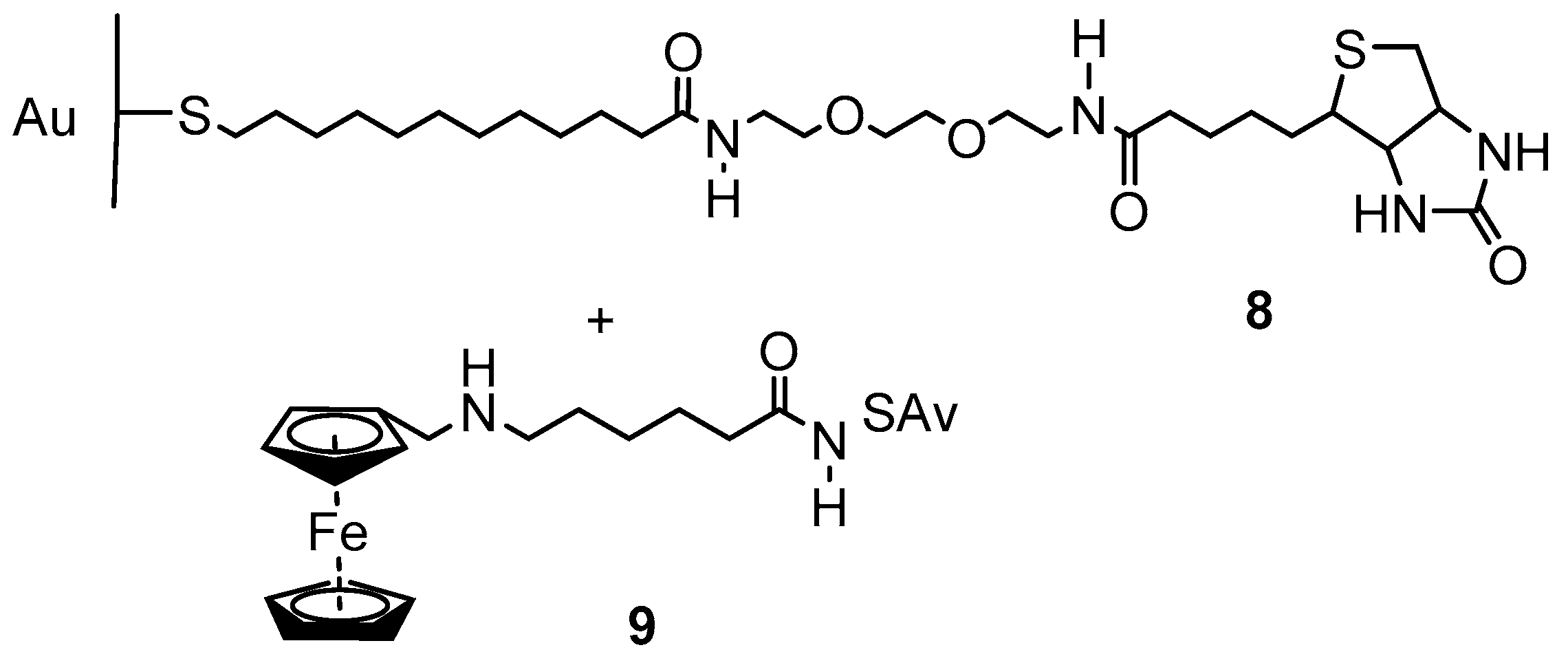
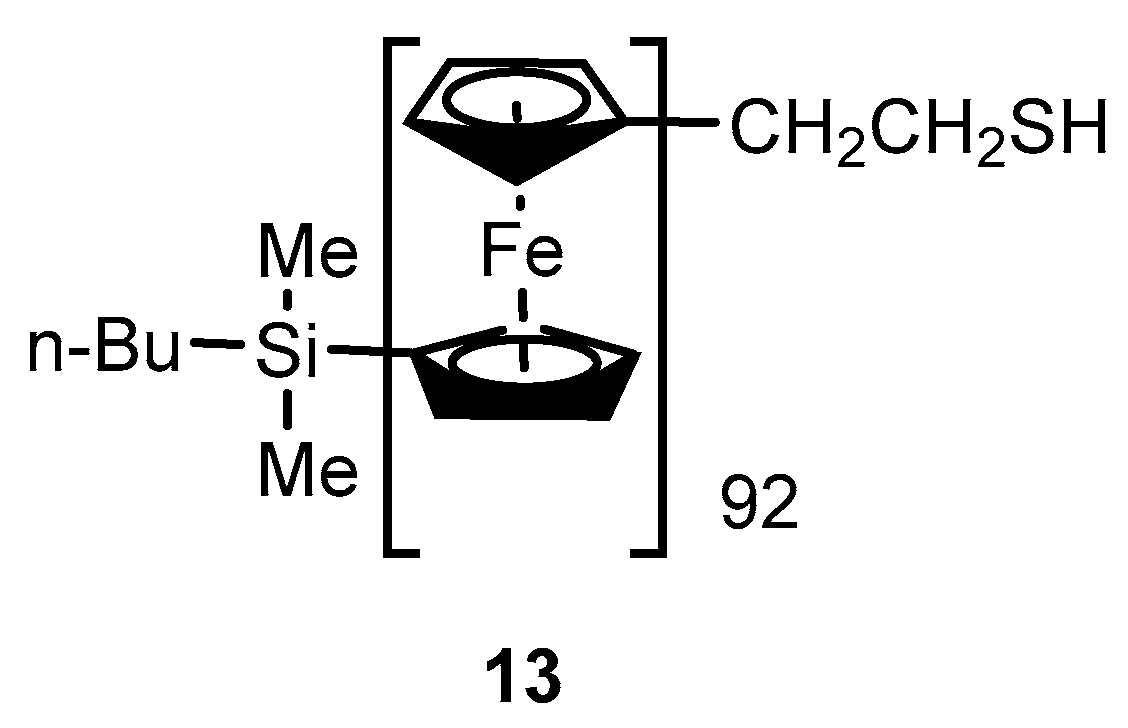
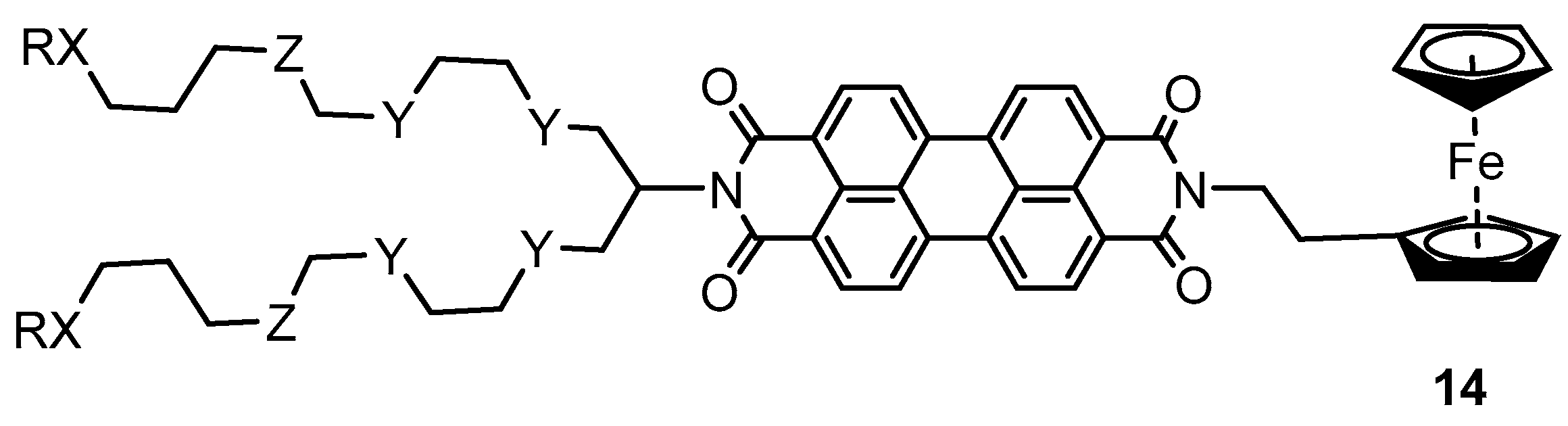
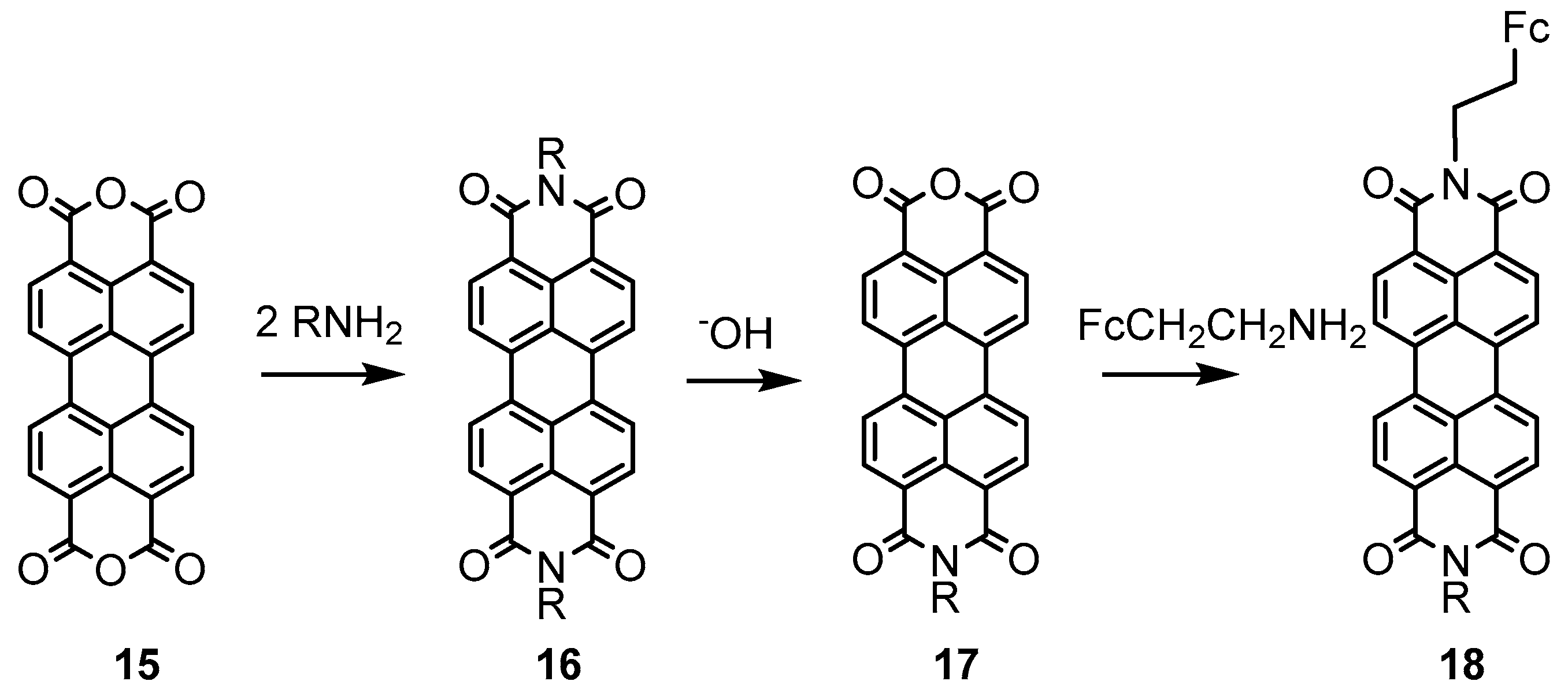
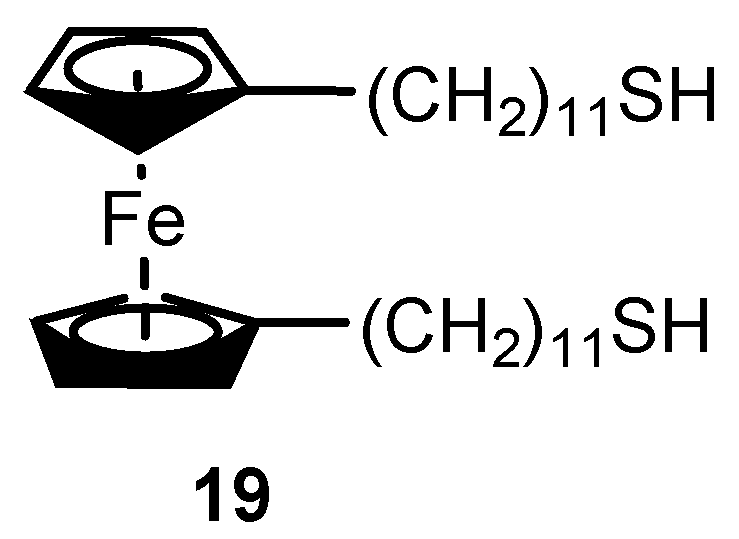
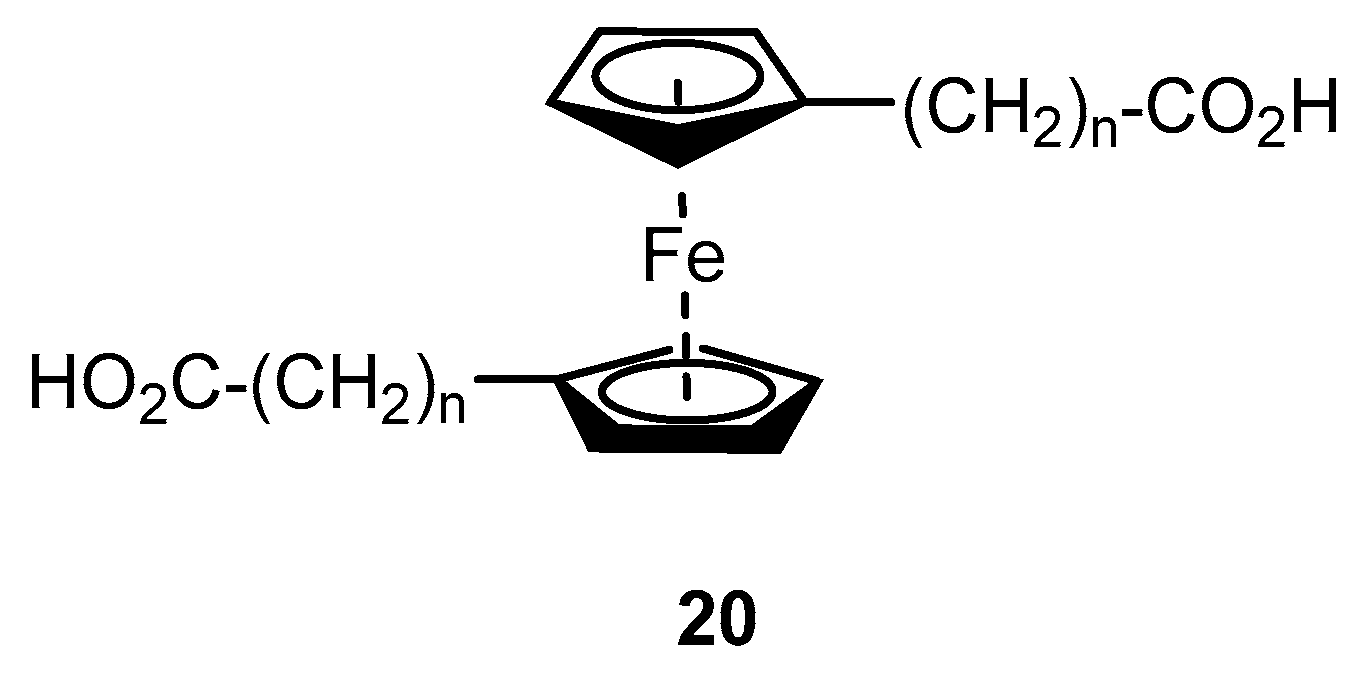
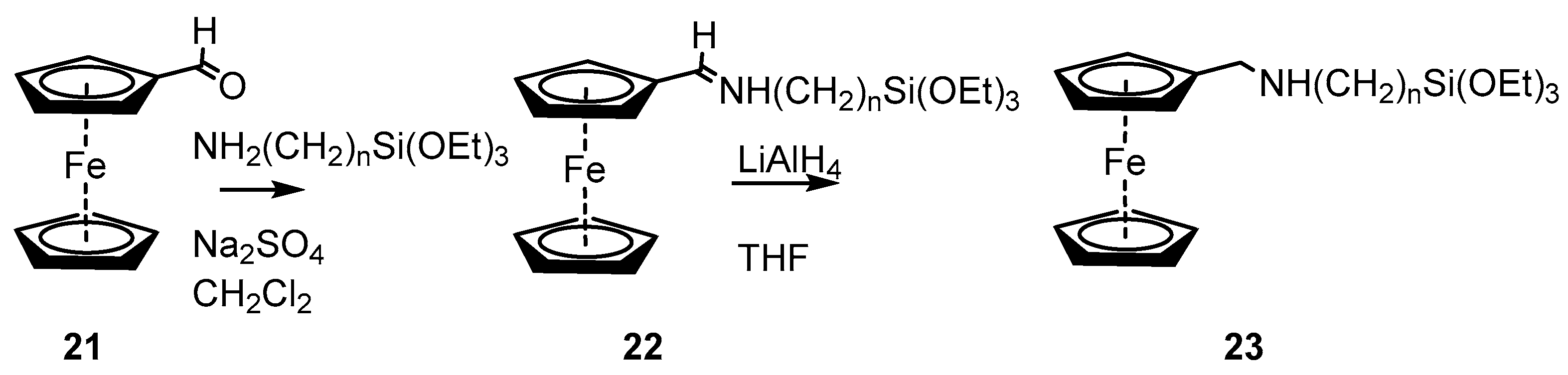
2.2. Experimental Work on Ferrocene-Containing Rectifiers
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Aviram, A.; Ratner, M.A. Molecular rectifiers. Chem. Phys. Lett. 1974, 29, 277. [Google Scholar] [CrossRef]
- Ng, M.K.; Lee, D.C.; Yu, L. Molecular diodes based on conjugated diblock co-oligomers. J. Am. Chem. Soc. 2002, 124, 11862–11863. [Google Scholar] [CrossRef] [PubMed]
- Lenfant, S.; Krzeminski, C.; Delerue, C.; Allan, G.; Vuillaume, D. Molecular rectifying diodes from self-assembly on silicon. Nano Lett. 2003, 3, 741–746. [Google Scholar] [CrossRef]
- Yuan, L.; Nerngchamnong, N.; Cao, L.; Hamoudi, H.; del Barco, E.; Roemer, M.; Sriramula, R.K.; Thompson, D.; Nijhuis, C.A. Controlling the direction of rectification in a molecular diode. Nat. Commun. 2015, 6, 6324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornilovitch, P.E.; Bratkovsky, A.M.; Williams, R.S. Current rectification by molecules with asymmetric tunneling barriers. Phys. Rev. B 2002, 66, 165436. [Google Scholar] [CrossRef]
- Yuan, L.; Breuer, R.; Jiang, L.; Schmittel, M.; Nijhuis, C.A. A molecular diode with a statistically robust rectification ratio of three orders of magnitude. Nano Lett. 2015, 15, 5506–5512. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Roemer, M.; Yuan, L.; Du, W.; Thompson, D.; del Barco, E.; Nijhuis, C.A. Molecular diodes with rectification ratios exceeding 105 driven by electrostatic interactions. Nat. Nanotechnol. 2017, 12, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Darancet, P.; Chen, Q.; Meisner, J.S.; Widawsky, J.R.; Neaton, J.B.; Nuckolls, C.; Venkataraman, L. Tuning rectification in single-molecular diodes. Nano Lett. 2013, 13, 6233–6237. [Google Scholar] [CrossRef] [PubMed]
- Mizuseki, H.; Belosludov, R.V.; Uehara, T.; Lee, S.U.; Kawazoe, Y. Transport properties of nanoscale materials for molecular wire applications: A case study of ferrocene dimers. J. Korean Phys. Soc. 2008, 52, 1197–1201. [Google Scholar] [CrossRef]
- Zhang, G.; Li, D.; Shang, Y.; Zhang, H.; Sun, M.; Liu, B.; Li, Z. Transport properties of double quantum dots formed by ferrocene units. J. Phys. Chem. C 2011, 115, 5257–5264. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, S.; Wang, Y.; Ling, Q. Effect of molecular structure on spin-dependent electron transport in biferrocene-based molecular junctions: a first-principles study. J. Comput. Electron. 2017, 16, 340–346. [Google Scholar] [CrossRef]
- Azzaroni, O.; Mir, M.; Álvarez, M.; Tiefenauer, L.; Knoll, W. Electrochemical rectification by redox-labeled bioconjugates: Molecular building blocks for the construction of biodiodes. Langmuir 2008, 24, 2878–2883. [Google Scholar] [CrossRef] [PubMed]
- Azzaroni, O.; Álvarez, M.; Mir, M.; Yameen, B.; Knoll, W. Redox mediation and electron transfer through supramolecular arrays of ferrocene-labeled streptavidin on biotinylated gold electrodes. J. Phys. Chem. C 2008, 112, 15850–15859. [Google Scholar] [CrossRef]
- Cortez, M.L.; Lorenzo, A.; Marmisollé, W.A.; von Bilderling, C.; Maza, E.; Pietrasanta, L.; Battaglini, F.; Ceolín, M.; Azzaroni, O. Highly-organized stacked multilayers via layer-by-layer assembly of lipid-like surfactants and polyelectrolytes. Stratified supramolecular structures for (bio)electrochemical nanoarchitectonics. Soft Matter 2018, 14, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, C.A.; Reus, W.F.; Whitesides, G.M. Molecular rectification in metal−SAM−metal oxide−metal junctions. J. Am. Chem. Soc. 2009, 131, 17814–17827. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, C.A.; Reus, W.F.; Barber, J.R.; Dickey, M.D.; Whitesides, G.M. Charge transport and rectification in arrays of SAM-based tunneling junctions. Nano Lett. 2010, 10, 3611–3619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimbush, K.S.; Reus, W.F.; van der Wiel, W.G.; Reinhoudt, D.N.; Whitesides, G.M.; Nijhuis, C.A.; Velders, A.H. Control over rectification in supramolecular tunneling junctions. Angew. Chem. Int. Ed. 2010, 49, 10176–10180. [Google Scholar] [CrossRef] [PubMed]
- Nerngchamnong, N.; Yuan, L.; Qi, D.C.; Li, J.; Thompson, D.; Nijhuis, C.A. The role of van der Waals forces in the performance of molecular diodes. Nat. Nanotechnol. 2013, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Nijhuis, C.A. Even the odd numbers help: Failure modes of SAM-based tunnel junctions probed via odd-even effects revealed in synchrotrons and supercomputers. Acc. Chem. Res. 2016, 49, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, A.R.; Wang, L.; del Barco, E.; Nijhuis, C.A. Electrostatic control over temperature-dependent tunnelling across a single-molecule junction. Nat. Commun. 2016, 7, 11595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrigues, A.R.; Yuan, L.; Wang, L.; Singh, S.; del Barco, E.; Nijhuis, C.A. Temperature dependent charge transport across tunnel junctions of single-molecules and self-assembled monolayers: A comparative study. Dalton Trans. 2016, 45, 17153–17159. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, A.R.; Yuan, L.; Wang, L.; Mucciolo, E.R.; Thompon, D.; del Barco, E.; Nijhuis, C.A. A single-level tunnel model to account for electrical transport through single molecule- and self-assembled monolayer-based junctions. Sci. Rep. 2016, 6, 26517. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yuan, L.; Roemer, M.; Jiang, L.; Nijhuis, C.A. Supramolecular vs electronic structure: The effect of the tilt angle of the active group in the performance of a molecular diode. J. Am. Chem. Soc. 2016, 138, 5769–5772. [Google Scholar] [CrossRef] [PubMed]
- Souto, M.; Yuan, L.; Morales, D.C.; Jiang, L.; Ratera, I.; Nijhuis, C.A.; Veciana, J. Tuning the rectification ratio by changing the electronic nature (open-shell and closed-shell) in donor–acceptor self-assembled monolayers. J. Am. Chem. Soc. 2017, 139, 4262–4265. [Google Scholar] [CrossRef] [PubMed]
- Sierra, M.A.; Sánchez, D.; Garrigues, A.R.; del Barco, E.; Wang, L.; Nijhuis, C.A. How to distinguish between interacting and noninteracting molecules in tunnel junctions. Nanoscale 2018, 10, 3904–3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Offenhäusser, A.; Mayer, D. Molecular rectification in metal–bridge molecule–metal junctions. Phys. Status Solidi A 2010, 207, 891–897. [Google Scholar] [CrossRef]
- Song, J.; Vancso, G.J. Responsive organometallic polymer grafts: Electrochemical switching of surface properties and current mediation behavior. Langmuir 2011, 27, 6822–6829. [Google Scholar] [CrossRef] [PubMed]
- Kota, R.; Samudrala, R.; Mattern, D.L. Synthesis of donor-σ-perylenebisimide-acceptor molecules having PEG swallowtails and sulfur anchors. J. Org. Chem. 2012, 77, 9641–9651. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.S.; Kota, R.; Mattern, D.L.; Metzger, R.M. Janus reversal and coulomb blockade in ferrocene-perylenebisimide and N,N,N′,N′-tetramethyl-para-phenylenediamine-perylenebisimide D-σ-A rectifiers. Langmuir 2016, 32, 6851–6859. [Google Scholar] [CrossRef] [PubMed]
- Mentovich, E.D.; Rosenberg-Shraga, N.; Kalifa, I.; Gozin, M.; Mujica, V.; Hansen, T.; Richter, S. Gated-controlled rectification of a self-assembled monolayer-based transistor. J. Phys. Chem. C 2013, 117, 8468–8474. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Peng, Z.L.; Hou, R.; Liang, J.-H.; Zheng, J.F.; Zhou, X.Y.; Zhou, X.S.; Jin, S.; Niu, Z.J.; Mao, B.W. Enhancing electron transport in molecular wires by insertion of a ferrocene center. Phys. Chem. Chem. Phys. 2014, 16, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kim, D.; Wang, G.; Park, S.; Lee, H.; Cho, K.; Hwang, W.T.; Yoon, M.H.; Jang, Y.H.; Song, H.; et al. Redox-induced asymmetric electrical characteristics of ferrocene-alkanethiolate molecular devices on rigid and flexible substrates. Adv. Funct. Mater. 2014, 24, 2472–2480. [Google Scholar] [CrossRef]
- Jeong, H.; Jang, Y.; Kim, D.; Hwang, W.-T.; Kim, J.W.; Lee, T. An in-depth study of redox-induced conformational changes in charge transport characteristics of a ferrocene-alkanethiolate molecular electronic junction: Temperature-dependent transition voltage spectroscopy analysis. J. Phys. Chem. C 2016, 120, 3564–3572. [Google Scholar] [CrossRef]
- Park, S.; Park, J.H.; Hwang, S.; Kwak, J. Programmable electrochemical rectifier based on a thin-layer cell. ACS Appl. Mater. Interfaces 2017, 9, 20955–20962. [Google Scholar] [CrossRef] [PubMed]
- Broadnax, A.D.; Lamport, Z.A.; Scharmann, B.; Jurchescu, O.D.; Welker, M.E. Ferrocenealkylsilane molecular rectifiers. J. Organomet. Chem. 2018, 856, 23–26. [Google Scholar] [CrossRef]
- Lamport, Z.A.; Broadnax, A.D.; Harrison, D.; Barth, K.J.; Mendenhall, L.; Hamilton, C.T.; Guthold, M.; Thonhauser, T.; Welker, M.E.; Jurchescu, O.D. Fluorinated benzalkylsilane molecular rectifiers. Sci. Rep. 2016, 6, 38092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.-P.; Mu, Y.Q.; Wei, M.Z.; Wang, S.; Huang, H.; Hu, G.C.; Li, Z.L.; Wang, C.K. Designing molecular rectifiers and spin valves using metallocene-functionalized undecanethiolates: One transition metal atom matters. J. Mater. Chem. C 2018, 6, 2105–2112. [Google Scholar] [CrossRef]
- Saha, P.; Yadav, K.; Chacko, S.; Philip, A.T.; Ramapanicker, R.; Gopakumar, T.G. Controlling growth to one dimension in nanoislands of ferrocene-sugar derivatives. J. Phys. Chem. C 2016, 120, 9223–9228. [Google Scholar] [CrossRef]



© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welker, M.E. Ferrocenes as Building Blocks in Molecular Rectifiers and Diodes. Molecules 2018, 23, 1551. https://doi.org/10.3390/molecules23071551
Welker ME. Ferrocenes as Building Blocks in Molecular Rectifiers and Diodes. Molecules. 2018; 23(7):1551. https://doi.org/10.3390/molecules23071551
Chicago/Turabian StyleWelker, Mark E. 2018. "Ferrocenes as Building Blocks in Molecular Rectifiers and Diodes" Molecules 23, no. 7: 1551. https://doi.org/10.3390/molecules23071551




