Fragilides K and L, New Briaranes from the Gorgonian Coral Junceella fragilis
Abstract
:1. Introduction
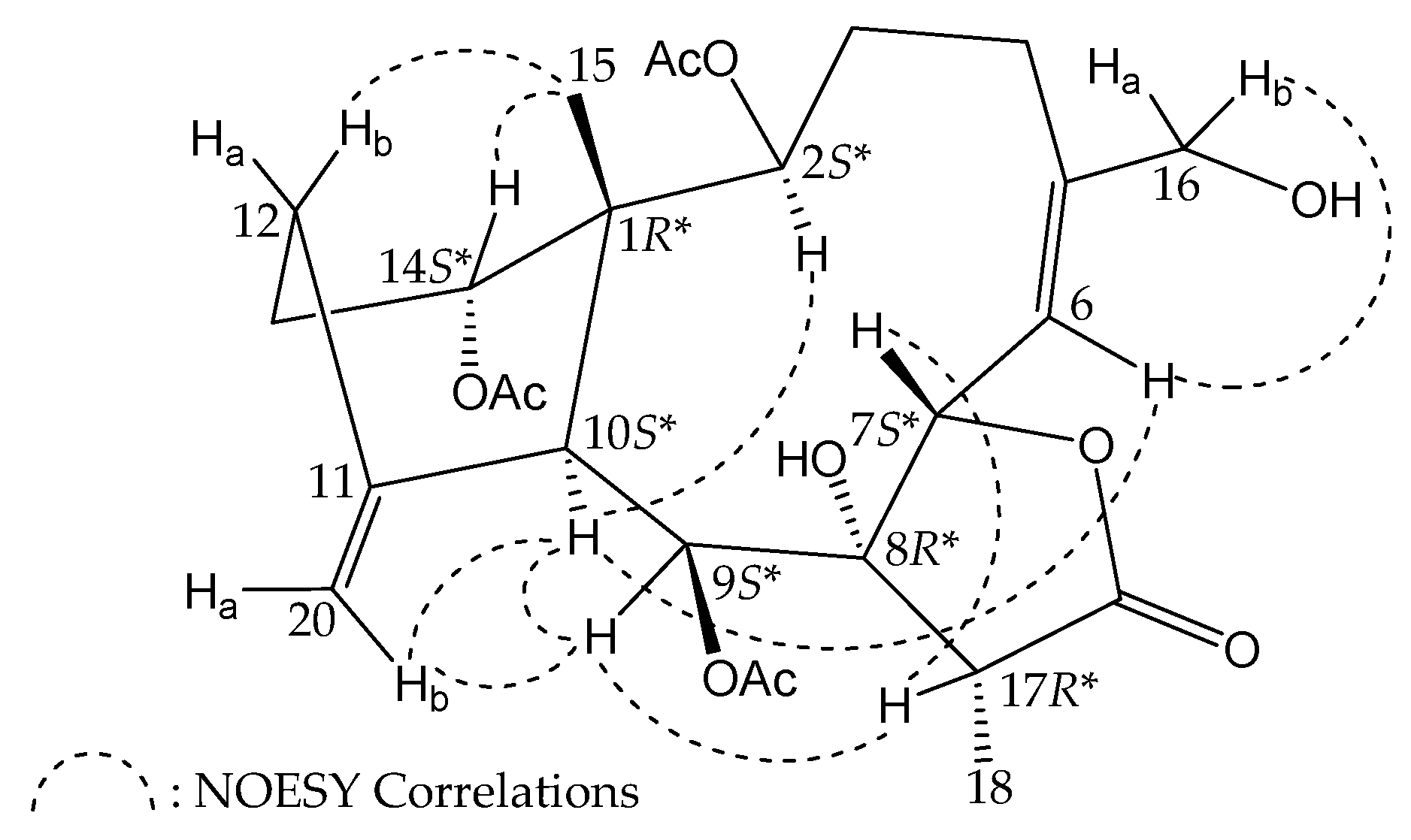
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Molecular Mechanics Calculations
3.5. In Vitro Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bayer, F.M. Key to the genera of Octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Chen, C.-C.; Chang, K.-H. Gorgonacea (Coelenterata: Anthozoa: Octocorallia) of Southern Taiwan. Bull. Inst. Zool. Acad. Sin. 1991, 30, 149–182. [Google Scholar]
- Bayer, F.M.; Grasshoff, M. The genus group taxa of the family Ellisellidae, with clarification of the genera established by J.E. Gray (Cnidaria: Octocorallia). Senckenberg. Biol. 1994, 74, 21–45. [Google Scholar]
- Sung, P.-J.; Gwo, H.-H.; Fan, T.-Y.; Li, J.-J.; Dong, J.; Han, C.-C.; Wu, S.-L.; Fang, L.-S. Natural product chemistry of gorgonian corals of the genus Junceella. Biochem. Syst. Ecol. 2004, 32, 185–196. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Su, J.-H.; Chou, T.-T.; Cheng, Y.-P.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H.; Li, J.-J.; Lu, M.-C.; et al. Natural product chemistry of gorgonian corals of the genus Junceella–Part II. Mar. Drugs 2011, 9, 2773–2792. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-C.; Lin, Y.-C.; Lin, Y.-S.; Chen, C.-H.; Hwang, T.-L.; Shen, Y.-C. Four new briarane diterpenoids from Taiwanese gorgonian Junceella fragilis. Mar. Drugs 2013, 11, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Sun, J.-F.; Han, Z.; Zhou, X.-F.; Yang, B.; Liu, Y. Fragilisinins A–L, new briarane-type diterpenoids from gorgonian Junceella fragilis. RSC Adv. 2014, 4, 5261–5271. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Yin, F.; van Ofwegen, L.; Li, W. Halogenated briarane diterpenes with acetyl migration from the gorgonian coral Junceella fragilis. Chem. Biodivers. 2017, 12, e1700053. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ji, M.; Li, X.; Ren, J.; Yin, F.; van Ofwegen, L.; Yu, S.; Chen, X.; Lin, W. Fragilolides A–Q norditerpenoid and briarane diterpenoids from the gorgonian coral Junceella fragilis. Tetrahedron 2017, 73, 2518–2528. [Google Scholar] [CrossRef]
- Li, C.; La, M.-P.; Tang, H.; Pan, W.-H.; Sun, P.; Krohn, K.; Yi, Y.-H.; Li, L.; Zhang, W. Bioactive briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Bioorg. Med. Chem. Lett. 2012, 22, 4368–4372. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Long, K.; Fang, Z. Studies of the chemical constituents of the Chinese gorgonia (III). Isolation and identification of a new polyacetoxy chlorine-containing diterpene lactone (praelolide). Acta Sci. Nat. Univ. Sunyatseni 1983, 1, 83–92. [Google Scholar]
- Dai, J.; Wan, Z.; Rao, Z.; Liang, D.; Fang, Z.; Luo, Y.; Long, K. Molecular structure and absolute configuration of the diterpene lactone, praelolide. Sci. Sin. B 1985, 28, 1132–1142. [Google Scholar] [PubMed]
- Shin, J.; Park, M.; Fenical, W. The junceellolides, new anti-inflammatory diterpenoids of the briarane class from the Chinese gorgonian Junceella fragilis. Tetrahedron 1989, 45, 1633–1638. [Google Scholar] [CrossRef]
- Subrahmanyam, C.; Kulatheeswaran, R.; Ward, R.S. Briarane diterpenes from the Indian Ocean gorgonian Gorgonella umbraculum. J. Nat. Prod. 1998, 61, 1120–1122. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.-J.; Fan, T.-Y.; Fang, L.-S.; Wu, S.-L.; Li, J.-J.; Chen, M.-C.; Cheng, Y.-M.; Wang, G.-H. Briarane derivatives from the gorgonian coral Junceella fragilis. Chem. Pharm. Bull. 2003, 51, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Zhang, S.; Huang, H.; Xiao, Z.-H.; Huang, J.-S.; Li, Q.-X. New briaranes from the South China Sea gorgonian Junceella juncea. J. Nat. Prod. 2004, 67, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Zhang, S.; Qian, P.-Y.; Xiao, Z.-H.; Li, M.-Y. Ten new antifouling briarane diterpenoids from the South China Sea gorgonian Junceella juncea. Tetrahedron 2006, 62, 9123–9130. [Google Scholar] [CrossRef]
- Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane diterpenoids isolated from Octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, Y.-P.; Hwang, T.-L.; Chiang, M.Y.; Fang, L.-S.; Sung, P.-J. Junceellolides J–L, 11,20-epoxybriaranes from the gorgonian coral Junceella fragilis. J. Nat. Prod. 2006, 69, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Y.-W.; Mollo, E.; Cimino, G. Junceellonoids A and B, two new briarane diterpenoids from the Chinese gorgonian Junceella fragilis Ridley. Helv. Chim. Acta 2004, 87, 2341–2345. [Google Scholar] [CrossRef]
- Sung, P.-J.; Wang, S.-H.; Chiang, M.Y.; Su, Y.-D.; Chang, Y.-C.; Hu, W.-P.; Tai, C.-Y.; Liu, C.-Y. Discovery of new chlorinated briaranes from Junceella fragilis. Bull. Chem. Soc. Jpn. 2009, 82, 1426–1432. [Google Scholar] [CrossRef]
- Xu, J.-H.; Lai, K.-H.; Su, Y.-D.; Chang, Y.-C.; Peng, B.-R.; Backlund, A.; Wen, Z.-H.; Sung, P.-J. Briaviolides K–N, new briarane-type diterpenoids from cultured Octocoral Briareum violaceum. Mar. Drugs 2018, 16, 75. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Chen, N.-F.; Chen, W.-F.; Hung, H.-C.; Lee, H.-P.; Lin, Y.-Y.; Wang, H.-M.; Sung, P.-J.; Sheu, J.-H.; Wen, Z.-H. Sinularin from indigenous soft coral attenuates nociceptive responses and spinal neuroinflammation in carrageenan-induced inflammatory rat model. Mar. Drugs 2012, 10, 1899–1919. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.-H.; Chen, W.-F.; Sung, C.-S.; Duh, C.-Y.; Huang, S.-Y.; Lin, C.-S.; Tai, M.-H.; Tzeng, S.-F.; Wen, Z.-H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury-induced neuropathic pain in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.-H.; Chen, W.-F.; Duh, C.-Y.; Huang, S.-Y.; Hsu, C.-H.; Lin, C.-S.; Sung, C.-S.; Chen, I.-M.; Wen, Z.-H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Lin, Y.-Y.; Jean, Y.-H.; Lu, Y.; Chen, W.-F.; Yang, S.-N.; Wang, H.-M.D.; Jang, I.-Y.; Chen, I.-M.; Su, J.-H.; et al. Anti-Inflammatory and analgesic effects of the marine-derived compound comaparvin isolated from the crinoid Comanthus bennetti. Molecules 2014, 19, 14667–14686. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Figueiredo, C.A.; Brito, C.; Stavroullakis, A.; Prakki, A.; da Silva Velozo, E.; Nogueira-Filho, G. Effect of Allium cepa L. on lipopolysaccharide-stimulated osteoclast precursor cell viability, count, and morphology using 4′,6-diamidino-2-phenylindole-staining. Int. J. Cell Biol. 2014, 2014, 535789. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compounds 1–7 are not available from the authors. |



| C/H | δH (J in Hz) | δC, Multiple | 1H–1H COSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 46.9, C | |||
| 2 | 5.50 d (6.8) | 72.6, CH | H-3 | C-1, C-3, C-4, C-15, acetate carbonyl |
| 3 | 6.22 dd (10.8, 6.8) | 63.7, CH | H-2, H-4 | C-1, C-4, C-5, acetate carbonyl |
| 4 | 4.49 d (10.8) | 78.9, CH | H-3 | C-3, C-5, C-6, C-8, C-16 |
| 5 | 134.1, C | |||
| 6 | 4.96 d (2.8) | 53.8, CH | H-7 | n. o. |
| 7 | 4.42 d (2.8) | 79.0, CH | H-6 | C-5 |
| 8 | 83.0, C | |||
| 9 | 5.60 s | 71.1, CH | n. o. a | C-1, C-10, C-11, C-17, acetate carbonyl |
| 10 | 3.32 s | 35.1, CH | n. o. | C-1, C-8, C-11, C-12, C-15, C-20 |
| 11 | 58.9, C | |||
| 12 | 3.49 br s | 72.2, CH | H2-13 | n. o. |
| 13α/β | 2.20 ddd (15.6, 3.2, 2.8); 1.98 ddd (15.6, 3.2, 3.2) | 30.3, CH2 | H-12, H-14 | n. o. |
| 14 | 5.01 dd (3.2, 3.2) | 74.2, CH | H2-13 | n. o. |
| 15 | 1.24 s | 15.2, CH3 | C-1, C-2, C-10, C-14 | |
| 16a/b | 5.36 d (1.6); 5.57 d (1.6) | 119.7, CH2 | C-4, C-6 | |
| 17 | 2.79 q (7.2) | 49.6, CH | H3-18 | C-18, C-19 |
| 18 | 1.37 d (7.2) | 7.4, CH3 | H-17 | C-8, C-17, C-19 |
| 19 | 174.2, C | |||
| 20a/b | 2.76 d (3.2); 2.56 d (3.2) | 50.6, CH2 | n. o. | |
| OAc-2 | 170.2, C | |||
| 2.05 s | 20.5, CH3 | Acetate carbonyl | ||
| OAc-3 | 169.9, C | |||
| 2.08 s | 21.1, CH3 | Acetate carbonyl | ||
| OAc-9 | 169.5, C | |||
| 2.32 s | 21.1, CH3 | Acetate carbonyl | ||
| OAc-14 | 169.9, C | |||
| 2.00 s | 20.3, CH3 | Acetate carbonyl |
| C/H | δH (J in Hz) | δC, Multiple | 1H–1HCOSY | HMBC (H→C) |
|---|---|---|---|---|
| 1 | 47.0, C | |||
| 2 | 4.83 dd (6.0, 1.2) | 75.9, CH | H2-3 | C-1, C-3, C-4, C-10, C-15, acetate carbonyl |
| 3α/β | 1.72 m; 2.49 m | 31.8, CH2 | H-2, H2-4 | C-1, C-4 |
| 4α/β | 2.26 m; 2.59 m | 26.2, CH2 | H2-3 | C-3, C-5, C-6, C-16 |
| 5 | 146.7, C | |||
| 6 | 5.94 d (10.0) | 121.7, CH | H-7 | C-4, C-16 |
| 7 | 5.30 d (10.0) | 77.3, CH | H-6 | C-5, C-6, C-8 |
| 8 | 83.2, C | |||
| 9 | 5.30 d (5.2) | 71.5, CH | H-10 | C-1, C-7, C-8, C-10, C-11, acetate carbonyl |
| 10 | 3.31 d (5.2) | 42.2, CH | H-9 | C-1, C-2, C-8, C-9, C-11, C-12, C-15, C-20 |
| 11 | 150.9, C | |||
| 12a/b | 2.26 m; 2.18 m | 26.4, CH2 | H2-13 | C-11, C-13, C-14, C-20 |
| 13a/b | 2.01 m; 1.76 m | 27.4, CH2 | H2-12, H-14 | C-1, C-11, C-14 |
| 14 | 4.72 dd (4.8, 1.6) | 73.8, CH | H2-13 | C-1, C-2, C-10, C-12, C-13, C-15, |
| acetate carbonyl | ||||
| 15 | 1.09 s | 15.1, CH3 | C-1, C-2, C-10, C-14 | |
| 16a/b | 4.28 dd (14.0, 5.2); 4.14 dd (14.0, 7.6) | 68.9, CH2 | OH-16 | C-5, C-6 |
| 17 | 2.47 q (7.2) | 42.6, CH | H3-18 | C-8, C-18, C-19 |
| 18 | 1.13 d (7.2) | 6.6, CH3 | H-17 | C-8, C-17, C-19 |
| 19 | 175.8, C | |||
| 20a/b | 4.90 s; 5.06 s | 113.1, CH2 | C-10, C-11, C-12 | |
| OAc-2 | 171.8, C | |||
| 2.01 s | 21.1, CH3 | Acetate carbonyl | ||
| OAc-9 | 169.4, C | |||
| 2.21 s | 21.8, CH3 | Acetate carbonyl | ||
| OAc-14 | 170.6, C | |||
| 1.91 s | 21.2, CH3 | Acetate carbonyl | ||
| OH-8 | 2.05 s | C-7, C-8, C-9, C-17 | ||
| OH-16 | 3.01 dd (7.6, 5.2) | H2-16 | n. o. a |
| Compound | iNOS | COX-2 | β-Actin |
|---|---|---|---|
| Expression (% of LPS Group) | Expression (% of LPS Group) | Expression (% of LPS Group) | |
| Control | 1.01 ± 0.15 | 7.07 ± 3.88 | 86.12 ± 8.75 |
| LPS | 100 ± 11.26 | 100 ± 3.36 | 100 ± 0.07 |
| 1 | 61.22 ± 3.82 | 88.09 ± 6.87 | 94.26 ± 2.3 |
| 2 | 49.13 ± 4.15 | 80.08 ± 7.98 | 110.11 ± 3.16 |
| 3 | 36.22 ± 13.28 | 43.64 ± 6.23 | 99.30 ± 16.53 |
| 4 | 61.63 ± 21.36 | 81.55 ± 22.66 | 117.99 ± 6.04 |
| 5 | 76.16 ± 21.90 | 58.94 ± 0.8 | 117.48 ± 13.63 |
| 6 | 43.33 ± 10.82 | 68.87 ± 11.08 | 129.76 ± 25.75 |
| 7 | 74.65 ± 18.02 | 47.49 ± 1.49 | 124.60 ± 18.10 |
| Dex. a | 30.83 ± 6.69 | 9.32 ± 3.47 | 100.88 ± 3.14 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.-G.; Chang, Y.-C.; Hu, C.-C.; Wen, Z.-H.; Wu, Y.-C.; Sung, P.-J. Fragilides K and L, New Briaranes from the Gorgonian Coral Junceella fragilis. Molecules 2018, 23, 1510. https://doi.org/10.3390/molecules23071510
Zheng L-G, Chang Y-C, Hu C-C, Wen Z-H, Wu Y-C, Sung P-J. Fragilides K and L, New Briaranes from the Gorgonian Coral Junceella fragilis. Molecules. 2018; 23(7):1510. https://doi.org/10.3390/molecules23071510
Chicago/Turabian StyleZheng, Li-Guo, Yu-Chia Chang, Chiung-Chih Hu, Zhi-Hong Wen, Yang-Chang Wu, and Ping-Jyun Sung. 2018. "Fragilides K and L, New Briaranes from the Gorgonian Coral Junceella fragilis" Molecules 23, no. 7: 1510. https://doi.org/10.3390/molecules23071510







