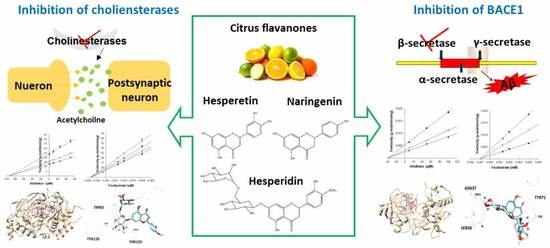
In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones
Abstract
:1. Introduction
2. Results
2.1. Inhibiting Multiple Enzyme Targets of Hesperetin, Naringenin, and Hesperidin
2.2. Enzyme Kinetics of Hesperetin, Naringenin, and Hesperidin
2.3. Molecular Docking Study of the Inhibitory Activity of Major Citrus Flavanones
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Enzymatic Assessment for Biological Evaluation
4.3. Assessment of Inhibition Kinetics on BACE1, AChE, and BChE
4.4. Molecular Docking Studies of BACE1, AChE, and BChE
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Selkoe, D.J. Aβ Oligomers—A decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Götz, J.; Schild, A.; Hoerndli, F.; Pennanen, L. Amyloid-induced neurofibrillary tangle formation in Alzheimer’s disease: Insight from transgenic mouse and tissue-culture models. Int. J. Dev. Neurosci. 2004, 22, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Bardini, P.; Obbili, A.; Vitali, A.; Borghi, R.; Zaccheo, D.; Pronzato, M.A.; Danni, O.; Smith, M.A.; Perry, G.; et al. Oxidative stress increases expression and activity of BACE in NT2 neurons. Neurobiol. Dis. 2002, 10, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Onyewuchi, O.; Yang, S.; Liu, R.; Simpkins, J.W. Increased β-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004, 1009, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, K.; Wang, R.; Cui, J.; Lipton, S.A.; Liao, F.F.; Xu, H.; Zhang, Y.W. Hypoxia-inducible factor 1α (HIF-1α)-mediated hypoxia increases BACE1 expression and β-amyloid generation. J. Biol. Chem. 2007, 282, 10873–10880. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, H.K.; Park, C.H.; Yokozawa, T.; Tanaka, T.; Jung, H.A.; Choi, J.S. Potential anti-cholinesterase and β-site amyloid precursor protein cleaving enzyme 1 inhibitory activities of cornuside and gallotannins from Cornus officinalis fruits. Arch. Pharm. Res. 2017, 40, 836–853. [Google Scholar] [CrossRef] [PubMed]
- Ciro, A.; Park, J.; Burkhard, G.; Yan, N.; Geula, C. Biochemical differentiation of cholinesterases from normal and Alzheimer’s disease cortex. Curr. Alzheimer Res. 2012, 9, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.C.; Pérez, A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med. Sci. Monit. 2007, 12, RA214–RA221. [Google Scholar]
- Cirmi, S.; Ferlazzo, N.; Lombardo, G.E.; Ventura-Spagnolo, E.; Gangemi, S.; Calapai, G.; Navarra, M. Neurodegerative disease: Might citrus flavonoids play a protective role? Molecules 2016, 21, 1312. [Google Scholar] [CrossRef] [PubMed]
- Nalini, N.; Aranganathan, S.; Kabalimurthy, J. Chemopreventive efficacy of hesperetin (citrus flavonone) against 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Toxicol. Mech. Methods 2012, 22, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, G. Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zug, C.; Qu, H.C.; Schluesener, H.; Zhang, Z.Y. Hesperidin ameliorates behavioral impairments and neuropathology of transgenic APP/PS1 mice. Behav. Brain Res. 2015, 281, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Tsai, S.Y.; Lin, J.A.; Wu, C.H.; Yen, G.C. Cytoprotective effects of hesperetin and hesperidin against amyloid β-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol. Nutr. Food Res. 2012, 56, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Liu, L.; Zhu, X.Y.; Wu, W.L.; Wang, Y. Hesperidin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress in a mouse model of Alzheimer’s disease. Cell. Mol. Neurobiol. 2014, 34, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Su, S.H.; Pradeep, P.; Jung, H.A.; Choi, J.S. Structure related inhibition of enzyme systems in cholinesterases and BACE1 in vitro by naturally occurring naphthopyrone and its glycosides isolated from Cassia obtusifolia. Molecuels 2018, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Senol, F.S.; Ankli, A.; Reich, E.; Orhan, I.E. HPTLC fingerprinting and cholinesterase inhibitory and metal-chelating capacity of various Citrus cultivars and Olea europaea. Food Technol. Biotechnol. 2016, 54, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hunag, A.-L.; Zhang, Y.-L.; Li, Z.; Ding, H.-W.; Huang, C.; Meng, X.-M.; Li, J. Design, synthesis and evaluation of hesperetin derivatives as potential multifunctional anti-Alzheimer agents. Molecules 2017, 22, 1067. [Google Scholar] [CrossRef] [PubMed]
- Remya, C.; Dileep, K.V.; Tintu, I.; Variyar, E.J.; Sadasivan, C. Flavanone glycosides as acetylcholinesterase inhibitors: Computational and experimental evidence. Indian J. Pharm. Sci. 2014, 76, 567–570. [Google Scholar] [PubMed]
- Heo, H.J.; Kim, D.O.; Shin, S.C.; Kim, M.J.; Kim, B.G.; Shin, D.H. Effect of antioxidant flavanone, naringenin, from Citrus junos on neuroprotection. J. Agric. Food Chem. 2004, 52, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ikoma, Y.; Sugiura, M.; Yano, M.; Hasegawa, Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004, 52, 6653–6659. [Google Scholar] [CrossRef] [PubMed]
- Bredsdorff, L.; Nielsen, I.L.; Rasmussen, S.E.; Cornett, C.; Barron, D.; Bouisset, F.; Offord, E.; Williamson, G. Absorption, conjugation and excretion of the flavanones, naringenin and hesperetin from a-rhamnosidase-treated orange juice in human subjects. Br. J. Nutr. 2010, 103, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, S.C.; Senthil Kumar, K.J.; Yu, K.N.; Lee Chao, P.D.; Tsai, S.Y.; Hou, Y.C.; Hseu, Y.C. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J. Agric. Food Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.W.; Cheng, F.C.; Huang, Y.T.; Chen, C.F.; Tsai, T.H. Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J. Chromatogr. 1998, 714, 369–374. [Google Scholar] [CrossRef]
- Tsai, T.H.; Chen, Y.F. Determination of unbound hesperetin in rat blood and brain by microdialysis coupled to microbore liquid chromatography. J. Food Drug Anal. 2000, 8, 331–333. [Google Scholar]
- Carballo-Villalobos, A.I.; Gonzalez-Trujano, M.E.; Psellicer, F.; López-Muñoz, F.J. Antihyperalgesic Effect of Hesperidin Improves with Diosmin in Experimental Neuropathic Pain. Biomed. Res. Int. 2016, 2016, 8263463. [Google Scholar] [CrossRef] [PubMed]
- Mogami, S.; Sadakane, C.; Nahata, M.; Mizuhara, Y.; Yamada, C.; Hattori, T.; Takeda, H. CRF receptor 1 antagonism and brain distribution of active components contribute to the ameliorative effect of rikkunshito on stress-induced anorexia. Sci. Rep. 2016, 6, 27516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- kheradmand, E.; Moghaddam, A.H.; Zare, M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed. Pharmacother. 2018, 97, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Yu, Y.; Lee, J.; Jeong, W.S.; Ho, C.T.; Jun, M. Polymethoxyflavones: Novel β-Secretase (BACE1) Inhibitors from Citrus Peels. Nutrients 2017, 9, 973. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Callaway, E. Erythrocyte cholinesterase-levels in mental patients. Nature 1961, 192, 1216. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, A.; Mcgaughey, G.B.; Sheridan, R.P.; Good, A.C.; Warren, G.; Mathieu, M.; Muchmore, S.W.; Brown, S.P.; Grant, J.A.; Haigh, J.A.; et al. Molecular Shape and Medicinal Chemistry: A Perspective. J. Med. Chem. 2010, 53, 3862. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Štěpánková, Š.; Vorčáková, K.; Švarcová, M.; Vinšová, J. Novel Cholinesterase Inhibitors Based on O-Aromatic N,N-Disubstituted Carbamates and Thiocarbamates. Molecules 2016, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| Compounds | IC50 (µM) 1 | ||
|---|---|---|---|
| BACE1 | AChE | BChE | |
| Hesperetin | 22.13 ± 1.81 | 45.70 ± 2.69 | >100 |
| Naringenin | 30.31 ± 2.06 | 42.66 ± 4.30 | >100 |
| Hesperidin | 16.99 ± 1.25 | 22.80 ± 2.78 | 48.09 ± 0.74 |
| Resveratrol 2 | 14.59 ± 0.79 | - | - |
| Galanthamine 3 | - | 1.59 ± 0.03 | 10.93 ± 0.69 |
| Sample (μM) | TACE | Trypsin | Chymotrypsin | Elastase |
|---|---|---|---|---|
| Hesperetin | ||||
| 10 | 8.66 ± 0.70 | 5.31 ± 0.39 | 4.91 ± 1.40 | 4.84 ± 0.46 |
| 100 | 11.31 ± 1.37 | 4.72 ± 0.45 | 9.21 ± 0.33 | 9.95 ± 1.28 |
| Naringenin | ||||
| 10 | 5.15 ± 0.98 | 7.80 ± 1.25 | 8.77 ± 0.74 | 9.74 ± 0.41 |
| 100 | 6.95 ± 1.52 | 5.84 ± 0.75 | 6.88 ± 0.89 | 10.51 ± 1.17 |
| Hesperidin | ||||
| 10 | 8.53 ± 1.05 | 2.27 ± 0.30 | 3.19 ± 0.28 | 6.91 ± 0.83 |
| 100 | 9.19 ± 1.20 | 1.41 ± 0.22 | 4.11 ± 0.34 | 6.00 ± 1.33 |
| Enzyme | Ligands | Lowest Energy (Kcal/mol) | H-Bonds No. | Residues | Bond Distance (Å) |
|---|---|---|---|---|---|
| BACE1 | Hesperetin | −8.3 | - | - | - |
| Naringenin | −8.1 | - | - | - | |
| Hesperidin | −10.1 | 3 | SER36 TYR71 ASN37 | 3.372 2.837 2.820 |
| Enzyme | Ligands | Lowest Energy (Kcal/mol) | H-Bonds No. | Residues | Bond Distance (Å) |
|---|---|---|---|---|---|
| AChE | Hesperetin | −8.4 | 3 | TYR124 PHE295 TYR337 | 2.927 2.913 2.699 |
| Naringenin | −8.7 | 1 | ASP74 | 2.738 | |
| Hesperidin | −9.8 | 2 | SER125 SER203 | 2.810 3.094 | |
| BChE | Hesperidin | −10.3 | 5 | THR120 TYR128 TRP82 HIS438 TYR332 | 2.929 2.696 3.366 2.617 3.006 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Youn, K.; Lim, G.; Lee, J.; Jun, M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules 2018, 23, 1509. https://doi.org/10.3390/molecules23071509
Lee S, Youn K, Lim G, Lee J, Jun M. In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones. Molecules. 2018; 23(7):1509. https://doi.org/10.3390/molecules23071509
Chicago/Turabian StyleLee, Seungeun, Kumju Youn, GyuTae Lim, Jinhyuk Lee, and Mira Jun. 2018. "In Silico Docking and In Vitro Approaches towards BACE1 and Cholinesterases Inhibitory Effect of Citrus Flavanones" Molecules 23, no. 7: 1509. https://doi.org/10.3390/molecules23071509