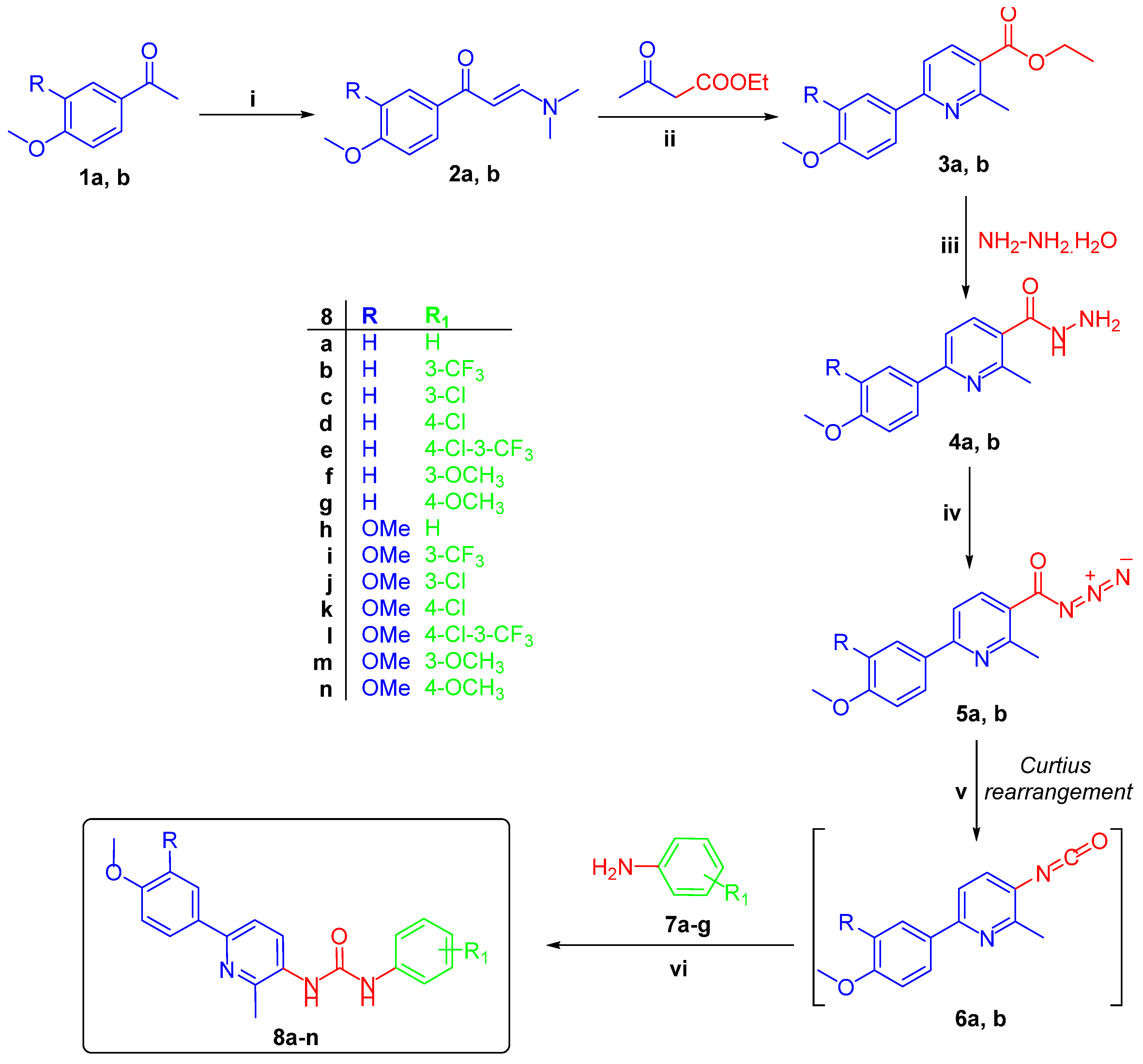
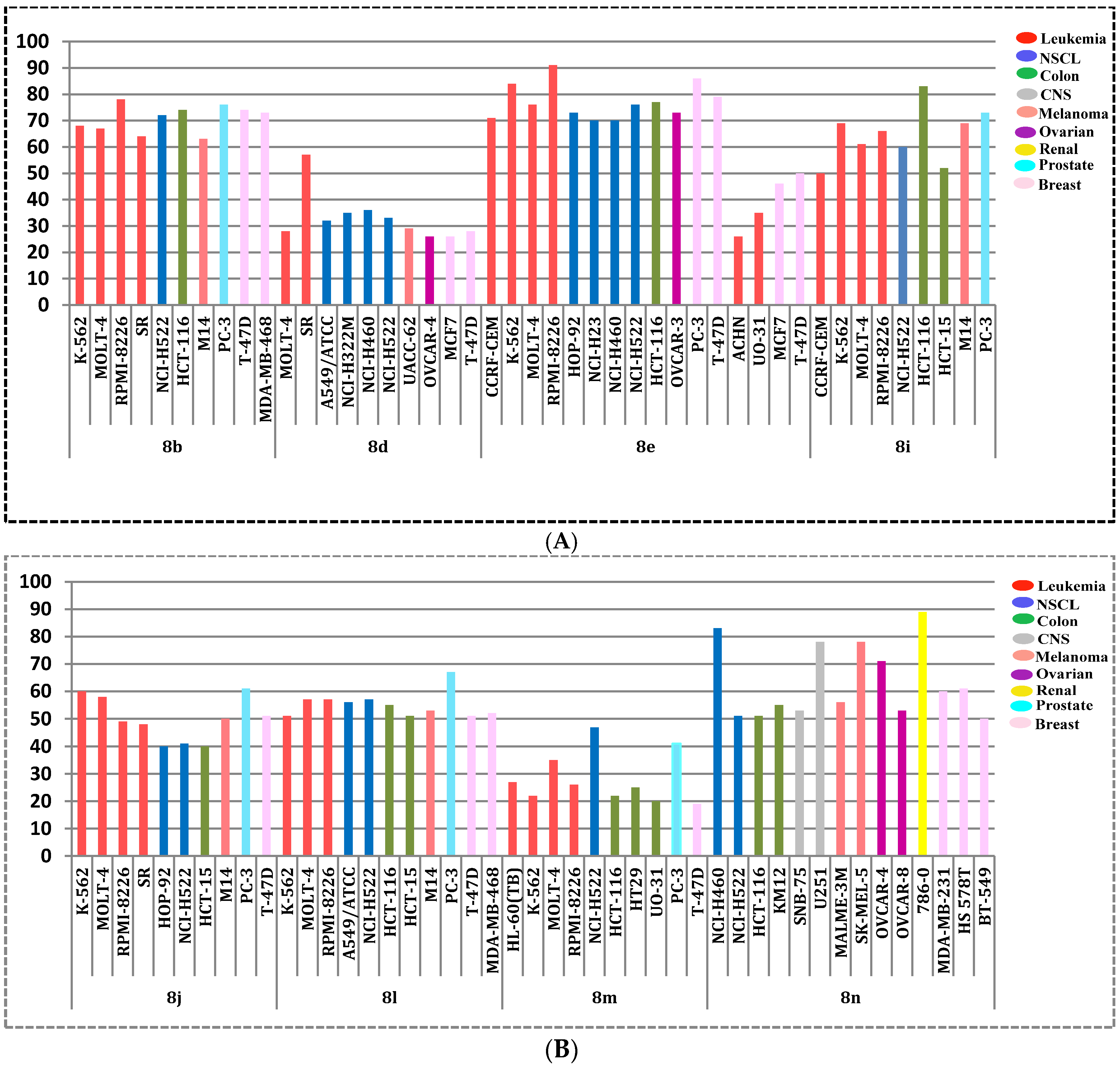
3.1.2. General Procedure for the Preparation of Target Pyridine-Ureas 8a–n
In an ice bath, a mixture of hydrazides 4a,b (5 mmol) and sodium nitrite (0.5 g, 7 mmol) was stirred in glacial acetic acid for 1 h, then stirring was continued at room temperature for another 1 h. The obtained solid was collected by filtration, washed with cold water and air-dried to furnish 6-(4-methoxyphenyl/3,4-dimethoxyphenyl)-2-methylnicotinoyl azide 5a,b, which used in the next reaction without further purification. Then, the appropriate nicotinoyl azide 5a,b was heated under reflux in dry xylene for 1 h before addition of anilines 7a–g. The reaction mixture was refluxed for 3 h then allowed to cool to room temperature. The formed precipitate was filtered off, washed with cold acetone, dried and recrystallized from dioxane to furnish the target pyridines 8a–n.
1-(6-(4-Methoxyphenyl)-2-methylpyridin-3-yl)-3-phenylurea (8a). White crystals (yield 72%), m.p. 205–207 °C; IR (KBr, ν cm−1) 3387 (NH), 1653 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.51 (s, 3H, -CH3), 3.86 (s, 3H, -OCH3), 6.29 (s, 1H, NH, D2O exchangeable); 6.40 (s, 1H, NH, D2O exchangeable), 6.98 (d, 2H, J = 8.8 Hz, Ar-H), 7.16 (m, 1H, Ar-H), 7.38 (m, 4H, Ar-H), 7.55 (d, 1H, J = 8.4 Hz, Ar-H), 7.93 (d, 2H, J = 8.8 Hz, Ar-H), 8.05 (d, 1H, J = 8.4 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.38 (CH3), 55.18 (OCH3), 113.99, 116.96, 118.10, 121.96, 127.24, 128.25, 128.88, 131.17, 132.13, 139.59, 147.36, 149.00, 152.62 (C=O), 159.51 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C20H20N3O2): 334.15500, found: 334.15499; Anal. Calcd. for C20H20N3O2 (333.39): C, 72.05; H, 5.74; N, 12.60; found C, 72.04; H, 5.71; N, 12.61.
1-(6-(4-Methoxyphenyl)-2-methylpyridin-3-yl)-3-(3-(trifluoromethyl)phenyl) Urea (8b). White crystals (yield 75%), m.p. 189–191 °C; IR (KBr, ν cm−1) 3405 (NH), 1716 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.60 (s, 3H, -CH3), 3.87 (s, 3H, -OCH3), 6.28 (s, 1H, NH, D2O exchangeable); 6.62 (s, 1H, NH, D2O exchangeable), 6.99 (d, 2H, J = 8.8 Hz, Ar-H), 7.35 (d, 1H, J = 7.7 Hz, Ar-H), 7.44 (t, 1H, J = 8 Hz, Ar-H), 7.58 (d, 1H, J = 8.4 Hz, Ar-H), 7.61 (d, 1H, J = 8 Hz, Ar-H), 7.68 (s, 1H, Ar-H), 7.89–7.99 (m, 3H, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.34 (CH3), 55.18 (OCH3), 114.00, 116.97, 118.20, 121.70, 123.12, 125.28, 127.32, 128.97, 129.45, 129.70, 130.03, 131.09, 131.70, 140.49, 148.03, 149.52, 152.67 (C=O), 159.59 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C21H19N3O2F3): 402.14239, found: 302.14220; Anal. Calcd. for C21H19N3O2F3 (401.39): C, 62.84; H, 4.52; N, 10.47; found C, 62.81; H, 4.52; N, 10.44.
1-(3-Chlorophenyl)-3-(6-(4-methoxyphenyl)-2-methylpyridin-3-yl) Urea (8c). White crystals (yield 81%), m.p. 212–214 °C; IR (KBr, ν cm−1) 3395 (NH), 1733 (C=O); 1H-NMR (MeOD-d4) δ ppm: 2.50 (s, 3H, CH3), 3.78 (s, 3H, -OCH3), 6.28 (s, 1H, NH, D2O exchangeable); 6.62 (s, 1H, NH, D2O exchangeable), 6.89–6.99 (m, 3H, Ar-H), 7.15–7.25 (m, 2H, Ar-H), 7.52 (dd, 2H, J = 2.1 Hz, J = 8.4 Hz, Ar-H), 7.80 (d, 2H, J = 8.8 Hz, Ar-H), 8.09 (d, 1H, J = 8.4 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.35 (CH3), 55.18 (OCH3), 114.00, 116.55, 116.97, 117.47, 121.57, 127.30, 128.76, 130.49, 131.10, 131.77, 133.26, 141.15, 147.85, 149.40, 152.52 (C=O), 159.57 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C20H19N3O2Cl): 368.11603, found: 368.11605; Anal. Calcd. for C20H19N3O2Cl (367.83): C, 65.31; H, 4.93; N, 11.42; found C, 65.34; H, 4.90; N, 11.41.
1-(4-Chlorophenyl)-3-(6-(4-methoxyphenyl)-2-methylpyridin-3-yl) Urea (8d). White crystals (yield 83%), m.p. 231–232 °C; IR (KBr, ν cm−1) 3387 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.57 (s, 3H, -CH3), 3.87 (s, 3H, -OCH3), 6.18 (s, 1H, NH, D2O exchangeable); 6.37 (s, 1H, NH, D2O exchangeable), 6.99 (d, 2H, J = 8.8 Hz, Ar-H), 7.27–7.38 (m, 3H, Ar-H), 7.52–7.60 (m, 2H, Ar-H), 7.91–8.00 (m, 3H, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.36 (CH3), 55.18 (OCH3), 113.99, 116.97, 119.63, 125.44, 127.27, 128.71, 131.12, 131.91, 138.61, 147.68, 149.24, 152.55 (C=O), 159.54 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C20H19N3O2Cl): 368.11603, found: 368.11603; Anal. Calcd. for C20H19N3O2Cl (367.83): C, 65.31; H, 4.93; N, 11.42; found C, 65.30; H, 4.92; N, 11.40.
1-(4-Chloro-3-(trifluoromethyl)phenyl)-3-(6-(4-methoxyphenyl)-2-methylpyridin-3-yl)urea (8e). White crystals (yield 74%), m.p. 195–197 °C; IR (KBr, ν cm−1) 3394 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.61 (s, 3H, -CH3), 3.87 (s, 3H, -OCH3), 6.25 (s, 1H, NH, D2O exchangeable); 6.60 (s, 1H, NH, D2O exchangeable), 6.99 (d, 2H, J = 8.5 Hz, Ar-H), 7.44 (d, 1H, J = 8.7 Hz, Ar-H), 7.57–7.71 (m, 3H, Ar-H), 7.90–7.95 (m, 3H, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.32 (CH3), 55.18 (OCH3), 114.01, 116.60, 116.98, 121.72, 122.37, 122.92, 123.86, 126.88, 127.35, 129.34, 131.05, 131.50, 132.10, 139.26, 148.37, 149.77, 152.59 (C=O), 159.62 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C21H18N3O2ClF3): 436.10342, found: 436.10332; Anal. Calcd. for C21H18N3O2ClF3 (435.83): C, 57.87; H, 3.93; N, 9.64; found C, 57.82; H, 3.90; N, 9.61.
1-(3-Methoxyphenyl)-3-(6-(4-methoxyphenyl)-2-methylpyridin-3-yl) Urea (8f). White crystals (yield 83%), m.p. 227–230 °C; IR (KBr, ν cm−1) 3395 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.49 (s, 3H, CH3), 3.81 (s, 3H, -OCH3), 3.83 (s, 3H, -OCH3), 6.30 (s, 1H, NH, D2O exchangeable), 6.60 (s, 1H, NH, D2O exchangeable), 6.91–7.00 (m, 3H, Ar-H), 7.15-7.27 (m, 2H, Ar-H), 7.53 (dd, 2H, J = 2.1 Hz, J = 8.4 Hz, Ar-H), 7.80 (d, 2H, J = 8.8 Hz, Ar-H), 8.09 (d, 1H, J = 8.4 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.32 (CH3), 55.18 (2 OCH3), 114.01, 116.60, 116.98, 121.72, 122.37, 122.92, 123.86, 126.88, 127.35, 129.34, 131.05, 131.50, 132.10, 139.26, 148.37, 149.77, 152.59 (C=O), 159.62 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C21H22N3O3): 364.16557, found: 364.16565.
1-(4-Methoxyphenyl)-3-(6-(4-methoxyphenyl)-2-methylpyridin-3-yl) Urea (8g). White crystals (yield 80%), m.p. 241–242 °C; IR (KBr, ν cm−1) 3387 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.42 (s, 3H, -CH3), 3.83 (s, 3H, -OCH3), 3.85 (s, 3H, -OCH3), 6.22 (s, 1H, NH, D2O exchangeable), 6.60 (s, 1H, NH, D2O exchangeable), 6.91–7.00 (m, 4H, Ar-H), 7.53 (d, 2H, J = 8.4 Hz, Ar-H), 7.91 (d, 2H, J = 8.8 Hz, Ar-H), 8.13 (d, 2H, J = 8.4 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.37 (CH3), 55.17 (2 OCH3), 159.47 (CO), 113.97, 114.07, 116.94, 119.87, 127.20, 128.02, 131.20, 132.33, 132.61, 147.15, 148.77, 152.77, 154.51 (C=O), 159.47 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C21H22N3O3): 364.16557, found: 364.16597; Anal. Calcd. for C21H22N3O3 (363.42): C, 69.41; H, 5.82; N, 11.56; found C, 69.44; H, 5.80; N, 11.56.
1-(6-(3,4-Dimethoxyphenyl)-2-methylpyridin-3-yl)-3-phenylurea (8h). White crystals (yield 77%), m.p. 238–239 °C; IR (KBr, ν cm−1) 3398 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.42 (s, 3H, CH3), 3.83 (s, 3H, -OCH3), 3.85 (s, 3H, -OCH3), 6.32 (s, 1H, NH, D2O exchangeable); 6.44 (s, 1H, NH, D2O exchangeable), 6.94 (d, 1H, J = 8.6 Hz, Ar-H), 7.39–7.42 (m, 5H, Ar-H), 7.51–7.62 (m, 3H, Ar-H), 8.08 (d, 1H, J = 8.8 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.42 (CH3), 55.52 (2 OCH3), 109.48, 111.73, 117.20, 118.12, 118.50, 121.96, 128.13, 128.88, 131.43, 132.20, 139.59, 147.25, 148.83, 149.00, 149.19, 152.63 (C=O); HRMS (ESI) m/z calcd for [M + H]+ (C21H22N3O3): 364.16557, found: 364.16528; Anal. Calcd. for C21H22N3O3 (363.42): C, 69.41; H, 5.82; N, 11.56; found C, 69.40; H, 5.80; N, 11.52.
1-(6-(3,4-Dimethoxyphenyl)-2-methylpyridin-3-yl)-3-(3-(trifluoromethyl)phenyl) Urea (8i). White crystals (yield 80%), m.p. 235–237 °C; IR (KBr, ν cm−1) 3396 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.55 (s, 3H, CH3), 3.93 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 6.56 (s, 1H, NH, D2O exchangeable), 6.93 (d, 1H, J = 8.6 Hz, Ar-H), 7.00 (s, 1H, Ar-H), 7.33 (d, 1H, J = 7.6 Hz, Ar-H), 7.40 (t, 1H, J = 7.6 Hz, Ar-H), 7.47 (dd, 1H, J = 2.0 Hz, J = 8.5 Hz, Ar-H), 7.52-7.62 (m, 2H, Ar-H), 7.65 (s, 1H, Ar-H), 7.97 (d, 1H, J = 8.6 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.38 (CH3), 55.53 (2 OCH3), 109.53, 111.73, 114.02, 117.21, 118.60, 121.72, 123.12, 125.28, 128.82, 129.70, 130.04, 131.33, 131.77, 140.47, 147.89, 148.84, 149.28, 149.52, 152.65 (C=O); HRMS (ESI) m/z calcd for [M + H]+ (C22H21N3O3F3): 432.15295, found: 432.15283; Anal. Calcd. for C22H21N3O3F3 (431.42): C, 61.25; H, 4.67; N, 9.74; found C, 61.22; H, 4.65; N, 9.71.
1-(3-Chlorophenyl)-3-(6-(3,4-dimethoxyphenyl)-2-methylpyridin-3-yl) Urea (8j). White crystals (yield 76%), m.p. 249–250 °C; IR (KBr, ν cm−1) 3373 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.57 (s, 3H, -CH3), 3.94 (s, 3H, -OCH3), 3.99 (s, 3H, -OCH3), 6.37 (s, 1H, NH, D2O exchangeable), 6.62 (s, 1H, NH, D2O exchangeable), 6.94 (d, 1H, J = 8.4 Hz, Ar-H), 7.05–7.13 (m, 1H, Ar-H), 7.45–7.53 (m, 4H, Ar-H), 7.56 (d, 1H, J = 8.4 Hz, Ar-H), 7.63 (s, 1H, Ar-H), 7.99 (d, 1H, J = 8.4 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.41 (CH3), 55.54 (2 OCH3), 109.52, 111.74, 116.57, 117.22, 117.48, 118.58, 121.59, 128.63, 130.51, 131.35, 131.85, 133.27, 141.16, 147.73, 148.84, 149.26, 149.41, 152.53 (C=O); HRMS (ESI) m/z calcd for [M + H]+ (C21H21N3O3Cl): 398.12660, found: 398.12642; Anal. Calcd. for C21H21N3O3Cl (397.86): C, 63.40; H, 5.07; N, 10.56; found C, 63.41; H, 5.02; N, 10.54.
1-(4-Chlorophenyl)-3-(6-(3,4-dimethoxyphenyl)-2-methylpyridin-3-yl) Urea (8k). White crystals (yield 81%), m.p. 266–267 °C; IR (KBr, ν cm−1) 3388 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.57 (s, 3H, CH3), 3.94 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 6.22 (s, 1H, NH, D2O exchangeable), 6.41 (s, 1H, NH, D2O exchangeable), 6.95 (d, 1H, J = 8.5 Hz, Ar-H), 7.30–7.35 (m, 4H, Ar-H), 7.51 (d, 1H, J = 8.8 Hz, Ar-H), 7.54–7.65 (m, 2H, Ar-H), 7.99 (d, 1H, J = 8.5 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.41 (CH3), 55.51 (2 OCH3), 109.50, 111.73, 117.20, 118.54, 119.64, 125.43, 128.46, 128.70, 131.38, 131.99, 138.63, 147.59, 148.83, 149.25, 152.56 (C=O); HRMS (ESI) m/z calcd for [M + H]+ (C21H21N3O3Cl): 398.12660, found: 398.12673; Anal. Calcd. for C21H21N3O3Cl (397.86): C, 63.40; H, 5.07; N, 10.56; found C, 63.40; H, 5.04; N, 10.52.
1-(4-Chloro-4-(trifluoromethyl)cyclohexa-2,5-dien-1-yl)-3-(6-(3,4-dimethoxyphenyl)-2-methylpyridin-3-yl)urea (8l). White crystals (yield 79%), m.p. 261–263 °C; IR (KBr, ν cm−1) 3393 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.51 (s, 3H, CH3), 3.92 (s, 3H, -OCH3), 3.96 (s, 3H, -OCH3), 6.22 (s, 1H, NH, D2O exchangeable), 6.74 (s, 1H, NH, D2O exchangeable), 6.91 (d, 1H, J = 8.6 Hz, Ar-H), 7.28 (s, 1H, Ar-H), 7.35 (d, 1H, J = 9.0 Hz, Ar-H), 7.50 (dd, 2H, J = 2.5, J = 8.7 Hz, Ar-H), 7.58 (d, 1H, J = 2.0 Hz, Ar-H), 7.65 (d, 1H, J = 2.4 Hz, Ar-H), 7.91 (d, 1H, J = 8.6 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.38 (CH3), 55.53 (2 OCH3), 109.56, 111.73, 117.22, 118.65, 121.74, 122.34, 122.93, 129.30, 131.32, 131.62, 132.10, 139.34, 148.33, 148.84, 149.32, 149.78, 152.65 (C=O); HRMS (ESI) m/z calcd for [M + H]+ (C22H20N3O3ClF3): 466.11398, found: 466.11398; Anal. Calcd. for C22H20N3O3ClF3 (465.86): C, 56.72; H, 4.11; N, 9.02; found C, 59.51; H, 4.61; N, 9.20
1-(6-(3,4-Dimethoxyphenyl)-2-methylpyridin-3-yl)-3-(3-methoxyphenyl) Urea (8m). White crystals (yield 80%), m.p. 243–245 °C; IR (KBr, ν cm−1) 3391 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.51 (s, 3H, -CH3), 3.80 (s, 3H, -OCH3), 3.92 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 6.22 (s, 1H, NH, D2O exchangeable), 6.56 (s, 1H, NH, D2O exchangeable), 6.66–6.75 (m, 2H, Ar-H), 6.83–6.96 (m, 2H, Ar-H), 7.05 (t, 1H, J = 2.3 Hz, Ar-H), 7.20–7.29 (m, 2H, Ar-H), 7.60 (d, 1H, J = 2.0 Hz, Ar-H), 8.06 (d, 1H, J = 8.5 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.40 (CH3), 54.93 (OCH3), 55.53 (2 OCH3), 103.87, 107.38, 109.49, 110.42, 111.73, 117.19, 118.51, 128.19, 129.65, 131.42, 132.13, 140.81, 147.28, 148.83, 149.05, 149.20, 152.55 (C=O); HRMS (ESI) m/z calcd for [M + H]+ (C22H24N3O4): 394.17613, found: 394.17608; Anal. Calcd. for C22H24N3O4 (393.44): C, 67.16; H, 5.89; N, 10.68; found C, 67.12; H, 5.89; N, 10.67.
1-(6-(3,4-Dimethoxyphenyl)-2-methylpyridin-3-yl)-3-(4-methoxyphenyl) Urea (8n). White crystals (yield 80%), m.p. 253–254 °C; IR (KBr, ν cm−1) 3392 (NH), 1733 (C=O); 1H-NMR (CDCl3-d) δ ppm: 2.42 (s, 3H, -CH3), 3.83 (s, 3H, -OCH3), 3.92 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 6.27 (s, 1H, NH, D2O exchangeable), 6.56 (s, 1H, NH, D2O exchangeable), 6.94 (dd, 3H, J = 5.1 Hz, J = 8.8 Hz, Ar-H), 7.29 (d, 2H, J = 7.4 Hz, Ar-H), 7.47 (d, 1H, J = 7.9 Hz, Ar-H), 7.54 (d, 1H, J = 8.2 Hz, Ar-H), 7.59 (s, 1H, Ar-H) 8.15 (d, 1H, J = 8.0 Hz, Ar-H); 13C-NMR (DMSO-d6) δ ppm: 21.43 (CH3), 55.18 (OCH3), 55.53 (2 OCH3), 109.47, 111.73, 114.06, 117.18, 118.47, 119.89, 127.96, 131.48, 132.43, 132.66, 147.09, 148.79, 148.83, 149.16, 152.81 (C=O), 154.51 (=C-O-CH3); HRMS (ESI) m/z calcd for [M + H]+ (C22H24N3O4): 394.17613, found: 394.17598; Anal. Calcd. for C22H24N3O4 (393.44): C, 67.16; H, 5.89; N, 10.68; found C, 67.11; H, 5.87; N, 10.65.