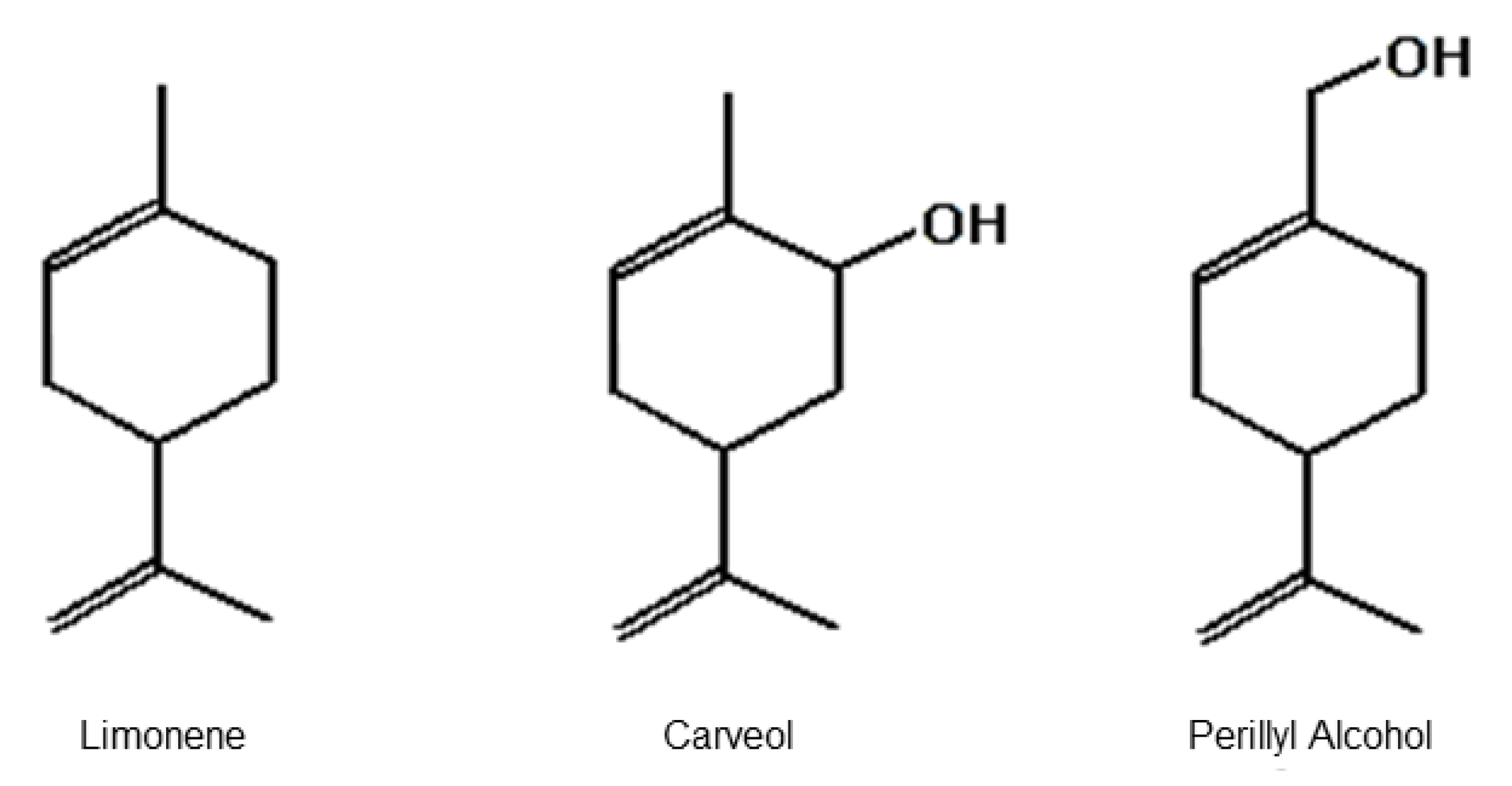
Hydroxyl Group and Vasorelaxant Effects of Perillyl Alcohol, Carveol, Limonene on Aorta Smooth Muscle of Rats
Abstract
:1. Introduction
2. Results
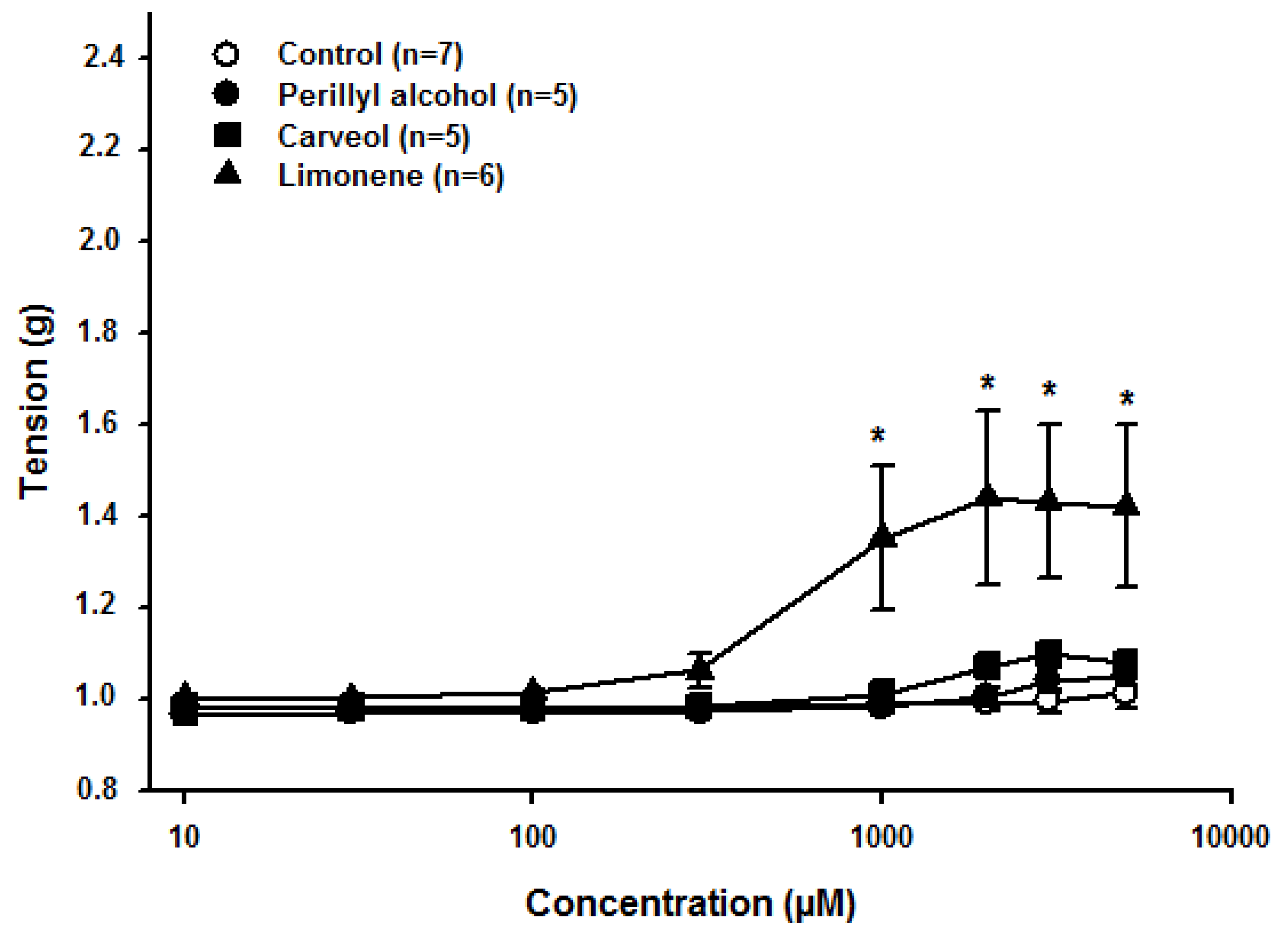
2.1. Effect of Limonene, Carveol, and Perillyl Alcohol on the Resting Tonus of Rat Aortic Rings
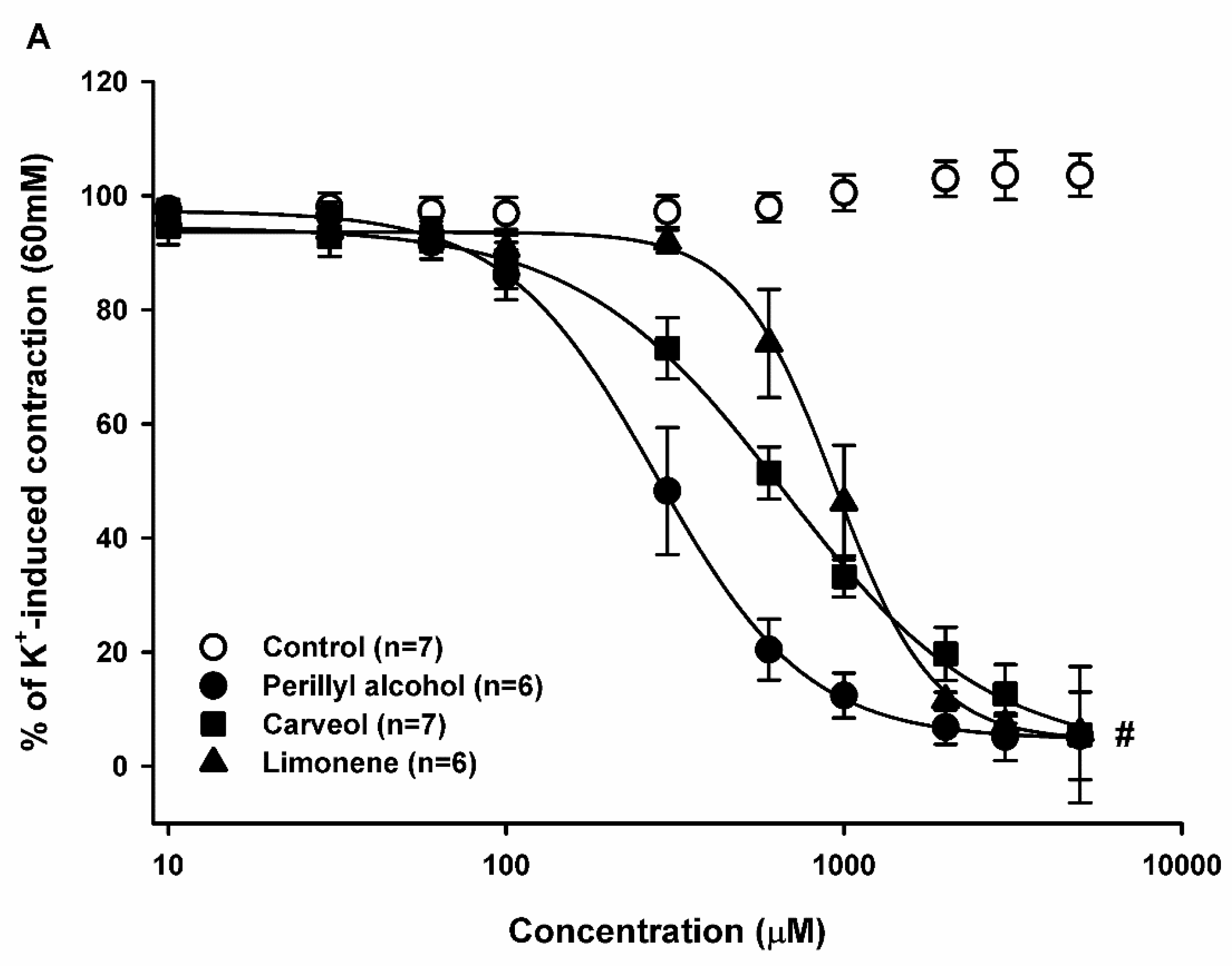
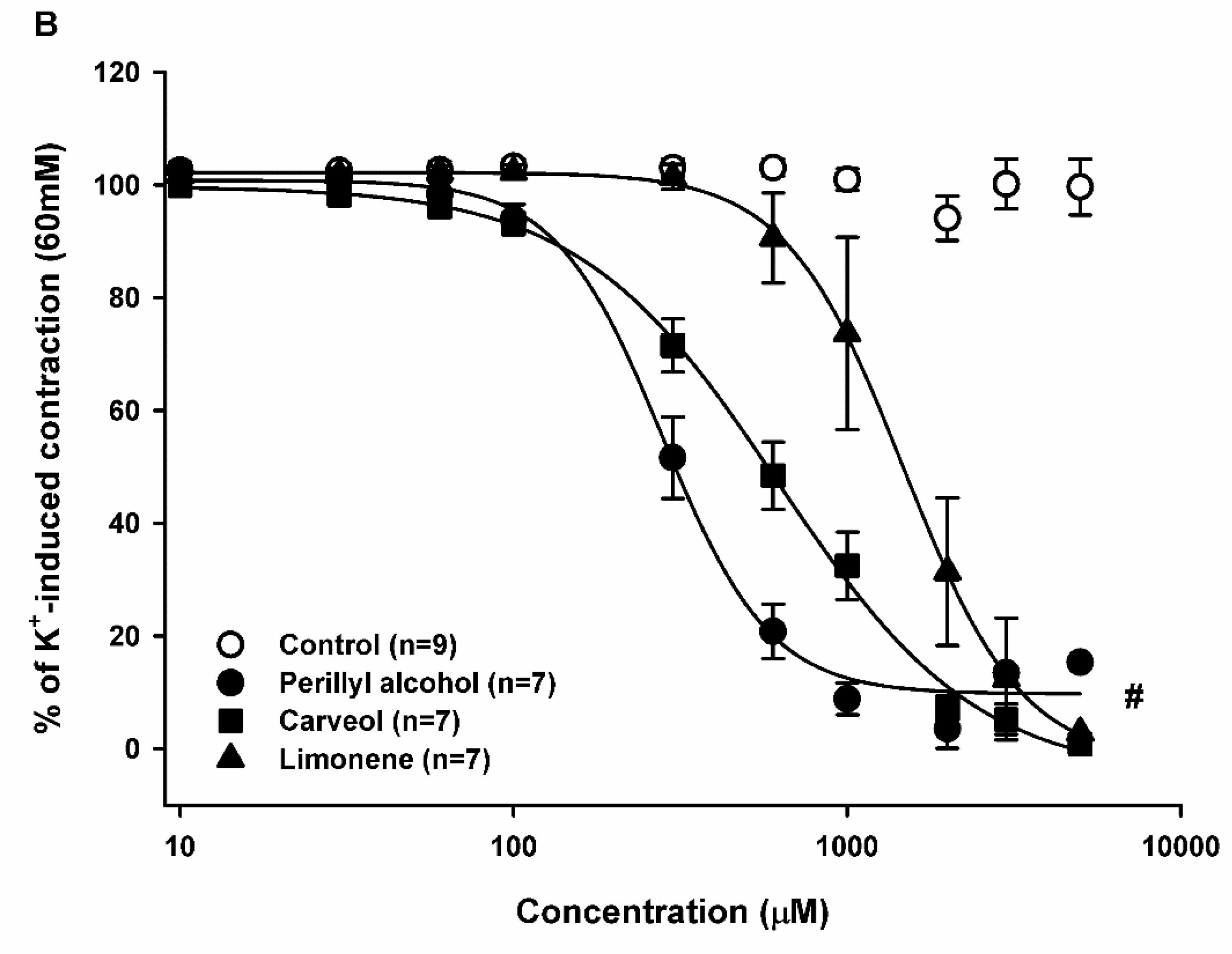
2.2. Relaxant Effect of Limonene, Carveol, and Perillyl Alcohol on the Sustained Contraction Induced by KCl in Rat Aortic Rings
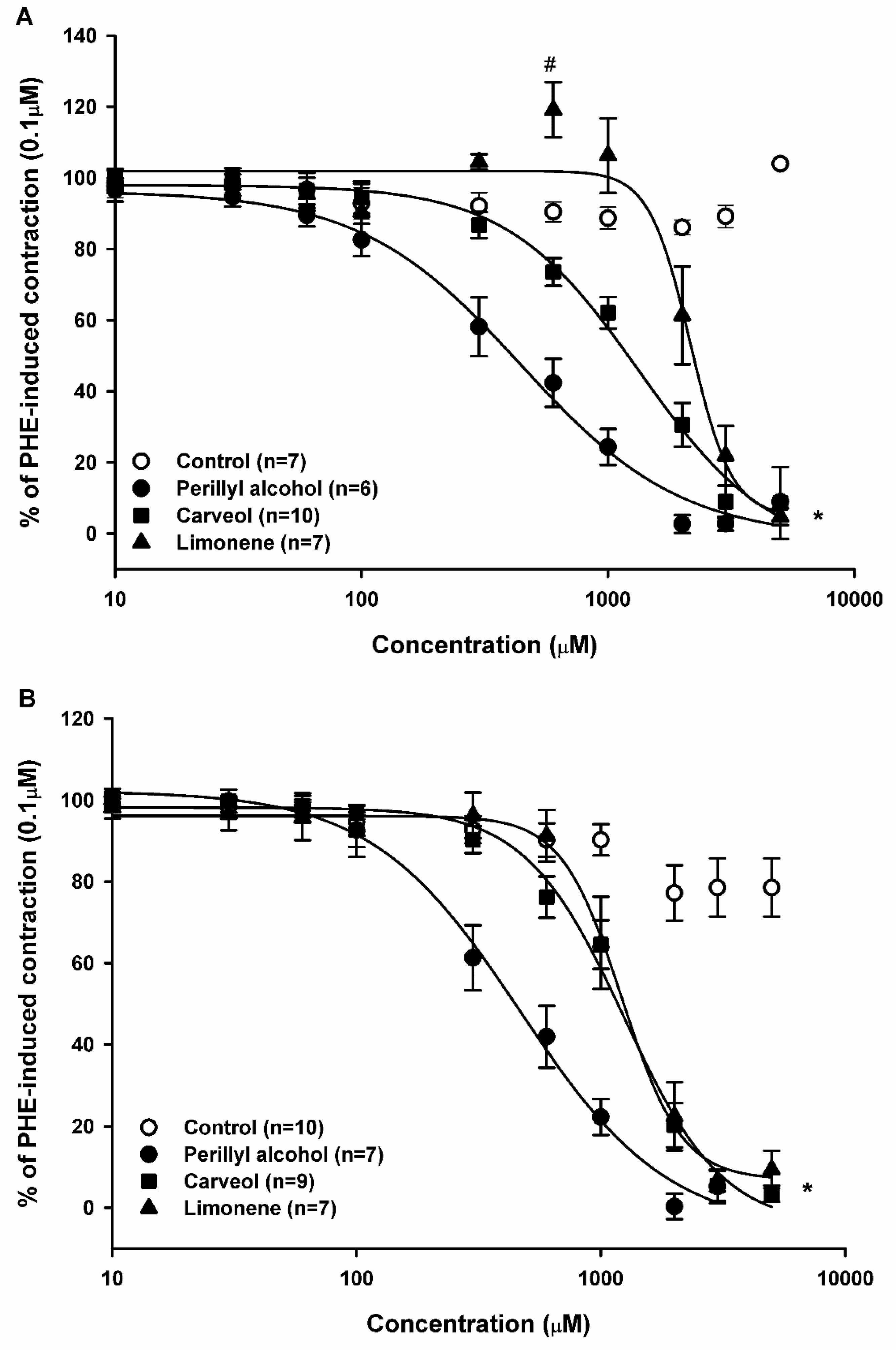
2.3. Relaxant Effect of Limonene, Carveol, and Perillyl Alcohol on the Sustained Contraction Induced by Phenylephrine (PHE) in Rat Aortic Rings
2.4. Relaxant Effect of Perillyl Alcohol, Carveol, and Limonene on Sustained Contractions Induced by BayK-8644
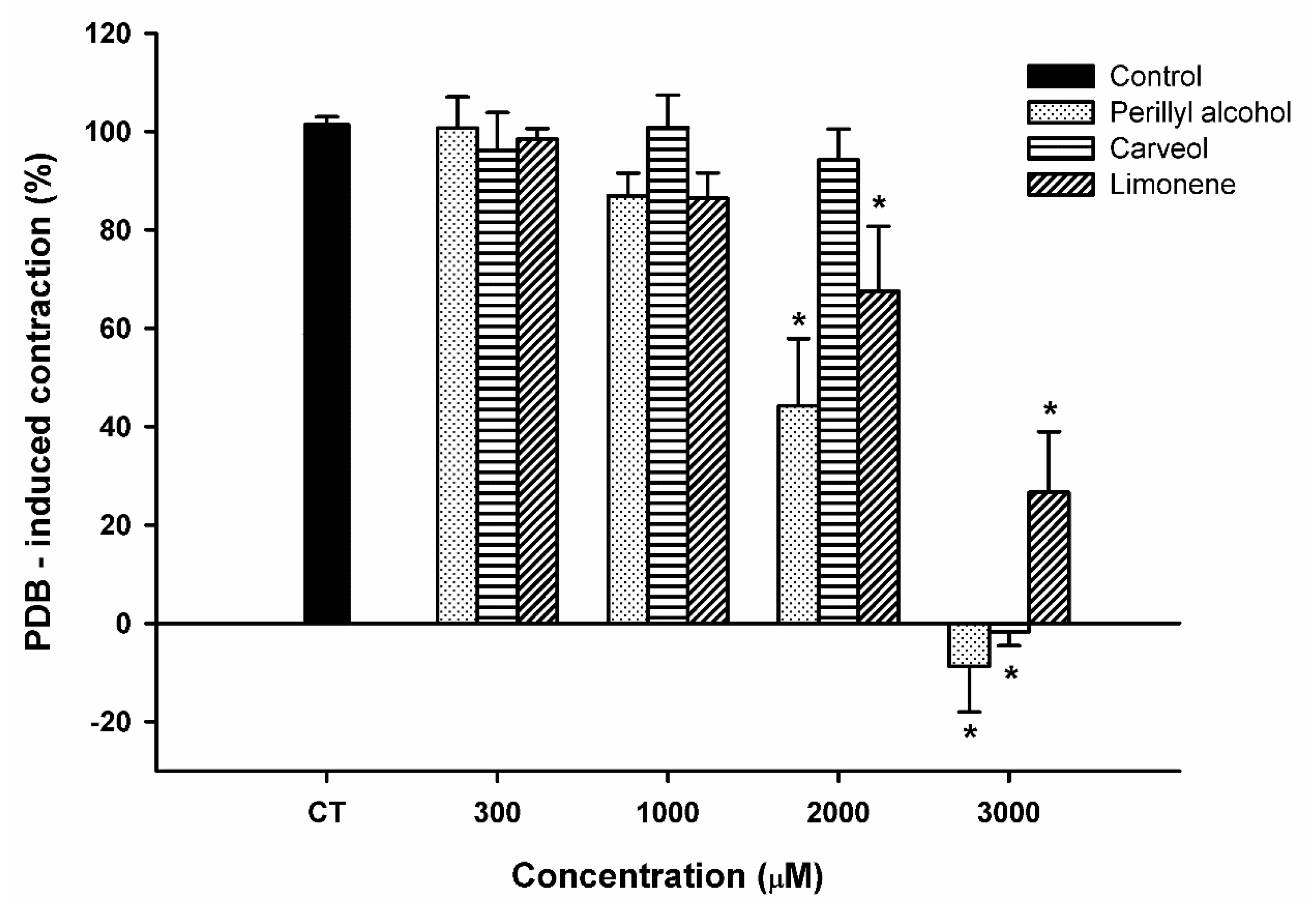
2.5. Effect of Limonene, Carveol, and Perillyl Alcohol on the Sustained Contraction Induced by Phorbol 12,13-Dibutyrate (PDB) in Rat Aortic Rings
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Solutions and Drugs
4.3. Isometric Tension Recording
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chappell, J. The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol. 1995, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.H.; Elmadfa, I. Biological relevance of terpenoids: Overview focusing on mono-, di- and tetraterpenes. Ann. Nutr. Metab. 2003, 47, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Da Rocha, M.L.; Oliveira, L.E.G.; Patrício Santos, C.C.M.; de Sousa, D.P.; de Almeida, R.N.; Araújo, D.A.M. Antinociceptive and anti-inflammatory effects of the monoterpene α,β-epoxy-carvone in mice. J. Nat. Med. 2013, 67, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Ponte, E.; Sousa, P.; Rocha, M.; Soares, P.; Coelho-de-Souza, A.; Leal-Cardoso, J. Comparative study of the anti-edematogenic effects of anethole and estragole. Pharmacol. Rep. 2012, 64, 984–990. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Lahlou, S.; Barreto, J.E.F.; Yum, M.E.M.; Oliveira, A.C.; Oliveira, H.D.; Celedônio, N.R.; Feitosa, R.G.; Duarte, G.P.; Santos, C.F.; et al. Essential oil of Croton zehntneri and its major constituent anethole display gastroprotective effect by increasing the surface mucous layer. Fundam. Clin. Pharmacol. 2013, 27, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Leal-Cardoso, J.H.; Fonteles, M.C. Pharmacological effects of essential oils of plants of the northeast of Brazil. An. Acad. Bras. Cienc. 1999, 71, 207–213. [Google Scholar] [PubMed]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.P.; Souza, D.P.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.M.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Maia-Joca, R.P.M.; Joca, H.C.; Ribeiro, F.J.P.; Do Nascimento, R.V.; Silva-Alves, K.S.; Cruz, J.S.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Investigation of terpinen-4-ol effects on vascular smooth muscle relaxation. Life Sci. 2014, 115, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Interaminense, L.F.L.; Leal-Cardoso, J.H.; Caldas Magalhães, P.J.; Pinto, D.G.; Lahlou, S. Enhanced hypotensive effects of the essential oil of Ocimum gratissimum leaves and its main constituent, eugenol, in DOCA-salt hypertensive conscious rats. Planta Med. 2005, 71, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Interaminense, L.F.L.; Jucá, D.M.; Magalhães, P.J.C.; Leal-Cardoso, J.H.; Duarte, G.P.; Lahlou, S. Pharmacological evidence of calcium-channel blockade by essential oil of Ocimum gratissimum and its main constituent, eugenol, in isolated aortic rings from DOCA-salt hypertensive rats. Fundam. Clin. Pharmacol. 2007, 21, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Interaminense, L.F.L.; Magalhães, P.J.C. Cardiovascular Effects of Eugenol, a Phenolic Compound Present in Many Plant Essential Oils, in Normotensive Rats. J. Cardiovasc. Pharmacol. 2004, 43, 250–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, N.V.; Assreuy, A.M.; Coelho-de-Souza, A.N.; Ceccatto, V.M.; Magalhães, P.J.C.; Lahlou, S.; Leal-Cardoso, J.H. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1, 8-cineole in rats. Phytomedicine 2009, 16, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Bastos, J.F.A.; Moreira, I.J.A.; Ribeiro, T.P.; Medeiros, I.A.; Antoniolli, A.R.; De Sousa, D.P.; Santos, M.R. Hypotensive and vasorelaxant effects of citronellol, a monoterpene alcohol, in rats. Basic Clin. Pharmacol. Toxicol. 2010, 106, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Figueiredo, A.F.; Magalhães, P.J.C.; Leal-Cardoso, J.H. Cardiovascular effects of 1,8-cineole, a terpenoid oxide present in many plant essential oils, in normotensive rats. Can. J. Physiol. Pharmacol. 2002, 80, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso-Lima, T.; Mota, M.M.; Barbosa-Filho, J.M.; Viana Dos Santos, M.R.; De Sousa, D.P. Structural relationships and vasorelaxant activity of monoterpenes. Daru 2012, 20, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sousa, D.P.; Júnior, G.A.S.; Andrade, L.N.; Calasans, F.R.; Nunes, X.P.; Barbosa-Filho, J.M.; Batista, J.S. Structure and spasmolytic activity relationships of monoterpene analogues found in many aromatic plants. Z. Naturforsch. C 2008, 63, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Mizuta, K.; Fujita, T.; Kumamoto, E. Inhibition by menthol and its related chemicals of compound action potentials in frog sciatic nerves. Life Sci. 2013, 92, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Alves, A.; de Araújo, A.; Cruz, J.; Araújo, D. Distinct effects of carvone analogues on the isolated nerve of rats. Eur. J. Pharmacol. 2010, 645, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, B.; Haeseler, G.; Leuwer, M.; Dengler, R.; Krampfl, K.; Bufler, J. Structural requirements of phenol derivatives for direct activation of chloride currents via GABA A receptors. Eur. J. Pharmacol. 2001, 421, 85–91. [Google Scholar] [CrossRef]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.V.; da Silva Rocha, K.A.; Oliveira, L.C.A.; Kozhevnikova, E.F.; Kozhevnikov, I.V.; Gusevskaya, E.V. Heteropoly acid catalysts for the synthesis of fragrance compounds from biorenewables: The alkoxylation of monoterpenes. RSC Adv. 2016, 6, 43217–43222. [Google Scholar] [CrossRef]
- Murali, R.; Saravanan, R. Antidiabetic effect of d-limonene, a monoterpene in streptozotocin-induced diabetic rats. Biomed. Prev. Nutr. 2012, 2, 269–275. [Google Scholar] [CrossRef]
- Rozza, A.; Moraes, T.; Kushima, H.; Tanimoto, A.; Marques, M.; Bauab, T.; Hiruma-Lima, C.A.; Pellizzon, C.H. Gastroprotective mechanisms of Citrus lemon (Rutaceae) essential oil and its majority compounds limonene and α-pinene: Involvement of heat-shock protein-70, vasoactive intestinal peptide, glutathione, sulfhydryl compounds, nitric oxide and prostaglandin E. Chem. Biol. Interact. 2011, 189, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Hirota, R.; Roger, N.; Nakamura, H.; Song, H.; Sawamura, M.; Suganuma, N. Anti-inflammatory Effects of Limonene from Yuzu (Citrus junos Tanaka) Essential Oil on Eosinophils. J. Food Sci. 2010, 75, H87–H92. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P. Monoterpenes in breast cancer chemoprevention. Breast Cancer Res. Treat. 1997, 46, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Omolo, M.O.; Okinyo, D.; Ndiege, I.O.; Lwande, W.; Hassanali, A. Repellency of essential oils of some Kenyan plants against Anopheles gambiae. Phytochemistry 2004, 65, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Da Fonseca, C.O.; Schwartsmann, G.; Fischer, J.; Nagel, J.; Futuro, D.; Quirico-Santos, T.; Gattass, C.R. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg. Neurol. 2008, 70, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Wadsworth, R.M.; Wainwright, C.L. Effect of antiproliferative agents on vascular function in normal and in vitro balloon-injured porcine coronary arteries. Eur. J. Pharmacol. 2003, 481, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Okabe, T.; Inamori, Y.; Tsujibo, H.; Miyake, Y.; Hiraoka, K.; Ishida, N. The biological properties of monoterpenes: Hypotensive effects on rats and antifungal activities on plant pathogenic fungi of monoterpenes. Mokuzai Gakkaishi 1996, 42, 677–680. [Google Scholar]
- Baek, I.; Jeon, S.B.; Kim, J.; Seok, Y.M.; Song, M.-J.; Chae, S.C.; Jun, J.E.; Park, W.H.; Kim, I.K. A role for Rho-kinase in Ca-independent contractions induced by phorbol-12,13-dibutyrate. Clin. Exp. Pharmacol. Physiol. 2009, 36, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Treiman, M.; Caspersen, C.; Brøgger, S. A tool coming of age: Thapsigargin as an inhibitor of sarco- endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol. Sci. 1998, 19, 131–135. [Google Scholar] [CrossRef]
- Somlyo, A.P.; Somlyo, A.V. Flash Photolysis Studies of Excitation-Contraction Coupling, Regulation, and Contraction in Smooth Muscle. Annu. Rev. Physiol. 1990, 52, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Quast, U. Do the K+ channel openers relax smooth muscle by opening K+ channels? Trends Pharmacol. Sci. 1993, 14, 332–337. [Google Scholar] [CrossRef]
- Marom, M.; Hagalili, Y.; Sebag, A.; Tzvier, L.; Atlas, D. Conformational changes induced in voltage-gated calcium channel Cav1.2 by BayK 8644 or FPL64176 modify the kinetics of secretion independently of Ca2+ influx. J. Biol. Chem. 2010, 285, 6996–7005. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Da-Silva, L.; Mendes-Maia, P.V.; Teófilo, T.M.N.G.; Barbosa, R.; Ceccatto, V.M.; Coelho-De-Souza, A.N.; Santos-Cruz, J.; Leal-Cardoso, J.H. Trans-caryophyllene, a natural sesquiterpene, causes tracheal smooth muscle relaxation through blockade of voltage-dependent Ca2+ channels. Molecules 2012, 17, 11965–11977. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Neves, D.; Leal-Cardoso, J.H.; Jaggar, J.H. Eugenol Dilates Rat Cerebral Arteries by Inhibiting Smooth Muscle Cell Voltage-dependent Calcium Channels. J. Cardiovasc. Pharmacol. 2014, 64, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolton, T.B.; Prestwich, S.; Zholos, V.; Gordienko, D.V. Excitation-contraction coupling in gastrointestinal and other smooth muscles. Annu. Rev. Physiol. 1999, 61, 85–115. [Google Scholar] [CrossRef] [PubMed]
- Karaki, H.; Ozaki, H.; Hori, M. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997, 49, 157–230. [Google Scholar] [PubMed]
- Whitney, G.; Throckmorton, D.; Isales, C.; Takuwa, Y.; Yeh, J.; Rasmussen, H.; Brophy, C. Kinase activation and smooth muscle contraction in the presence and absence of calcium. J. Vasc. Surg. 1995, 22, 37–44. [Google Scholar] [CrossRef]
- Magalhães, P.J.C.; Criddle, D.N.; Tavares, R.; Melo, E.M.; Mota, T.L.; Leal-Cardoso, J.H. Intestinal myorelaxant and antispasmodic effects of the essential oil of Croton nepetaefolius and its constituents cineole, methyl-eugenol and terpineol. Phyther. Res. 1998, 12, 172–177. [Google Scholar] [CrossRef]
- Török, J. Participation of nitric oxide in different models of experimental hypertension. Physiol. Res. 2008, 57, 813–825. [Google Scholar] [PubMed]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R. Role of endothelium in responses of vascular smooth muscle. Circ. Res. 1983, 53, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Mitsui, M.; Karaki, H. Effects of felodipine, nifedipine and verapamil on cytosolic Ca2+ and contraction in vascular smooth muscle. Eur. J. Pharmacol. 1993, 234, 1–7. [Google Scholar] [CrossRef]
- Rembold, C.; Murphy, R. [Ca2+]-dependent myosin phosphorylation in phorbol diester stimulated smooth muscle contraction. Am. J. Physiol. Cell Physiol. 1988, 255, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Stankevicius, E.; Kevelaitis, E.; Vainorius, E.; Simonsen, U. Role of nitric oxide and other endothelium-derived factors. Medicina 2003, 39, 333–341. [Google Scholar] [PubMed]
- Ruan, Y.C.; Zhou, W.; Chan, H.C. Regulation of smooth muscle contraction by the epithelium: Role of prostaglandins. Physiology 2011, 26, 156–170. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.F.; Robbins, R.J.; McMurtry, I.F. Endothelial cells in culture produce a vasoconstrictor substance. J. Cell. Physiol. 1987, 132, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Contractile Agent | Perillyl Alcohol (µM) | Carveol (µM) | Limonene (µM) | |
|---|---|---|---|---|
| K+ | E+ | 277.7 ± 5.46 (6) | 662.1 ± 32.85 (7) a | 941.6 ± 28.02 (6) b |
| K+ | E− | 279.7 ± 22.01 (7) | 619.8 ± 37.15 (7) a | 1474.5 ± 27.08 (7) b,c |
| PHE | E+ | 443.3 ± 66.83 (6) d | 1333.3 ± 225.20 (10) a,e | 2159.1 ± 203.62 (7) b,f |
| PHE | E− | 433.3 ± 44.31 (7) d | 1237.34 ± 117.90 (9) a,e | 1216.7 ± 57.50 (7) c,f |
| BayK | E− | 221.4 ± 4.09 (5) | 598.2 ± 42.05 (5) a | 439.0 ± 31.76 (5) b,g |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso-Teixeira, A.C.; Ferreira-da-Silva, F.W.; Peixoto-Neves, D.; Oliveira-Abreu, K.; Pereira-Gonçalves, Á.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Hydroxyl Group and Vasorelaxant Effects of Perillyl Alcohol, Carveol, Limonene on Aorta Smooth Muscle of Rats. Molecules 2018, 23, 1430. https://doi.org/10.3390/molecules23061430
Cardoso-Teixeira AC, Ferreira-da-Silva FW, Peixoto-Neves D, Oliveira-Abreu K, Pereira-Gonçalves Á, Coelho-de-Souza AN, Leal-Cardoso JH. Hydroxyl Group and Vasorelaxant Effects of Perillyl Alcohol, Carveol, Limonene on Aorta Smooth Muscle of Rats. Molecules. 2018; 23(6):1430. https://doi.org/10.3390/molecules23061430
Chicago/Turabian StyleCardoso-Teixeira, Ana Carolina, Francisco Walber Ferreira-da-Silva, Dieniffer Peixoto-Neves, Klausen Oliveira-Abreu, Átila Pereira-Gonçalves, Andrelina Noronha Coelho-de-Souza, and José Henrique Leal-Cardoso. 2018. "Hydroxyl Group and Vasorelaxant Effects of Perillyl Alcohol, Carveol, Limonene on Aorta Smooth Muscle of Rats" Molecules 23, no. 6: 1430. https://doi.org/10.3390/molecules23061430
APA StyleCardoso-Teixeira, A. C., Ferreira-da-Silva, F. W., Peixoto-Neves, D., Oliveira-Abreu, K., Pereira-Gonçalves, Á., Coelho-de-Souza, A. N., & Leal-Cardoso, J. H. (2018). Hydroxyl Group and Vasorelaxant Effects of Perillyl Alcohol, Carveol, Limonene on Aorta Smooth Muscle of Rats. Molecules, 23(6), 1430. https://doi.org/10.3390/molecules23061430





