Correlation between the Potency of Flavonoids on Mushroom Tyrosinase Inhibitory Activity and Melanin Synthesis in Melanocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibitory Effect of Flavonoids on the o-Diphenolase Activity of Mushroom Tyrosinase
2.2. Inhibitory Effect of Flavonoids on the o-Diphenolase Activity of Murine Tyrosinase
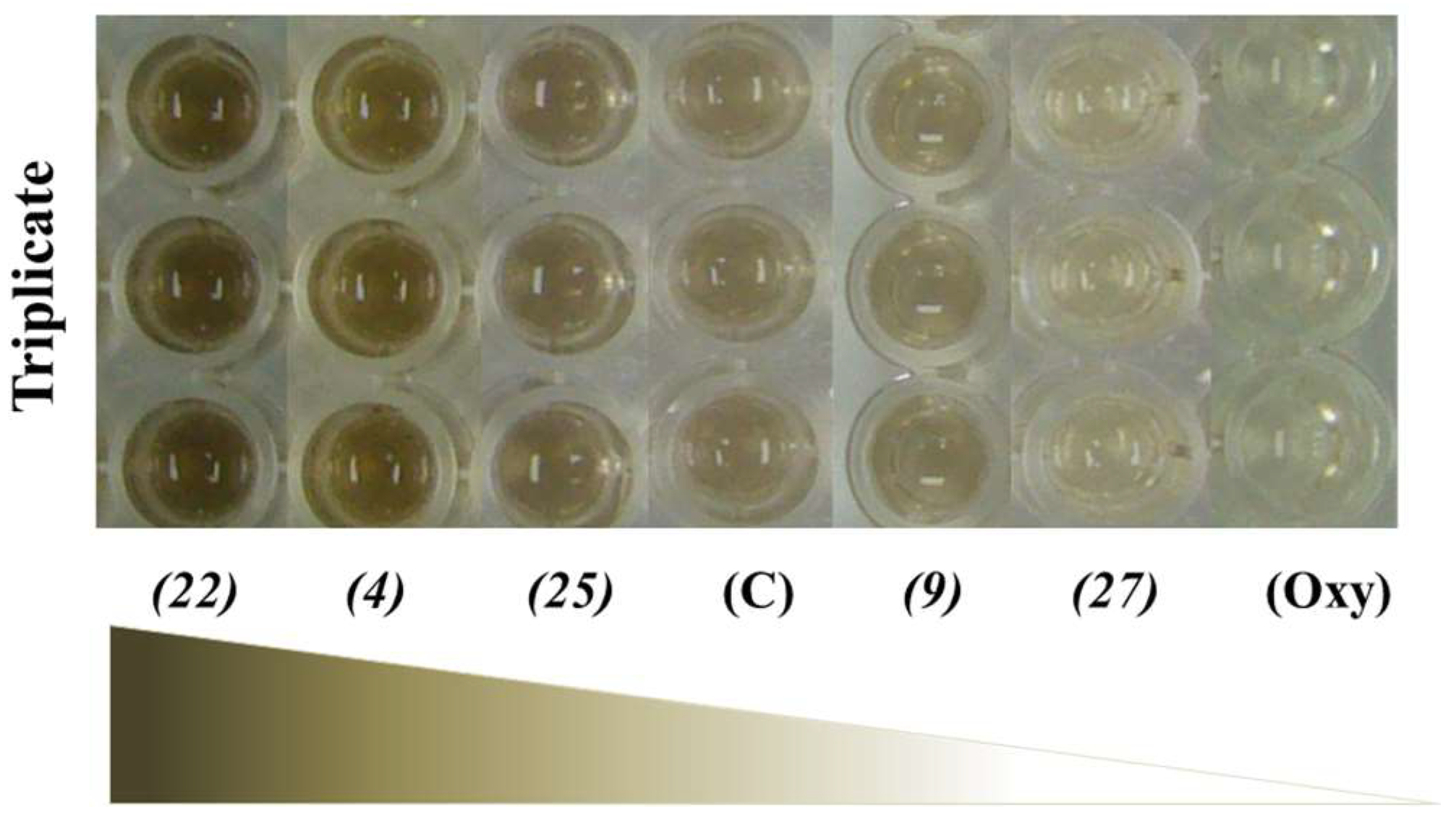
2.3. Effects of the Flavonoids on Cell Viability and Melanogenesis of B16-F10 Melanoma Cells
3. Materials and Methods
3.1. Chemicals
3.2. Mushroom Tyrosinase Inhibitory Assay
3.3. Cell Cultures
3.4. Murine Tyrosinase Inhibitory Assay
3.5. Cell Viability and Total Melanin Content Assay
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Martinez, M.V.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Kang, M.; Chung, H.S.; Bae, H. Naturally occurring tyrosinase inhibitors: Mechanism and applications in skin health, cosmetics and agriculture industries. Phytother. Res. 2007, 21, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.Y.; Sharma, V.K.; Sharma, N. Mushroom tyrosinase: Recent prospects. J. Agric. Food Chem. 2003, 51, 2837–2853. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Park, J.; Kim, J.; Han, C.; Yoon, J.; Kim, N.; Seo, J.; Lee, C. Flavonoids as mushroom tyrosinase inhibitors: A fluorescence quenching study. J. Agric. Food Chem. 2006, 54, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I. Flavonols from saffron flower: Tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Hirata, N.; Masuda, M.; Naruto, S.; Murata, K.; Wakabayashi, K.; Matsuda, H. Inhibitory effects of Citrus hassaku extract and its flavanone glycosides on melanogenesis. Biol. Pharm. Bull. 2009, 32, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, K.T.; Lee, H.S.; Kim, M.; Lim, Y.H. Evaluation of the inhibition of mushroom tyrosinase and cellular tyrosinase activities of oxyresveratrol: Comparison with mulberroside A. J. Enzym. Inhib. Med. Chem. 2012, 27, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Nemoto, K.; Matsushita, A.; Terada, E.; Monthakantirat, O.; De-Eknamkul, W.; Miyase, T.; Warashina, T.; Degawa, M.; Noguchi, H. Flavonoids from the heartwood of the Thai medicinal plant Dalbergia parviflora and their effects on estrogenic-responsive human breast cancer cells. J. Nat. Prod. 2009, 72, 2163–2168. [Google Scholar] [CrossRef] [PubMed]
- Umehara, K.; Nemoto, K.; Kimijima, K.; Matsushita, A.; Terada, E.; Monthakantirat, O.; De-Eknamkul, W.; Miyase, T.; Warashina, T.; Degawa, M.; et al. Estrogenic constituents of the heartwood of Dalbergia parviflora. Phytochemistry 2008, 69, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Promden, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Structure and antioxidant activity relationships of isoflavonoids from Dalbergia parviflora. Molecules 2014, 19, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, M.R.; Lee, E.S.; Lee, C.H. Inhibitory effects of calycosin isolated from the root of Astragalus membranaceus on melanin biosynthesis. Biol. Pharm. Bull. 2009, 32, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.P.; Chen, Q.X.; Huang, H.; Wang, H.Z.; Zhang, R.Q. Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry 2003, 68, 487–491. [Google Scholar] [PubMed]
- Chang, T.S.; Ding, H.Y.; Lin, H.C. Identifying 6,7,4′-trihydroxyisoflavone as a potent tyrosinase inhibitor. Biosci. Biotechnol. Biochem. 2005, 69, 1999–2001. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.S. Two potent suicide substrates of mushroom tyrosinase: 7,8,4′-trihydroxyisoflavone and 5,7,8,4′-tetrahydroxyisoflavone. J. Agric. Food Chem. 2007, 55, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Kubo, I. Resveratrol as a kcat type inhibitor for tyrosinase: Potentiated melanogenesis inhibitor. Bioorganic Med. Chem. 2012, 20, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P.; Berthon, J.Y. Resveratrol: An original mechanism on tyrosinase inhibition. Int. J. Cosmet. Sci. 2000, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Gasowska-Bajger, B.; Wojtasek, H. Reactions of flavonoids with o-quinones interfere with the spectrophotometric assay of tyrosinase activity. J. Agric. Food Chem. 2016, 64, 5417–5427. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Thody, A.J. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 1996, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Nitoda, T.; Nihei, K. Effects of quercetin on mushroom tyrosinase and B16-F10 melanoma cells. Molecules 2007, 12, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Takekoshi, S.; Takeyama, R.; Homma, T.; Osamura, R.Y. Quercetin enhances melanogenesis by increasing the activity and synthesis of tyrosinase in human melanoma cells and in normal human melanocytes. Pigment Cell Res. 2004, 17, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Ohguchi, K.; Akao, Y.; Nozawa, Y. Stimulation of melanogenesis by the citrus flavonoid naringenin in mouse B16 melanoma cells. Biosci. Biotechnol. Biochem. 2006, 70, 1499–1501. [Google Scholar] [CrossRef] [PubMed]
- Bouzaiene, N.N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar] [PubMed]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, E.; Decker, H. Tyrosinases from crustaceans form hexamers. Biochem. J. 2003, 371, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lerch, K. Neurospora tyrosinase: Structural, spectroscopic and catalytic properties. Mol. Cell. Biochem. 1983, 52, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, K. Particulate tyrosinase of human malignant melanoma. Solubilization, purification following trypsin treatment, and characterization. Eur. J. Biochem. 1978, 85, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Strothkamp, K.G.; Jolley, R.L.; Mason, H.S. Quaternary structure of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1976, 70, 519–524. [Google Scholar] [CrossRef]
- Song, T.Y.; Chen, C.H.; Yang, N.C.; Fu, C.S.; Chang, Y.T.; Chen, C.L. The correlation of in vitro mushroom tyrosinase activity with cellular tyrosinase activity and melanin formation in melanoma cells A2058. J. Food Drug Anal. 2009, 17, 156–162. [Google Scholar]
- Likhitwitayawuid, K.; Sritularak, B. A new domeric stilbene with tyrosinase inhibitory activity from Artocarpus gomezianus. J. Nat. Prod. 2001, 64, 1457–1459. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: All samples are available from the authors. |
 | No. | Isoflavones | R5 | R6 | R7 | R2′ | R3′ | R4′ | R5′ |
| 1 | Formononetin | H | H | OH | H | H | OMe | H | |
| 2 | Calycosin | H | H | OH | H | OH | OMe | H | |
| 3 | Biochanin A | OH | H | OH | H | H | OMe | H | |
| 4 | Genistein | OH | H | OH | H | H | OH | H | |
| 5 | Khrinone B | OH | H | OH | OH | H | OMe | OH | |
| 6 | 3′-O-Methylorobol | OH | H | OH | H | OMe | OH | H | |
| 7 | Khrinone C | OH | H | OH | OMe | OH | OMe | H | |
| 8 | Tectorigenin | OH | OMe | OH | H | H | OH | H | |
| 9 | Cajanin | OH | H | OMe | OH | H | OH | H | |
 | No. | Isoflavanones | R5 | R6 | R7 | R2′ | R3′ | R4′ | R5′ |
| 10 | (3R)-7,3′-Dihydroxy-4′-methoxy-isoflavanone | H | H | OH | H | OH | OMe | H | |
| 11 | Onogenin | H | H | OH | OMe | H | OCH2O | ||
| 12 | Dalparvin | H | H | OH | OMe | H | OMe | OH | |
| 13 | Dalparvin B | H | H | OH | OH | OMe | OMe | H | |
| 14 | (3S)-Sativanone | H | H | OH | OMe | H | OMe | H | |
| 15 | (3R,S)-3′-O-Methylviolanone | H | H | OH | OMe | OMe | OMe | H | |
| 16 | (3R,S)-Kenusanone G | OH | H | OH | H | OH | OMe | H | |
| 17 | (3S)-Secundiflorol H | OH | H | OH | OMe | OH | OMe | H | |
| 18 | Dalparvin A | OH | H | OH | OMe | H | OMe | OH | |
 | No. | Isoflavans | R6 | R7 | R8 | R2′ | R3′ | R4′ | R5′ |
| 19 | (3R)-Vestitol | H | OH | H | OH | H | OMe | H | |
| 20 | (3R)(+)-Mucronulatol | H | OH | H | OMe | OH | OMe | H | |
| 21 | (3R,S)-3′-Hydroxy-8-methoxy vestitol | H | OH | OMe | OH | OH | OMe | H | |
| 22 | Duartin | H | OH | OMe | OMe | OH | OMe | H | |
| 23 | (3S)-8-Demethylduartin | H | OH | OH | OMe | OH | OMe | H | |
 | No. | Flavanones | R3 | R5 | R6 | R7 | R4′ | R5′ | R6′ |
| 24 | (2S)-Liquiritigenin | H | H | H | OH | OH | H | H | |
| 25 | (2S)-Naringenin | H | OH | H | OH | OH | H | H | |
| 26 | Alpinetin | H | OMe | H | OH | H | H | H | |
 | No. | Pterocarpan | R5 | R6 | R7 | R2′ | R3′ | R4′ | R5′ |
| 27 | (6aR,11aR)-3,8-Dihydroxy-9-methoxy pterocarpan | H | H | OH | H | OH | OMe | H | |
| No. | Compound | Mushroom Tyrosinase Inhibition | |
|---|---|---|---|
| % Inhibition (at 200 μM) | IC50 (μM) | ||
| (control) | Oxyresveratrol | 98.4 ± 1.1 | 0.19 ± 0.1 |
| (control) | Kojic acid | 93.4 ± 1.7 | 16.8 ± 4.6 |
| 27 | (6aR,11aR)-3,8-Dihydroxy-9-methoxy pterocarpan | 84.6 ± 0.6 | 16.7 ± 5.0 |
| 5 | Khrinone B | 72.7 ± 2.2 | 54.0 ± 6.0 |
| 21 | (3RS)-3′-Hydroxy-8-methoxy vestitol | 64.1 ± 1.3 | 67.8 ± 5.8 |
| 9 | Cajanin | 65.0 ± 1.6 | 67.9 ± 6.2 |
| 10 | (3R)-7,3′-Dihydroxy-4′-methoxy-isoflavanone | 52.1 ± 0.4 | 176.7 ± 16.3 |
| 24 | (2S)-Liquiritigenin | 52.1 ± 1.4 | 178.1 ± 14.0 |
| 20 | (3R)(+)-Mucronulatol | 48.3 ± 1.6 | 228.9 ± 22.2 |
| 17 | (3S)-Secundiflorol H | 44.0 ± 3.7 | 278.1 ± 54.5 |
| 26 | Alpinetin | 36.9 ± 0.5 | 450.0 ± 48.5 |
| 19 | (3R)-Vestitol | 35.6 ± 2.2 | 473.0 ± 60.9 |
| 12 | Dalparvin | 31.6 ± 0.5 | 906.1 ± 43.6 |
| 6 | 3′-O-Methylorobol | 14.3 ± 1.1 | N.D. |
| 3 | Biochanin A | 9.2 ± 0.4 | N.D. |
| 1 | Formononetin | 10% (at 300 μM) | N.D. |
| 7 | Khrinone C | 2% (at 300 μM) | N.D. |
| 2 | Calycosin | 0% (at 300 μM) | N.D. |
| 11 | Onogenin | 0% (at 300 μM) | N.D. |
| 15 | (3R,S)-3′-O-Methylviolanone | 0% (at 300 μM) | N.D. |
| 13 | Dalparvin B | 4% (at 500 μM) | N.D. |
| 14 | (3S)-Sativanone | 13% (at 500 μM) | N.D. |
| 16 | (3R,S)-Kenusanone G | 0% (at 500 μM) | N.D. |
| 18 | Dalparvin A | 0% (at 500 μM) | N.D. |
| 22 | Duartin | 0% (at 500 μM) | N.D. |
| 4 | Genistein | S* | N.D. |
| 8 | Tectorigenin | S* | N.D. |
| 23 | (3S)-8-Demethylduartin | S* | N.D. |
| 25 | (2S)-Naringenin | S* | N.D. |
| No. | Isoflavones | Murine Tyrosinase Inhibition, % (at 200 μM) | 15 μM | 30 μM | ||
|---|---|---|---|---|---|---|
| % Melanin Content | % Cell Viability | % Melanin Content | % Cell Viability | |||
| 1 | Formononetin | - | 133.8 ± 2.7 | 96.0 ± 3.3 | 155.3 ± 15.4 | 92.8 ± 9.4 |
| 2 | Calycosin | - | 142.8 ± 8.2 | 99.1 ± 9.2 | 196.6 ± 6.4 | 101.3 ± 1.9 |
| 3 | Biochanin A | - | 165.2 ± 5.9 | 103.4 ± 4.5 | 168.0 ± 5.1 | 102.2 ± 4.8 |
| 4 | Genistein | 9.9 ± 3.1 | 165.1 ± 1.6 | 128.2 ± 3.5 | 191.7 ± 13.9 | 93.8 ± 5.7 |
| 5 | Khrinone B | 10.1 ± 3.0 | 84.1 ± 5.3 | 95.1 ± 5.4 | 34.7 ± 12.3 | 62.3 ± 15.1 |
| 6 | 3′-O-Methylorobol | - | 170.1 ± 10.5 | 106.6 ± 6.2 | 215.7 ± 15.7 | 101.5 ± 2.7 |
| 7 | Khrinone C | - | 107.6 ± 0.8 | 93.3 ± 2.0 | 154.0 ± 9.9 | 93.4 ± 2.0 |
| 8 | Tectorigenin | 10.2 ± 2.9 | 127.6 ± 2.8 | 129.7 ± 3.4 | 152.1 ± 4.7 | 119.3 ± 1.3 |
| 9 | Cajanin | 9.2 ± 1.5 | 65.8 ± 7.5 | 101.5 ± 6.6 | 61.6 ± 8.9 | 107.2 ± 3.3 |
| No. | Isoflavanones | |||||
| 10 | (3R)-7,3′-Dihydroxy-4′-methoxy-isoflavanone | - | 95.1 ± 3.0 | 94.9 ± 3.9 | 95.2 ± 2.7 | 96.5 ± 3.9 |
| 11 | Onogenin | - | 133.5 ± 4.6 | 100.0 ± 5.0 | 169.8 ± 15.4 | 92.0 ± 2.0 |
| 12 | Dalparvin | - | 133.8 ± 9.7 | 117.1 ± 8.9 | 199.6 ± 12.1 | 99.7 ± 6.7 |
| 13 | Dalparvin B | 9.8 ± 2.8 | 102.0 ± 2.7 | 82.3 ± 5.4 | 99.1 ± 2.3 | 74.1 ± 6.4 |
| 14 | (3S)-Sativanone | 9.5 ± 1.6 | 124.2 ± 4.3 | 106.7 ± 10.6 | 127.6 ± 6.0 | 64.3 ± 23.7 |
| 15 | (3R,S)-3′-O-Methylviolanone | - | 143.3 ± 8.4 | 74.2 ± 7.8 | 157.2 ± 11.5 | 72.9 ± 6.2 |
| 16 | (3R,S)-Kenusanone G | - | 132.6 ± 5.1 | 124.0 ± 1.7 | 158.5 ± 13.5 | 102.2 ± 2.8 |
| 17 | (3S)-Secundiflorol H | - | 117.6 ± 17.7 | 109.4 ± 6.6 | 146.4 ± 6.1 | 102.7 ± 4.1 |
| 18 | Dalparvin A | - | 132.0 ± 4.4 | 105.4 ± 4.8 | 184.2 ± 10.6 | 100.4 ± 7.1 |
| No. | Isoflavans | |||||
| 19 | (3R)-Vestitol | 12.4 ± 3.3 | 186.1 ± 1.6 | 142.1 ± 3.0 | 232.0 ± 9.3 | 119.7 ± 4.1 |
| 20 | (3R)(+)-Mucronulatol | 12.8 ± 2.1 | 178.6 ± 15.9 | 103.6 ± 6.9 | 263.7± 8.2 | 102.5 ± 2.7 |
| 21 | (3RS)-3′-Hydroxy-8-methoxy Vestitol | 11.4 ± 2.5 | 97.0 ± 6.3 | 102.9 ± 1.0 | 36.3 ± 0.1 | 62.4 ± 3.2 |
| 22 | Duartin | - | 301.1 ± 2.3 | 139.9 ± 7.5 | 326.8 ± 2.1 | 94.4 ± 2.8 |
| 23 | (3S)-8-Demethylduartin | - | 87.8 ± 2.0 | 93.5 ± 3.0 | 72.7 ± 4.3 | 98.3 ± 5.4 |
| No. | Flavanones | |||||
| 24 | (2S)-Liquiritigenin | 13.2 ± 2.7 | 102.9 ± 5.5 | 98.9 ± 10.7 | 124.5 ± 2.0 | 100.0 ± 3.5 |
| 25 | (2S)-Naringenin | - | 132.5 ± 14.5 | 94.3 ± 2.0 | 152.1 ± 3.8 | 98.1 ± 0.5 |
| 26 | Alpinetin | - | 89.7 ± 0.4 | 77.0 ± 12.7 | 92.3 ± 1.6 | 77.1 ± 10.2 |
| No. | Pterocarpan | |||||
| 27 | (6aR,11aR)-3,8-Dihydroxy-9-methoxy pterocarpan | 29.2 ± 2.9 | 41.9 ± 0.4 | 105.7 ± 4.9 | 53.5 ± 0.4 | 14.5 ± 0.9 |
| Others | ||||||
| Control | Kojic acid | 73.8 ± 1.0 | 68.9 ± 5.2 | 98.9 ± 4.7 | 74.2 ± 5.8 | 99.6 ± 2.3 |
| Control | Oxyresveratrol | 99.4 ± 1.0 | 24.3 ± 1.1 | 117.2 ± 2.2 | 28.4 ± 1.7 | 118.1 ± 5.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the Potency of Flavonoids on Mushroom Tyrosinase Inhibitory Activity and Melanin Synthesis in Melanocytes. Molecules 2018, 23, 1403. https://doi.org/10.3390/molecules23061403
Promden W, Viriyabancha W, Monthakantirat O, Umehara K, Noguchi H, De-Eknamkul W. Correlation between the Potency of Flavonoids on Mushroom Tyrosinase Inhibitory Activity and Melanin Synthesis in Melanocytes. Molecules. 2018; 23(6):1403. https://doi.org/10.3390/molecules23061403
Chicago/Turabian StylePromden, Worrawat, Wittawat Viriyabancha, Orawan Monthakantirat, Kaoru Umehara, Hiroshi Noguchi, and Wanchai De-Eknamkul. 2018. "Correlation between the Potency of Flavonoids on Mushroom Tyrosinase Inhibitory Activity and Melanin Synthesis in Melanocytes" Molecules 23, no. 6: 1403. https://doi.org/10.3390/molecules23061403





