Four New Sesquiterpenoids from the Roots of Diarthron Tianschanica with Their Antineoplastic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of Compounds 1–4
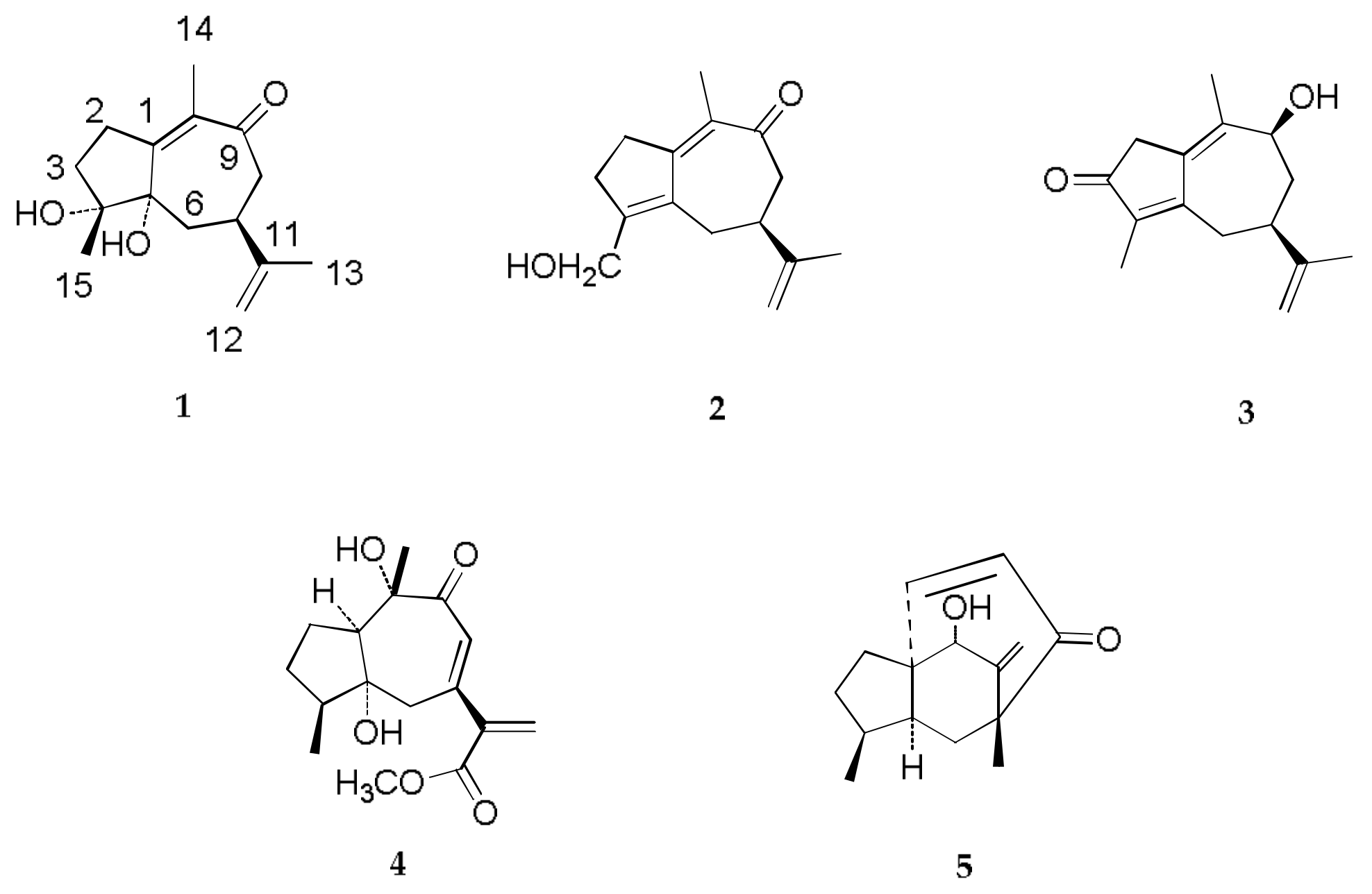
2.2. Structure Elucidation of Compounds 1–4
2.3. Antineoplastic Activity of Compounds 1–5
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation and Purification of Compounds 1–5
3.4. Characterization of Compounds 1–4
3.5. Cytotoxicity Assay of Compounds 1–5
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Editorial Committee of Flora of China. Flora of China; Beijing Science Press: Beijing, China, 1999; p. 400. [Google Scholar]
- Fan, J.W.; Yu, L.; Ma, L.Z.; Guo, N.; Gao, Q.S.; Zhao, Q.M.; Zhen, F.L.; Ge, F.; Wang, Q.K.; Deng, X.M.; et al. Antimycobacterial activity of 29 plants extracts. Chin. Agric. Sci. Bull. 2009, 25, 1–7. [Google Scholar]
- Ai, L.Y. Clinical observation on 297 cases of chronic bronchitis treated by stellera chamaejasme. J. Tradit. Chin. Med. 1994, 21, 138. [Google Scholar]
- Zha, K.J.; Xu, G.J.; Jin, R.L.; Xu, L.S.; Zhang, P.Z. Comparative observation on the inhibitory effect against tuberculous bacillus of Chinese drug Langdu. J. China Pharm. Univ. 1995, 26, 122–124. [Google Scholar]
- Shi, L.L.; Ma, G.X.; Gao, H.C.; Chen, Q.C.; Yang, J.S.; Jia, X.G.; Zhang, J. Diarylpentanol constituents from the aerial part of Stelleropsis tianschanica. J. Asian Nat. Prod. Res. 2016, 9, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Ma, G.X.; Yang, J.S.; Gulinar, S.; Jia, X.G. Chemical constituents from plant of Stelleropsis tianschanica. Chin. Tradit. Herbal Drugs 2016, 47, 223–226. [Google Scholar]
- Huang, S.Z.; Li, X.N.; Ma, Q.Y.; Dai, H.F.; Li, L.C.; Cai, X.H.; Liu, Y.Q.; Zhou, J.; Zhao, Y.X. Daphnauranols A-C, new antifeedant sesquiterpenoids with a 5/6/7 ring system from Daphne aurantiaca. Tetrahedron Lett. 2014, 55, 3693–3696. [Google Scholar] [CrossRef]
- Ishihara, M.; Tsuneya, T.; Uneyama, K. Guaiane sesquiterpenes from agarwood. Photochemistry 1991, 30, 3343–3347. [Google Scholar] [CrossRef]
- Xu, F.M.; Morikawa, T.; Matsuda, H.; Ninomiya, K.; Yoshikawa, M. Structures of new sesquiterpenes and hepatoprotective constituents from the Egyptian herbal medicine Cyperus longus. J. Nat. Prod. 2004, 67, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Ingert, N.; Bombarda, I.; Herbette, G.; Faure, R.; Moretti, C.; Raharivelomanana, P. Oleodaphnoic acid and coriaceol, two new natural products from the stem bark of wikstroemia coriacea. Molecules 2013, 18, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–4 and 5 are available from the authors. |

| Position | 1 | Position | 2 | ||
|---|---|---|---|---|---|
| δc Type | δH (J in Hz) | δc Type | δH (J in Hz) | ||
| 1 | 172.1 | 1 | 162.6 | ||
| 2 | 27.6 | 1.50 (1H, dd, 10.8, 9.6) 1.52 (1H, dd, 10.8, 2.4) | 2 | 39.9 | 2.26 (2H, m) |
| 3 | 39.5 | 1.99 (1H, m) 2.48 (1H, m) | 3 | 34.0 | 2.48 (2H, m) |
| 4 | 73.9 | 4 | 145.5 | ||
| 5 | 83.3 | 5 | 144.9 | ||
| 6 | 34.7 | 2.53 (1H, m) 2.65 (1H, m) | 6 | 39.6 | 2.48 (2H, m) |
| 7 | 42.2 | 2.67 (1H, m) | 7 | 41.5 | 3.28 (1H, m) |
| 8 | 47.9 | 2.16 (1H, dd, 12.0, 1.8) 2.60 (1H, dd, 12.0, 10.8) | 8 | 49.6 | 2.81 (2H, m) |
| 9 | 205.3 | 9 | 199.0 | ||
| 10 | 136.8 | 10 | 134.1 | ||
| 11 | 151.0 | 11 | 147.3 | ||
| 12 | 108.7 | 4.65 (1H, d, 1.8) 4.71 (1H, d, 1.8) | 12 | 110.8 | 4.77 (1H, d, 1.8) 4.79 (1H, d, 1.8) |
| 13 | 20.1 | 1.72 (3H, s) | 13 | 20.1 | 1.75 (3H, s) |
| 14 | 7.9 | 1.54 (3H, s) | 14 | 2.3 | 1.80 (3H, s) |
| 15 OH | 27.5 | 1.19 (3H, s) 4.31 (br s) 5.27 (br s) | 15 OH | 58.8 | 4.15 (1H, dd, 11.5, 5.4) |
| Position | 3 | Position | 4 | ||
|---|---|---|---|---|---|
| δc Type | δH (J in Hz) | δc Type | δH (J in Hz) | ||
| 1 | 138.2 | 1 | 47.6 | 2.33 (1H, m) | |
| 2 | 40.4 | 2.90 (1H, m) 3.20 (1H, m) | 2 | 29.1 | 1.09 (1H, m) 1.23 (1H, m) |
| 3 | 202.8 | 3 | 30.9 | 1.43 (1H, m) 1.85 (1H, m) | |
| 4 | 137.3 | 4 | 34.7 | 2.56 (1H, m) | |
| 5 | 165.5 | 5 | 78.4 | ||
| 6 | 35.3 | 2.63 (1H, m) 2.80 (1H, m) | 6 | 35.5 | 1.76 (2H, m) |
| 7 | 37.2 | 2.65 (1H, m) | 7 | 154.3 | |
| 8 | 41.0 | 1.93 (1H, m) 1.89 (1H, m) | 8 | 124.5 | 5.63 (1H, d, 1.2) |
| 9 | 70.0 | 4.33 (1H, m) | 9 | 202.1 | |
| 10 | 130.4 | 10 | 84.3 | ||
| 11 | 149.8 | 11 | 146.3 | ||
| 12 | 110.1 | 4.71 (1H, s) 4.76 (1H, s) | 12 | 125.6 | 6.09 (1H, d, 1.2) 6.12 (1H, d, 0.6) |
| 13 | 20.2 | 1.75 (1H, s) | 13 | 165.8 | |
| 14 | 17.0 | 1.87 (1H, s) | 14 | 15.9 | 1.92 (3H, s) |
| 15 | 8.2 | 1.66 (1H, s) | 15 MeO | 22.2 51.7 | 0.90 (3H, d, 7.2) 3.62 (3H, s) |
| Compound | HepG-2 | MCF-7 | HeLa |
|---|---|---|---|
| IC50 (μM) * | |||
| Paclitaxel | 1.80 ± 0.26 | 3.80 ± 0.31 | 4.25 ± 0.52 |
| 1 | 18.9 ± 0.02 | 48.7 ± 0.39 | >50 |
| 2 | 41.3 ± 0.13 | >50 | 39.6 ± 0.53 |
| 3 | 22.5 ± 0.09 | >50 | >50 |
| 4 | >50 | >50 | >50 |
| 5 | 20.3 ± 0.24 | >50 | 29.6 ± 0.61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.-X.; Zhao, D.; Wei, H.-Y.; Ma, X.-L.; Shi, L.-L.; Zhang, J. Four New Sesquiterpenoids from the Roots of Diarthron Tianschanica with Their Antineoplastic Activity. Molecules 2018, 23, 1383. https://doi.org/10.3390/molecules23061383
Sun D-X, Zhao D, Wei H-Y, Ma X-L, Shi L-L, Zhang J. Four New Sesquiterpenoids from the Roots of Diarthron Tianschanica with Their Antineoplastic Activity. Molecules. 2018; 23(6):1383. https://doi.org/10.3390/molecules23061383
Chicago/Turabian StyleSun, Dong-Xue, Dan Zhao, Hong-Yan Wei, Xiao-Ling Ma, Lei-Ling Shi, and Jing Zhang. 2018. "Four New Sesquiterpenoids from the Roots of Diarthron Tianschanica with Their Antineoplastic Activity" Molecules 23, no. 6: 1383. https://doi.org/10.3390/molecules23061383




