Functional Characterisation of New Sesquiterpene Synthase from the Malaysian Herbal Plant, Polygonum Minus
Abstract
:1. Introduction
2. Results
2.1. Screening and Isolation of Sesquiterpene Synthase Gene from P. minus
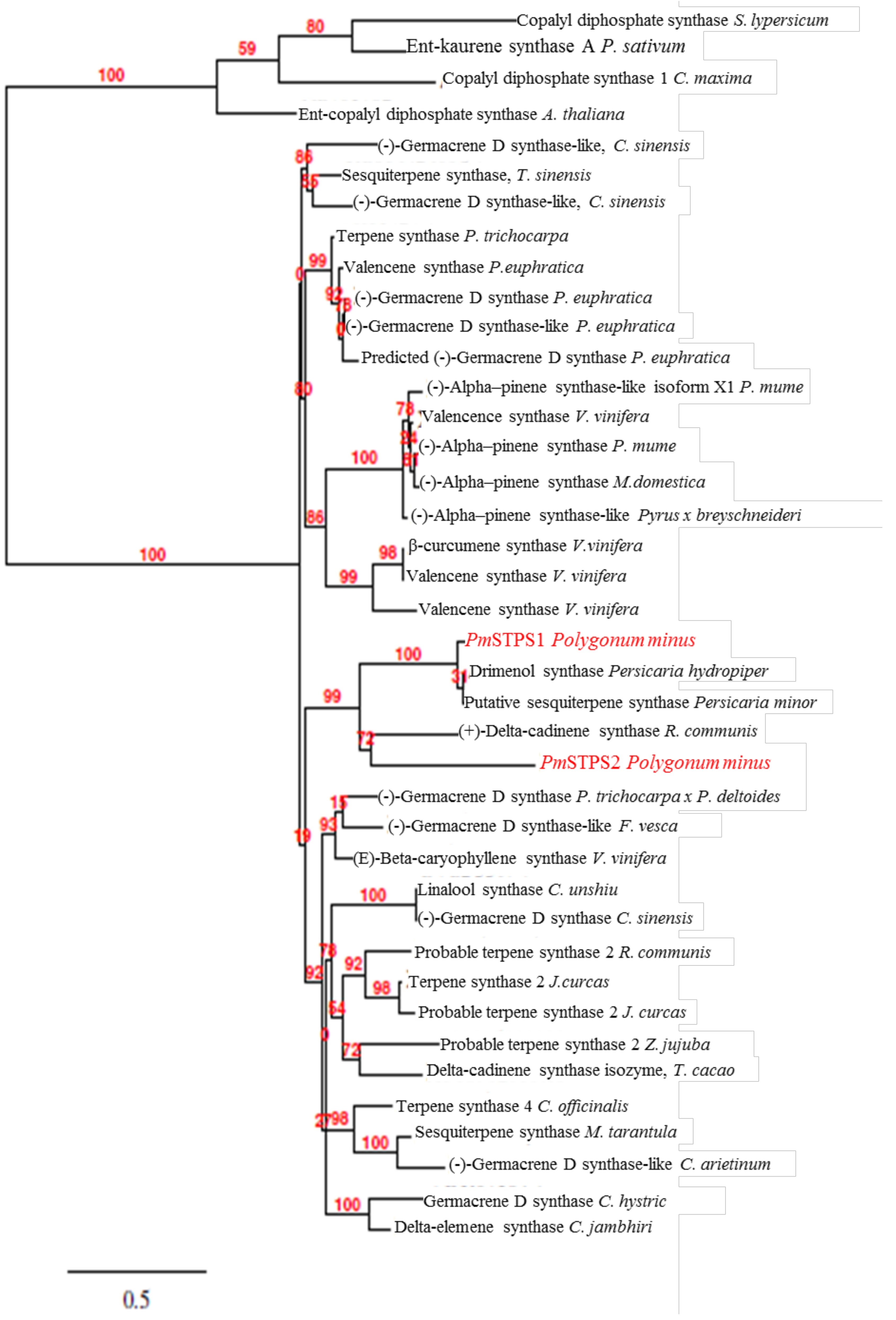
2.2. Phylogenetic Analysis of P. minus Sesquiterpene Synthase (PmSTPS)
2.3. Expression of PmSTPS1 and PmSTPS2 in E. coli
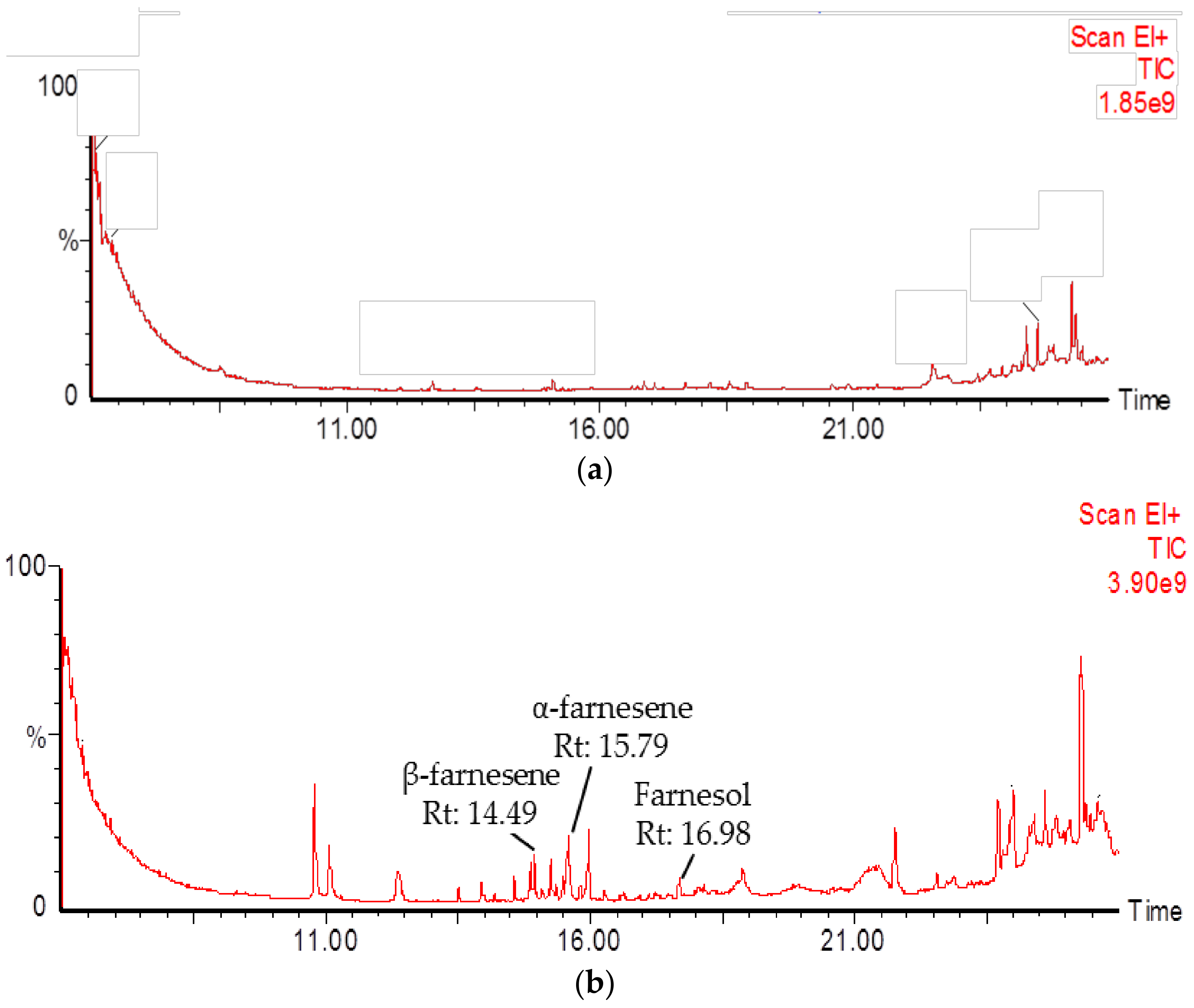
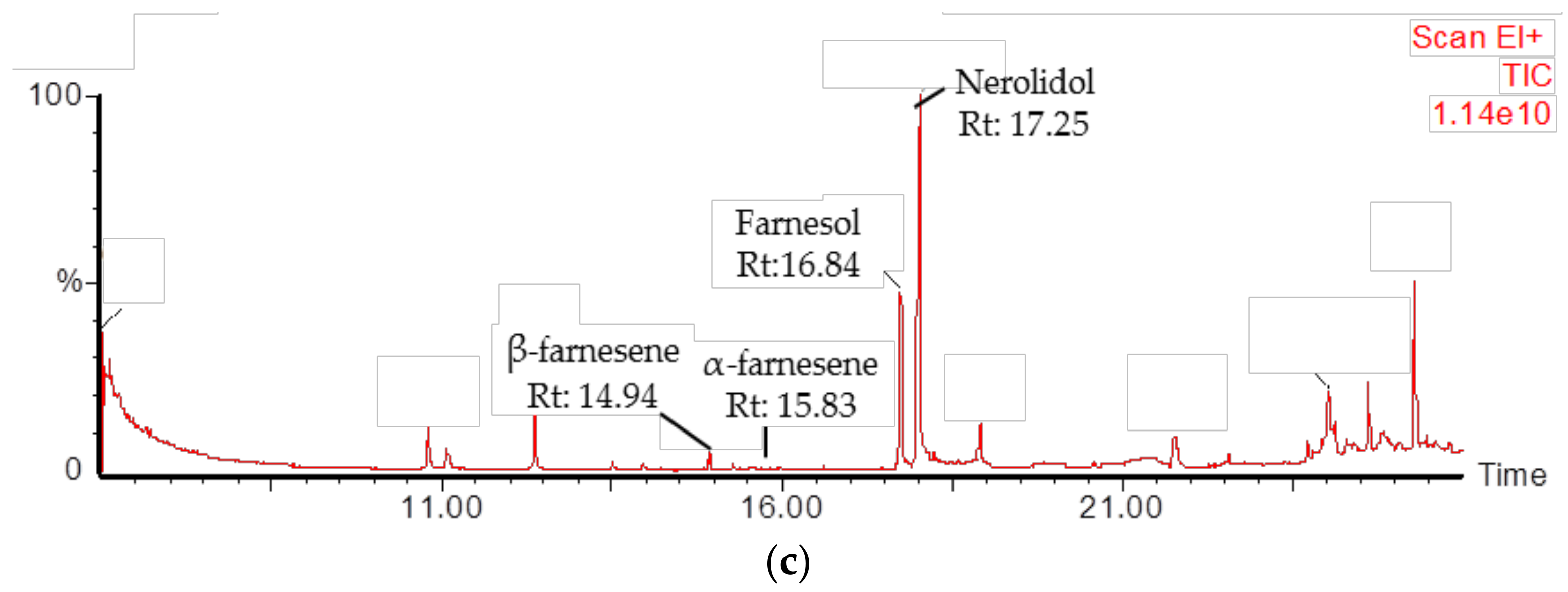
2.4. Identification of PmSTPS1 and PmSTPS2 Assay Products
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. RNA Isolation and cDNA Synthesis
4.3. Candidate Gene Selection and Isolation of Full-Length PmSTPS1 and PmSTPS2
4.4. Full-Length cDNA Sequence Analysis and Phylogenetic Tree Construction
4.5. Phylogenetic Analysis
4.6. Expression of PmSTPS1 and PmSTPS2 in E. coli
4.7. Enzyme Assay
4.8. Detection of Sesquiterpenes Using GC-MS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCaskill, D.R.C. Prospects for the Bioengineering of Isoprenpid Biosynthesis. In Biotechnology of Aroma Compounds. Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 1997; pp. 107–146. [Google Scholar]
- Lange, B.M.; Ahkami, A. Metabolic Engineering of Plant Monoterpenes, Sesquiterpenes and Diterpenes-Current Status and Future Opportunities. Plant Biotechnol. J. 2013, 11, 169–196. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.B.; Yu, R.C.; Yu, Y.Y.; Fan, Y.P. Molecular Cloning and Expression of Hedychium coronarium Farnesyl Pyrophosphate Synthase Gene and Its Possible Involvement in the Biosynthesis of Floral and Wounding/herbivory Induced Leaf Volatile Sesquiterpenoids. Gene 2013, 518, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, J.; Meyer-Gauen, G.; Croteau, R. Plant Terpenoid Synthases: Molecular Biology and Phylogenetic Analysis. Proc. Natl. Acad. Sci. USA 1998, 95, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and Sesquiterpene Synthases and the Origin of Terpene Skeletal Diversity in Plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef] [PubMed]
- Tholl, D.; Lee, S. Terpene Specialized Metabolism in Arabidopsis thaliana. Arabidopsis Book 2011, 9, e0143. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed]
- Kuzuyama, T. Mevalonate and Nonmevalonate Pathways for the Biosynthesis of Isoprene Units. Biosci. Biotechnol. Biochem. 2002, 66, 1619–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenreich, W.; Bacher, A.; Arigoni, D.; Rohdich, F. Biosynthesis of Isoprenoids via the Non-Mevalonate Pathway. Cell. Mol. Life Sci. 2004, 61, 1401–1426. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. Regulation of the Mevalonate Pathway. Nature 1993, 295, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, M. The Mevalonate-Independent Methylerythritol 4-Phosphate (MEP) Pathway for Isoprenoid Biosynthesis, Including Carotenoids. Pure Appl. Chem. 1999, 71, 2279–2284. [Google Scholar] [CrossRef]
- Chandran, S.S.; Kealey, J.T.; Reeves, C.D. Microbial Production of Isoprenoids. Process Biochem. 2011, 46, 1703–1710. [Google Scholar] [CrossRef]
- Keasling, J.D. Synthetic Biology and the Development of Tools for Metabolic Engineering. Metab. Eng. 2012, 14, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Yahya, P.P.; Ouellet, M.; Chan, R.; Mukhopadhyay, A.; Keasling, J.D.; Lee, T.S. Identification and Microbial Production of a Terpene-Based Advanced Biofuel. Nat. Commun. 2011, 2, 483. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-Derived Flavor Compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.L.; Daramwar, P.P.; Krithika, R.; Pandreka, A.; Shankar, S.S.; Thulasiram, H.V. Functional Characterization of Novel Sesquiterpene Synthases from Indian Sandalwood, Santalum album. Sci. Rep. 2015, 5, 10095. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Keasling, J.D. Semi-Synthetic Artemisinin: A Model for the use of Synthetic Biology in Pharmaceutical Development. Nat. Rev. Microbiol. 2014, 12, 355. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, A.-K.; Kwon, M.; Conrad, J.; Ro, D.-K.; Spring, O. Identification and Characterization of Two Bisabolene Synthases from Linear Glandular Trichomes of Sunflower (Helianthus annuus L., Asteraceae). Phytochemistry 2016, 124, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Pazouki, L.; Memari, H.R.; Kännaste, A.; Bichele, R.; Niinemets, Ü. Germacrene A Synthase in Yarrow (Achillea millefolium) is an Enzyme with Mixed Substrate Specificity: Gene Cloning, Functional Characterization and Expression Analysis. Front. Plant Sci. 2015, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Cochrane, S.A.; Vederas, J.C.; Ro, D.K. Molecular Cloning and Characterization of Drimenol Synthase from Valerian Plant (Valeriana Officinalis). FEBS Lett. 2014, 588, 4597–4603. [Google Scholar] [CrossRef] [PubMed]
- Parveen, I.; Wang, M.; Zhao, J.; Chittiboyina, A.G.; Tabanca, N.; Ali, A.; Baerson, S.R.; Techen, N.; Chappell, J.; Khan, I.A.; et al. Investigating Sesquiterpene Biosynthesis in Ginkgo biloba: Molecular Cloning and Functional Characterization of (E,E)-Farnesol and α-Bisabolene Synthases. Plant Mol. Biol. 2015, 89, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Okamto, S.; Nakasone, K.; Adachi, K.; Matsuda, S.; Harada, H.; Misawa, N.; Utsumi, R. Molecular Cloning and Functional Characterization of α-Humulene Synthase, a Possible Key Enzyme of Zerumbone Biosynthesis in Shampoo Ginger (Zingiber zerumbet Smith). Planta 2008, 227, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.; Kim, S.; Ro, D. Catalyzing the First Step in the Biosynthesis of the Natural Sweetener, Hernandulcin, in Lippia dulcis. Arch. Biochem. Biophys. 2012, 527, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Jayaramaiah, R.H.; Anand, A.; Beedkar, S.D.; Dholakia, B.B.; Punekar, S.A.; Kalunke, R.M.; Gade, W.N.; Thulasiram, H.V.; Giri, A.P. Functional Characterization and Transient Expression Manipulation of a New Sesquiterpene Synthase Involved in β-Caryophyllene Accumulation in Ocimum. Biochem. Biophys. Res. Commun. 2016, 473, 265–271. [Google Scholar] [CrossRef] [PubMed]
- König, J.C.; Scheper, T.; Beutel, S. Heterologous Expression, Purification, and Biochemical Characterization of α-Humulene Synthase from Zingiber zerumbet Smith. Appl. Biochem. Biotechnol. 2016, 178, 474–489. [Google Scholar]
- Picaud, S.; Olsson, M.E.; Brodelius, M.; Brodelius, P.E. Cloning, Expression, Puri W Cation and Characterization of Recombinant (+)-Germacrene D Synthase from Zingiber officinale. Arch. Biochem. Biophys. 2006, 452, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.A.; Zulkifli, R.M.; Nur, W.; Wan, A.; Husni, A.; Shariff, M.; Ahmad, N.; Nik, N.; Zakaria, Z.; Ahmad, F. Antibacterial Properties of Persicaria minor (Huds.) Ethanolic and Aqueous-Ethanolic Leaf Extracts. J. Appl. Pharm. Sci. 2015, 5 (Suppl. 2), 50–56. [Google Scholar]
- Christapher, P.V.; Parasuraman, S.; Christina, J.M.A.; Asmawi, M.Z.; Vikneswaran, M. Review on Polygonum minus. Huds, a Commonly Used Food Additive in Southeast Asia. Pharmacogn. Res. 2014, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Chinnappan, S.; Choudhary, Y.; Bommu, P.; Sridhar, M. Immunomodulatory Activity of an Aqueous Extract of Polygonum minus Huds on Swiss Albino Mice Using Carbon Clearance Assay. Asian Pac. J. Trop. Dis. 2014, 4, 398–400. [Google Scholar] [CrossRef]
- Qader, S.W.; Abdulla, M.A.; Chua, L.S. Potential Bioactive Property of Polygonum minus Huds (Kesum) Review. Sci. Res. Essays 2012, 7, 90–93. [Google Scholar]
- Ahmad, R.; Baharum, S.N.; Bunawan, H.; Lee, M.; Noor, N.M.; Rohani, E.R.; Ilias, N.; Zin, N.M. Volatile Profiling of Aromatic Traditional Medicinal Plant, Polygonum minus in Different Tissues and Its Biological Activities. Molecules 2014, 19, 19220–19242. [Google Scholar] [CrossRef] [PubMed]
- Baharum, S.N.; Bunawan, H.; Ghani, A.; Aida, W.; Mustapha, W.; Noor, N.M. Analysis of the Chemical Composition of the Essential Oil of Polygonum minus Huds. Using Two-Dimensional Gas Chromatography-Time-of-Flight Mass Spectrometry (GC-TOF MS). Molecules. [CrossRef] [PubMed]
- Yaacob, K.B.; Noor Salleh, A.; Joulain, D. Studies on Polygonum Spp. In Proceedings of the 12th International Congress of Flavours Fragrances and Essential Oils, Vienna, Austria, 4–8 October 1992; pp. 491–498. [Google Scholar]
- Rusdi, N.A.; Goh, H.H.; Baharum, S.N. GC-MS/Olfactometric Characterisation and Aroma Extraction Dilution Analysis of Aroma Active Compounds in Polygonum minus Essential Oil. Plant Omics 2016, 9, 289–294. [Google Scholar] [CrossRef]
- Tan, E.; Othman, R. Characterization of α-Farnesene Synthase Gene from Polygonum minus. Trans. Malaysian Soc. Plant Physiol. 2012, 20, 155–157. [Google Scholar]
- Song, A.A.-L.; Abdullah, J.O.; Abdullah, M.P.; Shafee, N.; Othman, R.; Tan, E.-F.; Noor, N.M.; Raha, A.R. Overexpressing 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase (HMGR) in the Lactococcal Mevalonate Pathway for Heterologous Plant Sesquiterpene Production. PLoS ONE 2012, 7, e52444. [Google Scholar] [CrossRef] [PubMed]
- Ee, S.; Othman, R.; Shaharuddin, N.A.; Ismail, I.; Zainal, Z. Functional Characterization of Sesquiterpene Synthase from Polygonum minus. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kiat, C.J.; Ashraf, M.F.; Naeem-Ul-Hassan, M.; Che Mohd Zain, C.R.; Zainal, Z.; Ismail, I. Molecular Cloning, Characterization and Expression Pattern Analysis of a Jasmonic Acid Responsive Sesquiterpene Synthase Gene from Persicaria minor. Plant Omics 2016, 9, 360–368. [Google Scholar] [CrossRef]
- Ker, D.-S.; Pang, S.L.; Othman, N.F.; Kumaran, S.; Tan, E.F.; Krishnan, T.; Chan, K.G.; Othman, R.; Hassan, M.; Ng, C.L. Purification and Biochemical Characterization of Recombinant Persicaria Minor β -Sesquiphellandrene Synthase. PeerJ 2017, 5, e2961. [Google Scholar] [CrossRef] [PubMed]
- Loke, K.; Rahnamaie-tajadod, R.; Yeoh, C.; Goh, H.; Mohamed-Hussein, Z.; Mohd, N.; Zainal, Z.; Ismail, I. Genomics Data RNA-Seq Analysis for Secondary Metabolite Pathway Gene Discovery in Polygonum minus. GDATA 2016, 7, 12–13. [Google Scholar]
- Chen, X.; Wang, Y.; Sun, J.; Wang, J.; Xun, H.; Tang, F. Cloning, Expression and Functional Characterization of Two Sesquiterpene Synthase Genes from Moso Bamboo (Phyllostachys edulis). Protein Expr. Purif. 2016, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Ma, L.T.; Wang, S.Y.; Chu, F.H. Cloning and Expression of a Sesquiterpene Synthase Gene from Taiwania cryptomerioides. Holzforschung 2015, 69, 1041–1048. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gutensohn, M.; Wilkerson, C.G.; Dudareva, N. Two Nearly Identical Terpene Synthases Catalyze the Formation of Nerolidol and Linalool in Snapdragon Flowers. Plant J. 2008, 55, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Yu, X.; Fan, J.; Pickett, J.A.; Jones, H.D.; Zhou, J.; Michael, A.; Caulfield, J.; Napier, J.A.; et al. Molecular Characterization of Two Isoforms of a Farnesyl Pyrophosphate Synthase Gene in Wheat and Their Roles in Sesquiterpene Synthesis and Inducible Defence against Aphid Infestation. New Phytol. 2015, 206, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Yahyaa, M.; Matsuba, Y.; Brandt, W.; Doron-Faigenboim, A.; Bar, E.; McClain, A.; Davidovich-Rikanati, R.; Lewinsohn, E.; Pichersky, E.; Ibdah, M. Identification, Functional Characterization, and Evolution of Terpene Synthases from a Basal Dicot. Plant Physiol. 2015, 169, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Alquézar, B.; Rodríguez, A.; de la Peña, M.; Peña, L. Genomic Analysis of Terpene Synthase Family and Functional Characterization of Seven Sesquiterpene Synthases from Citrus sinensis. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.C. Western Blot: Technique, Theory, and Trouble Shooting. N. Am. J. Med. Sci. 2012, 4. [Google Scholar]
- Jin, J.; Kim, M.J.M.J.; Dhandapani, S.; Tjhang, J.G.; Yin, J.-L.J.-L.; Wong, L.; Sarojam, R.; Chua, N.-H.N.-H.; Jang, I.-C. The Floral Transcriptome of Ylang Ylang (Cananga odorata Var. Fruticosa) Uncovers Biosynthetic Pathways for Volatile Organic Compounds and a Multifunctional and Novel Sesquiterpene Synthase. J. Exp. Bot. 2015, 66, 3959–3975. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Hu, T.; Liu, Y.; Tong, Y.; Guan, H.; Zhang, Y.; Zhou, J.; Huang, L.; Gao, W. Functional Characterization of NES and GES Responsible for the Biosynthesis of (E)-Nerolidol and (E,E)-Geranyllinalool in Tripterygium wilfordii. Sci. Rep. 2017, 7, 40851. [Google Scholar] [CrossRef] [PubMed]
- Keeling, C.I.; Bohlmann, J. Genes, Enzymes and Chemicals of Terpenoid Diversity in the Constitutive and Induced Defence of Conifers against Insects and Pathogens. New Phytol. 2006, 170, 657–675. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.C.; Croteau, R. Genomic Organization of Plant Terpene Synthases and Molecular Evolutionary Implications. Genetics 2001, 158, 811–832. [Google Scholar] [PubMed]
- Mercke, P.; Crock, J.; Croteau, R.; Brodelius, P.E. Cloning, Expression, and Characterization of Epi-Cedrol Synthase, a Sesquiterpene Cyclase from Artemisia annua L. Arch. Biochem. Biophys. 1999, 369, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Irmisch, S.; Krause, S.T.; Kunert, G.; Gershenzon, J.; Degenhardt, J.; Köllner, T.G. The Organ-Specific Expression of Terpene Synthase Genes Contributes to the Terpene Hydrocarbon Composition of Chamomile Essential Oils. BMC Plant Biol. 2012, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Chu, F.H. Plasticity Residues Involved in Secondary Cyclization of Terpene Synthesis in Taiwania cryptomerioides. Tree Genet. Genomes 2015, 11, 796. [Google Scholar] [CrossRef]
- Picaud, S.; Brodelius, M.; Brodelius, P.E. Expression, Purification and Characterization of Recombinant (E)-β-Farnesene Synthase from Artemisia annua. Phytochemistry 2005, 66, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kirby, J.; Keasling, J.D. Functional Characterization of Four Sesquiterpene Synthases from Ricinus communis (Castor Bean). Phytochemistry 2012, 78, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Green, S.A.; Chen, X.; Nieuwenhuizen, N.J.; Matich, A.J.; Wang, M.Y.; Bunn, B.J.; Yauk, Y.K.; Atkinson, R.G. Identification, Functional Characterization, and Regulation of the Enzyme Responsible for Floral (E)-Nerolidol Biosynthesis in Kiwifruit (Actinidia chinensis). J. Exp. Bot. 2012, 63, 1951–1967. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant Protein Expression in Escherichia Coli: Advances and Challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, L.; Chen, Y.; Bach, L.S.; Rattleff, S.; Maury, J.; Brix, S.; Nielsen, J.; Mortensen, U.H. Diversion of Flux toward Sesquiterpene Production in Saccharomyces cerevisiae by Fusion of Host and Heterologous Enzymes. Appl. Environ. Microbiol. 2011, 77, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, N.; Lu, X.; Yang, B.; Peters, R.J. The Application of Synthetic Biology to Elucidation of Plant Mono-, Sesqui-, and Diterpenoid Metabolism. Mol. Plant 2015, 8, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Tippmann, S.; Scalcinati, G.; Siewers, V.; Nielsen, J. Production of Farnesene and Santalene by Saccharomyces cerevisiae Using Fed-Batch Cultivations with R-Controlled Feed. Biotechnol. Bioeng. 2016, 113, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Šobotník, J.; Hanus, R.; Kalinová, B.; Piskorski, R.; Cvačka, J.; Bourguignon, T.; Roisin, Y. (E,E)-α-Farnesene, an Alarm Pheromone of the Termite Prorhinotermes canalifrons. J. Chem. Ecol. 2008, 34, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Sohdi, N.A.S.; Seman-Kamarulzaman, A.F.; Mohamed-Hussein, Z.A.; Hassan, M. Purification and Characterization of a Novel NAD (P)+-Farnesol Dehydrogenase from Polygonum minus Leaves. PLoS ONE 2015, 10, e0143310. [Google Scholar] [CrossRef] [PubMed]
- Lapczynski, A.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Cis-Nerolidol. Food Chem. Toxicol. 2008, 46, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; Gonçalves, P.; Martel, F.; Coimbra, M.A.; Rocha, S.M. Assessment of the Antioxidant and Antiproliferative Effects of Sesquiterpenic Compounds in in Vitro Caco-2 Cell Models. Food Chem. 2014, 156, 204–211. [Google Scholar] [CrossRef] [PubMed]
- AbouLaila, M.; Sivakumar, T.; Yokoyama, N.; Igarashi, I. Inhibitory Effect of Terpene Nerolidol on the Growth of Babesia Parasites. Parasitol. Int. 2010, 59, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Tan, L.T.H.; Chan, K.G.; Lee, L.H.; Goh, B.H. Nerolidol: A Sesquiterpene Alcohol with Multi-Faceted Pharmacological and Biological Activities. Molecules 2016, 529. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.N.; Oliveira, G.L.S.; De Carvalho, R.B.F.; De Sousa, D.P.; Freitas, R.M.; Pinto, P.L.S.; De Moraes, J. Antischistosomal Activity of the Terpene Nerolidol. Molecules 2014, 19, 3793–3803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunawan, H.; Choong, C.Y.; Md-Zain, B.M.; Baharum, S.N.; Noor, N.M. Molecular Systematics of Polygonum minus Huds. Based on ITS Sequences. Int. J. Mol. Sci. 2011, 12, 7626–7634. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, A.; Suleman, N.I.; Zakaria, W.A.; Goh, H.H.; Noor, N.M.; Aizat, W.M. RNA Extractions of Mangosteen (Garcinia mangostana L.) Pericarps for Sequencing. Sains Malaysiana 2017, 46, 1231–1240. [Google Scholar] [CrossRef]
- Kong, S.L.; Abdullah, S.N.A.; Ho, C.L.; Amiruddin, M.D. Molecular Cloning, Gene Expression Profiling and in Silico Sequence Analysis of Vitamin E Biosynthetic Genes from the Oil Palm. Plant Gene 2016, 5, 100–108. [Google Scholar] [CrossRef]
- Maille, P.E.O.; Chappell, J.; Noel, J.P. A Single-Vial Analytical and Quantitative Gas Chromatography—Mass Spectrometry Assay for Terpene Synthases. Anal. Biochem. 2004, 335, 210–217. [Google Scholar]
Sample Availability: Samples of the compounds β-farnesene and nerolidol are not available from the authors. |


| Description | Organism | Score | E-value | Identity (%) | Accession |
|---|---|---|---|---|---|
| Drimenol synthase | Persicaria hydropiper | 678 | 0.0 | 96 | AHF2284.1 |
| Putative sesquiterpene synthase | Persicaria minor | 664 | 0.0 | 95 | ALO69830.1 |
| Delta-cadinene synthase isozyme A | Theobroma cacao | 393 | 5 × 10−129 | 54 | XP_007021123.1 |
| Predicted: probable sesquiterpene synthase | Beta vulgaris subsp. vulgaris | 389 | 3 × 10−127 | 54 | XP_010694277.1 |
| Predicted: probable terpene synthase 2 | Rocinus communis | 381 | 3 × 10−124 | 52 | XP_002523635.1 |
| Probable sesquiterpene synthase | Santalum murrayanum | 379 | 1 × 10−123 | 54 | F6M8H1 |
| (−)-germacrene D synthase-like isoform X2 | Citrus sinensis | 379 | 1 × 10−123 | 52 | XP_015384843.1 |
| Description | Organism | Score | E-value | Identity (%) | Accession |
|---|---|---|---|---|---|
| Drimenol synthase | Persicaria hydropiper | 492 | 3 × 10−164 | 46 | AHF2284.1 |
| Probable sesquiterpene synthase | Beta vulgaris subsp. vulgaris | 492 | 3 × 10−164 | 43 | XP_010694277.1 |
| Valencene synthase-like | Vitis vinifera | 489 | 2 × 10−163 | 44 | NP_001268028.1 |
| Germacrene A synthase | Vitis vinifera | 489 | 2 × 10−163 | 44 | ADR66821.1 |
| Putative sesquiterpene synthase | Persicaria minor | 484 | 3 × 10−161 | 45 | ALO69830.1 |
| Predicted: (−)-germacrene D synthase | Vitis vinifera | 479 | 4 × 10−159 | 42 | XP_002282488.1 |
| (E)-beta-caryophyllene synthase | Vitis vinifera | 477 | 1 × 10−158 | 45 | ADR74194.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusdi, N.A.; Goh, H.-H.; Sabri, S.; Ramzi, A.B.; Mohd Noor, N.; Baharum, S.N. Functional Characterisation of New Sesquiterpene Synthase from the Malaysian Herbal Plant, Polygonum Minus. Molecules 2018, 23, 1370. https://doi.org/10.3390/molecules23061370
Rusdi NA, Goh H-H, Sabri S, Ramzi AB, Mohd Noor N, Baharum SN. Functional Characterisation of New Sesquiterpene Synthase from the Malaysian Herbal Plant, Polygonum Minus. Molecules. 2018; 23(6):1370. https://doi.org/10.3390/molecules23061370
Chicago/Turabian StyleRusdi, Nor Azizun, Hoe-Han Goh, Suriana Sabri, Ahmad Bazli Ramzi, Normah Mohd Noor, and Syarul Nataqain Baharum. 2018. "Functional Characterisation of New Sesquiterpene Synthase from the Malaysian Herbal Plant, Polygonum Minus" Molecules 23, no. 6: 1370. https://doi.org/10.3390/molecules23061370
APA StyleRusdi, N. A., Goh, H.-H., Sabri, S., Ramzi, A. B., Mohd Noor, N., & Baharum, S. N. (2018). Functional Characterisation of New Sesquiterpene Synthase from the Malaysian Herbal Plant, Polygonum Minus. Molecules, 23(6), 1370. https://doi.org/10.3390/molecules23061370






