Stomatal Complex Development and F-Actin Organization in Maize Leaf Epidermis Depend on Cellulose Synthesis
Abstract
:1. Introduction
2. Results
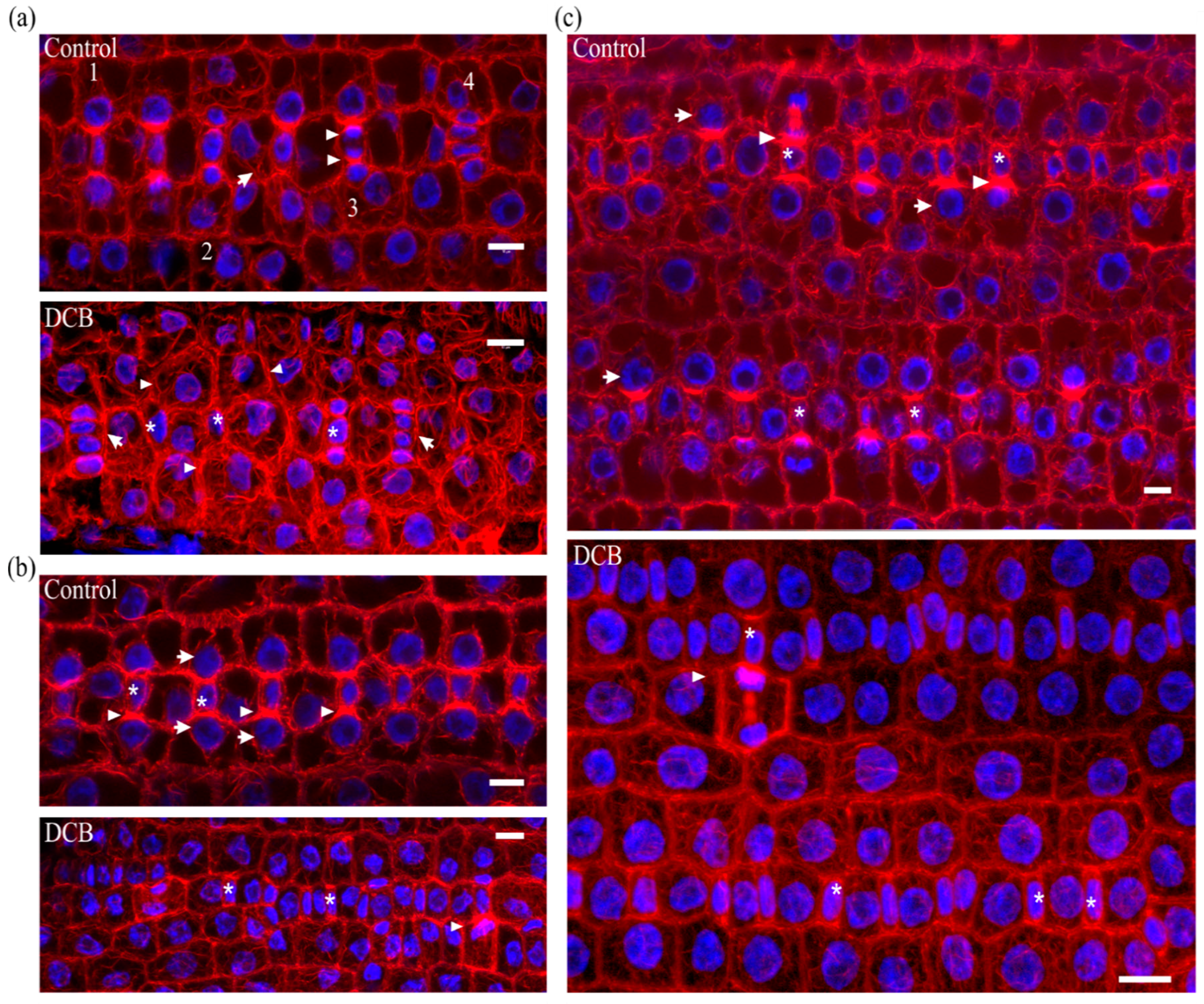
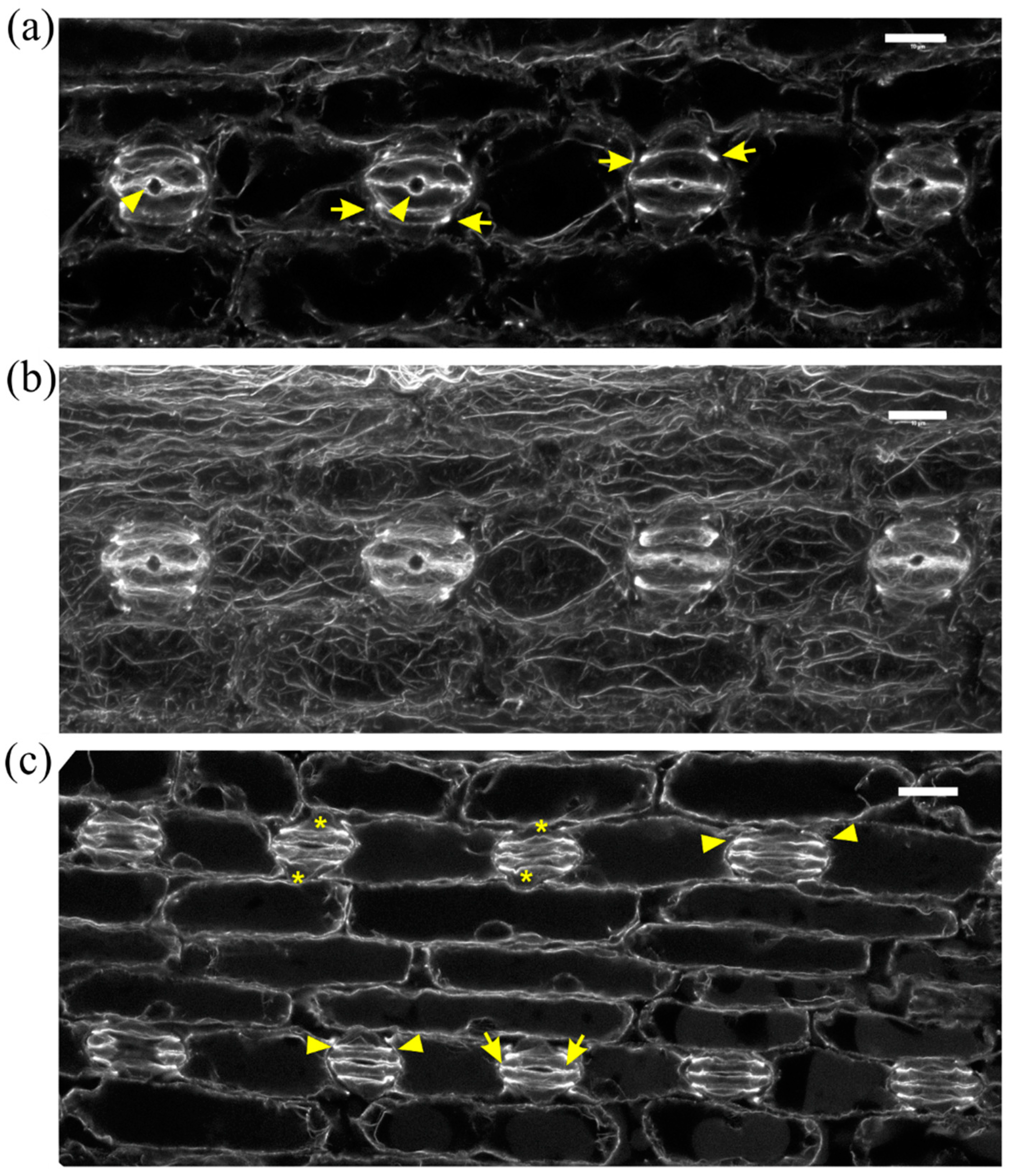
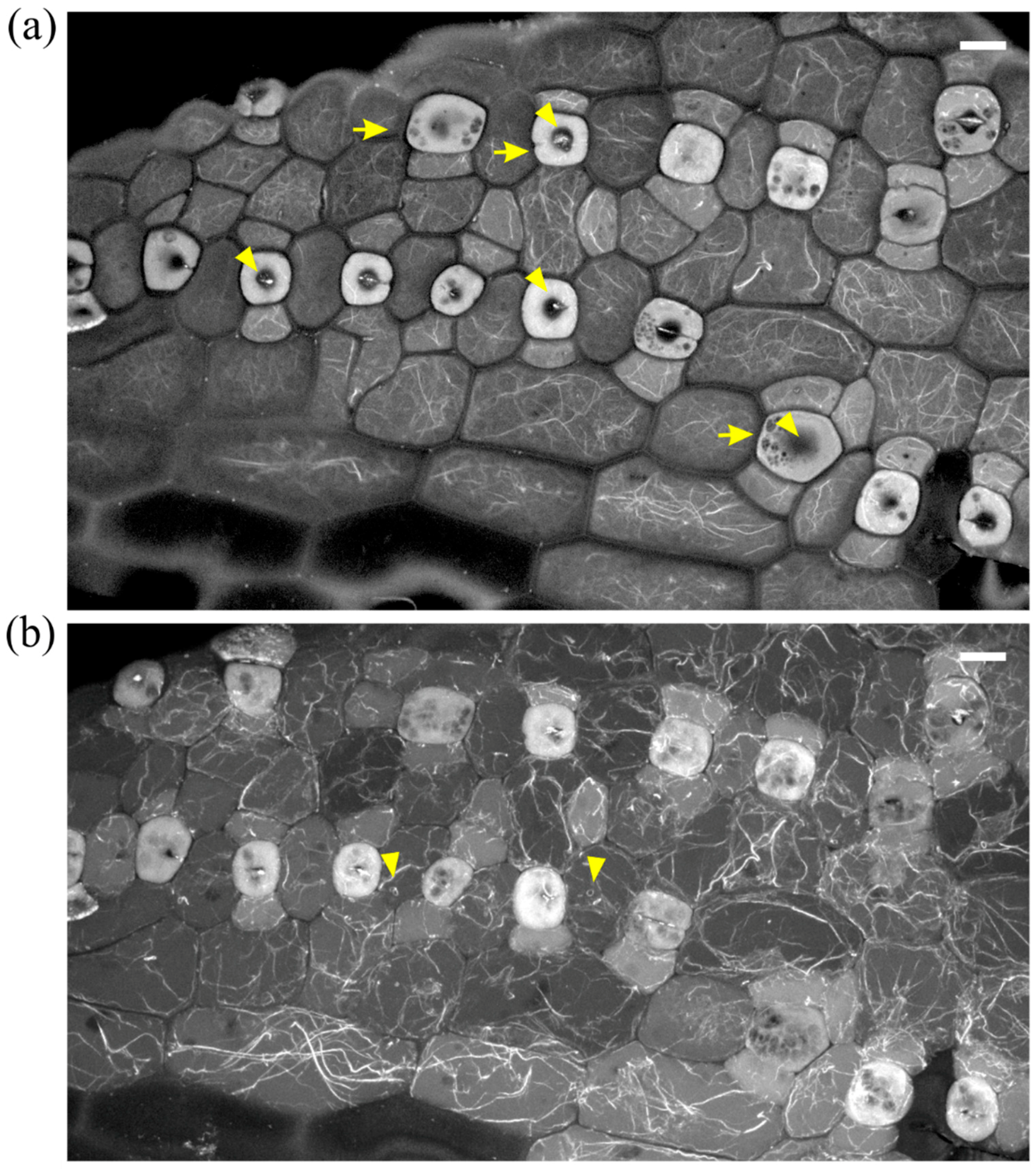
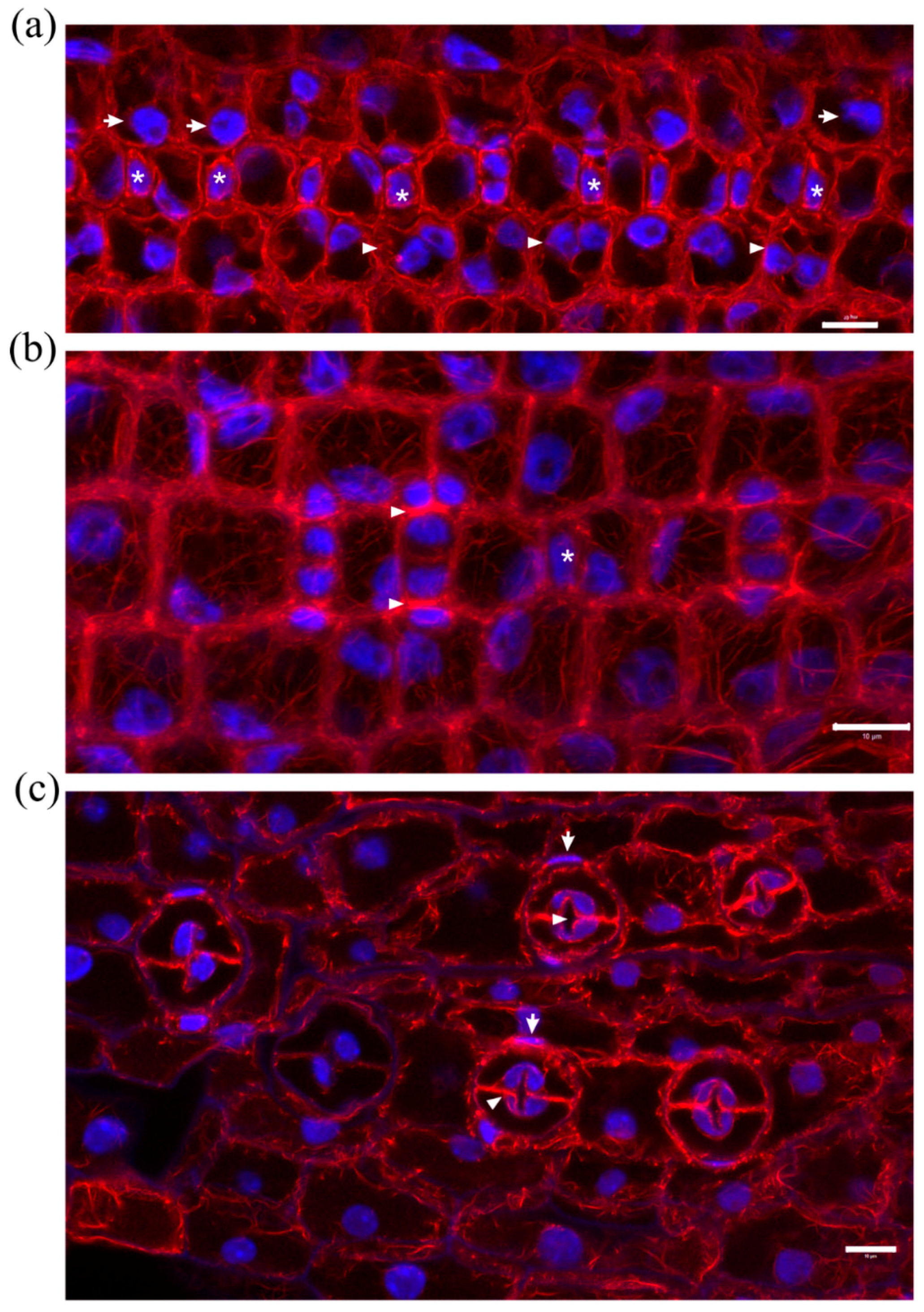
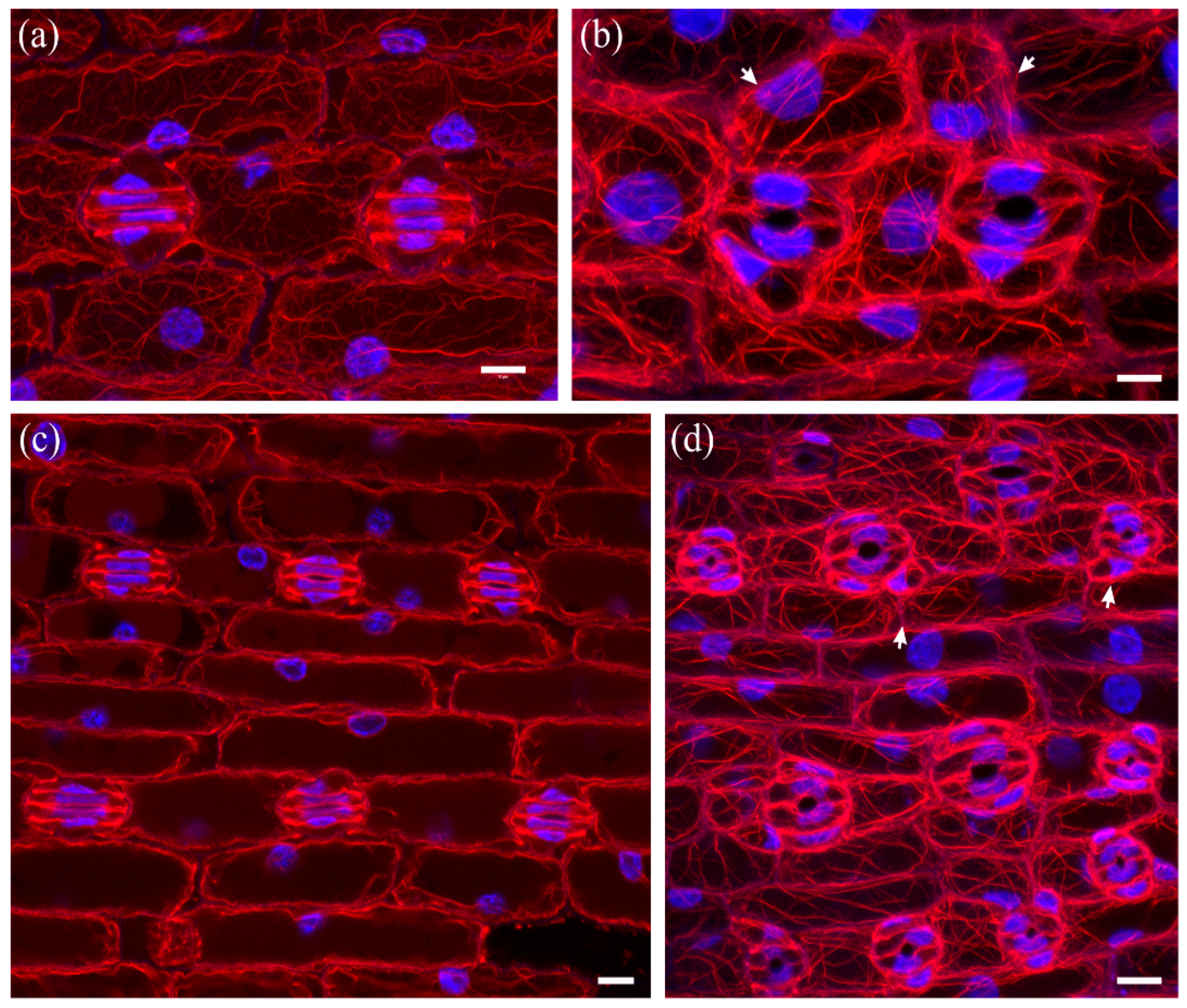
2.1. Seedlings Germinated under the Effect of DCB
2.2. Seedlings Treated with Cellulose Biosynthesis Inhibitors after Germination
3. Discussion
4. Materials and Methods
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Landrein, B.; Hamant, O. How mechanical stress controls microtubule behavior and morphogenesis in plants: History, experiments and revisited theories. Plant J. 2013, 75, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Paredez, A.R.; Somerville, C.R.; Ehrhardt, D.W. Visualization of cellulose synthase demonstrates functional association with microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Panteris, E.; Galatis, B. The morphogenesis of lobed plant cells in the mesophyll and epidermis: Organization and distinct roles of cortical microtubules and actin filaments. New Phytol. 2005, 167, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Martinière, A.; Gayral, P.; Hawes, C.; Runions, J. Building bridges: Formin1 of Arabidopsis forms a connection between the cell wall and the actin cytoskeleton. Plant J. 2011, 66, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Nebenfuhr, A.; Gallagher, L.A.; Dunahay, T.G.; Frohlick, J.A.; Mazurkiewicz, A.M.; Meehl, J.B.; Staehelin, L.A. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol. 1999, 121, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.; Shaw, S.L. Update: Plant Cortical Microtubule Arrays. Plant Physiol. 2018, 176, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Himmelspach, R.; Williamson, R.E.; Wasteneys, G.O. Cellulose microfibril alignment recovers from DCB-induced disruption despite microtubule disorganization. Plant J. 2003, 36, 565–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredez, A.R.; Persson, S.; Ehrhardt, D.W.; Somerville, C.R. Genetic evidence that cellulose synthase activity influences microtubule cortical array organization. Plant Physiol. 2008, 147, 1723–1734. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, L.; Cheng, X.; Fan, L.S.; Hao, H.Q. Disruption of cellulose synthesis by 2,6-dichlorobenzonitrile affects the structure of the cytoskeleton and cell wall construction in Arabidopsis. Plant Biol. 2013, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Panteris, E.; Adamakis, I.D.; Daras, G.; Hatzopoulos, P.; Rigas, S. Differential responsiveness of cortical microtubule orientation to suppression of cell expansion among the developmental zones of Arabidopsis thaliana root apex. PLoS ONE 2013, 8, e82442. [Google Scholar] [CrossRef] [PubMed]
- Panteris, E.; Adamakis, I.D.; Daras, G.; Rigas, S. Cortical microtubule patterning in roots of Arabidopsis thaliana primary cell wall mutants reveals the bidirectional interplay with cell expansion. Plant Signal. Behav. 2014, 9, e28737. [Google Scholar] [CrossRef] [PubMed]
- Tolmie, A.F.; Poulet, A.; McKenna, J.F.; Sassmann, S.; Graumann, K.; Deeks, M.; Runions, J. The cell wall of Arabidopsis thaliana influences actin network dynamics. J. Exp. Bot. 2017, 68, 4517–4527. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.; Smith, L.G. Roles for polarity and nuclear determinants in specifying daughter cell fates after an asymmetric cell division in the maize leaf. Curr. Biol. 2000, 10, 1229–1232. [Google Scholar] [CrossRef]
- Panteris, E.; Apostolakos, P.; Galatis, B. Cytoskeletal asymmetry in Zea mays subsidiary cell mother cells: A monopolar prophase microtubule half-spindle anchors the nucleus to its polar position. Cell Motil. Cytoskelet. 2006, 63, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Panteris, E.; Galatis, B.; Quader, H.; Apostolakos, P. Cortical actin filament organization in developing and functioning stomatal complexes of Zea mays and Triticum turgidum. Cell Motil. Cytoskelet. 2007, 64, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Montezinos, D.; Delmer, D.P. Characterization of inhibitors of cellulose synthesis in cotton fibers. Planta 1980, 148, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Heim, D.R.; Skomp, J.R.; Tschabold, E.E.; Larrinua, I.M. Isoxaben inhibits the synthesis of acid insoluble cell wall materials in Arabidopsis thaliana. Plant Physiol. 1990, 93, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Barlow, P.W.; Volkmann, D. Actin: A Dynamic Framework for Multiple Plant Cell Functions; Staiger, C.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; p. 457. [Google Scholar]
- Hamant, O.; Heisler, M.G.; Jonsson, H.; Krupinski, P.; Uyttewaal, M.; Bokov, P.; Corson, F.; Sahlin, P.; Boudaoud, A.; Meyerowitz, E.M.; et al. Developmental patterning by mechanical signals in Arabidopsis. Science 2008, 322, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Hamant, O.; Traas, J.; Boudaoud, A. Regulation of shape and patterning in plant development. Curr. Opin. Genet. Dev. 2010, 20, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Hamant, O. Widespread mechanosensing controls the structure behind the architecture in plants. Curr. Opin. Plant Biol. 2013, 16, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Yan, A.; Krupinski, P.; Meyerowitz, E.M. Physical forces regulate plant development and morphogenesis. Curr. Biol. 2014, 24, R475–R483. [Google Scholar] [CrossRef] [PubMed]
- Daras, G.; Rigas, S.; Penning, B.; Milioni, D.; McCann, M.C.; Carpita, N.C.; Fasseas, C.; Hatzopoulos, P. The thanatos mutation in Arabidopsis thaliana cellulose synthase 3 (AtCesA3) has a dominant-negative effect on cellulose synthesis and plant growth. New Phytol. 2009, 184, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Karyophyllis, D.; Katsaros, C.; Galatis, B. F-actin involvement in apical cell morphogenesis of Sphacelaria rigidula (Phaeophyceae): Mutual alignment between cortical actin filaments and cellulose microfibrils. Eur. J. Phycol. 2000, 35, 195–203. [Google Scholar] [CrossRef]
- Katsaros, C.; Karyophyllis, D.; Galatis, B. Cortical F-actin underlies microfibril patterning in brown algal cells. Phycologia 2002, 41, 178–183. [Google Scholar] [CrossRef]
- Katsaros, C.; Karyophyllis, D.; Galatis, B. Cytoskeleton and morphogenesis in brown algae. Ann. Bot. 2006, 97, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Blancaflor, E.B. Cortical actin filaments potentially interact with cortical microtubules in regulating polarity of cell expansion in primary roots of maize (Zea mays L.). J. Plant Growth Regul. 2000, 19, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Jasik, J.; Edelmann, H.G.; Salajová, T.; Volkmann, D. Latrunculin B-induced plant dwarfism: Plant cell elongation is F-actin dependent. Dev. Biol. 2001, 231, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Gutierrez, R.; McFarlane, H.E.; Bringmann, M.; Lindeboom, J.; Emons, A.M.; Samuels, L.; Ketelaar, T.; Ehrhardt, D.W.; Persson, S. Patterning and lifetime of plasma membrane-localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol. 2013, 162, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Livanos, P.; Galatis, B.; Apostolakos, P. Deliberate ROS production and auxin synergistically trigger the asymmetrical division generating the subsidiary cells in Zea mays stomatal complexes. Protoplasma 2016, 253, 1081–1099. [Google Scholar] [CrossRef] [PubMed]
- Livanos, P.; Giannoutsou, E.; Apostolakos, P.; Galatis, B. Auxin as an inducer of asymmetrical division generating the subsidiary cells in stomatal complexes of Zea mays. Plant Signal. Behav. 2015, 10, e984531. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, P.; Livanos, P.; Giannoutsou, E.; Panteris, E.; Galatis, B. The intracellular and intercellular cross-talk during subsidiary cell formation in Zea mays: Existing and novel components orchestrating cell polarization and asymmetric division. Ann. Bot. 2018, in press. [Google Scholar] [CrossRef]
- Giannoutsou, E.; Apostolakos, P.; Galatis, B. Spatio-temporal diversification of the cell wall matrix materials in the developing stomatal complexes of Zea mays. Planta 2016, 244, 1125–1143. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Y.; Herth, W. Chemical inhibition of cell wall formation and cytokinesis, but not of nuclear division, in protoplasts of Nicotiana tabacum L. cultivated in vitro. Planta 1978, 142, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Galatis, B.; Apostolakos, P. Microtubule organization and morphogenesis of stomata in caffeine-affected seedlings of Zea mays. Protoplasma 1991, 165, 11–26. [Google Scholar] [CrossRef]
- Rosero, A.; Žárský, V.; Cvrčková, F. AtFH1 formin mutation affects actin filament and microtubule dynamics in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Brabham, C.; Stork, J.; Barrett, M.; DeBolt, S. Grass cell walls have a role in the inherent tolerance of grasses to the cellulose biosynthesis inhibitor isoxaben. Pest Manag. Sci. 2018, 74, 878–884. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Control a | Application of 2 μΜ DCB upon Germination a | Treatment with 2 μΜ DCB after Germination a | Treatment with 10 μΜ Isoxaben after Germination a | ||
|---|---|---|---|---|---|
| ontogenesis of stomatal complexes | SMCs without F-actin patch | 7.55 ± 1.77% (32/420) | 24.19 ± 2.14% (92/380) *** | 19.65 ± 2.81% (81/410) *** | 21.85 ± 1.03% (85/390) *** |
| SMCs with unpolarized nucleus | 2.68 ± 1.03% (11/420) | 20.58 ± 1.56% (78/380) *** | 17.03 ± 2.33% (70/410) *** | 20.56 ± 0.95% (80/390) *** | |
| defective divisions of GMCs | 0% (0/350) | 16.51 ± 3.35% (68/400) *** | 0% (0/420) | 6.89 ± 2.24% (26/380) *** | |
| non-canonical stomatal rows b | 1.58 ± 0.12% (3/190) | 32.51 ± 2.43% (65/200) *** | 8.63 ± 0.94% (16/185) *** | 20.97 ± 4.68% (42/200) *** | |
| mature stomatal complexes | stomata without subsidiary cells | 0% (0/260) | 8.47 ± 1.27% (23/270) *** | 5.42 ± 1.06% (15/275) *** | 6.91 ± 0.89% (18/260) *** |
| stomata with one subsidiary cell | 5.38 ± 1.59% (14/260) | 16.87 ± 2.81% (46/270) *** | 9.05 ± 2.86% (25/275) * | 10.80 ± 2.96% (28/260) ** | |
| stomata with aberrant subsidiary cells | 1.91 ± 0.92% (5/260) | 23.57 ± 4.14% (64/270) *** | 19.01 ± 3.44% (52/275) *** | 21.69 ± 3.53% (56/260) *** | |
| stomata with swollen guard cells | 0% (0/260) | 16.54 ± 4.19% (45/270) *** | 14.60 ± 1.59% (40/275) *** | 17.85 ± 5.57% (46/260) *** | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panteris, E.; Achlati, T.; Daras, G.; Rigas, S. Stomatal Complex Development and F-Actin Organization in Maize Leaf Epidermis Depend on Cellulose Synthesis. Molecules 2018, 23, 1365. https://doi.org/10.3390/molecules23061365
Panteris E, Achlati T, Daras G, Rigas S. Stomatal Complex Development and F-Actin Organization in Maize Leaf Epidermis Depend on Cellulose Synthesis. Molecules. 2018; 23(6):1365. https://doi.org/10.3390/molecules23061365
Chicago/Turabian StylePanteris, Emmanuel, Theonymphi Achlati, Gerasimos Daras, and Stamatis Rigas. 2018. "Stomatal Complex Development and F-Actin Organization in Maize Leaf Epidermis Depend on Cellulose Synthesis" Molecules 23, no. 6: 1365. https://doi.org/10.3390/molecules23061365





