Roselle Anthocyanins: Antioxidant Properties and Stability to Heat and pH
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Anthocyanins and Antioxidant Capacity of Roselle Extract
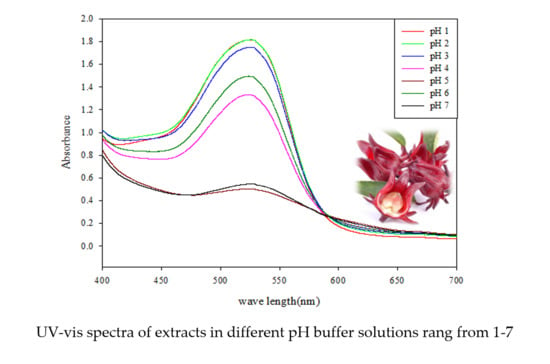
2.2. Color Stability
2.3. Thermal Kinetic Degradation
3. Materials and Methods
3.1. Materials and Reagents
3.2. Roselle Extract Preparation
3.3. Antioxidant Assays
3.3.1. Total Anthocyanin Concentration (TAC)
3.3.2. Total Polyphenol Concentration (TPC)
3.3.3. DPPH Method
3.3.4. ABTS Method
3.3.5. FRAP Method
3.4. Effect of Temperature and pH on the Roselle Extract
3.4.1. Extract in Different pH Buffers
3.4.2. Wavelength Scanning
3.4.3. Color Measurement
3.5. Kinetics of Degradation
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dhar, P.; Kar, C.S.; Ojha, D.; Pandey, S.K.; Mitra, J. Chemistry, phytotechnology, pharmacology and nutraceutical functions of kenaf (Hibiscus cannabinus L.) and roselle (Hibiscus sabdariffa L.) seed oil: An overview. Ind. Crops Prod. 2015, 77, 323–332. [Google Scholar] [CrossRef]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Ifie, I.; Abrankó, L.; Villa-Rodriguez, J.A.; Papp, N.; Ho, P.; Williamson, G.; Marshall, L.J. The effect of ageing temperature on the physicochemical properties, phytochemical profile and α-glucosidase inhibition of Hibiscus sabdariffa (roselle) wine. Food Chem. 2017. [Google Scholar] [CrossRef]
- Carvajal-Zarrabal, O.; Waliszewski, S.M.; Barradas-Dermitz, D.M.; Orta-Flores, Z.; Hayward-Jones, P.M.; Nolasco-Hipolito, C.; Angulo-Guerrero, O.; Sanchez-Ricano, R.; Infanzon, R.M.; Trujillo, P.R. The consumption of Hibiscus sabdariffa dried calyx ethanolic extract reduced lipid profile in rats. Plant Foods Hum. Nutr. 2005, 60, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Anthocyanins-nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Jalali-Khanabadi, B.A.; Afkhami-Ardekani, M.; Fatehi, F. Effects of sour tea (Hibiscus sabdariffa) on lipid profile and lipoproteins in patients with type II diabetes. J. Altern. Complem. Med. 2009, 15, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Peng, C.H.; Chan, K.C.; Yang, Y.S.; Huang, C.N.; Wang, C.J. The hypolipidemic effect of Hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J. Agric. Food Chem. 2010, 58, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Siro, I.; Kapolna, E.; Kapolna, B.; Lugasi, A. Functional food. product development, marketing and consumer acceptance-a review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Nair, S. Developing New Functional Food and Nutraceutical Products; Bagchi, D., Nair, S., Eds.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Bhardwaj, P.; Khanna, D. Green tea catechins: Defensive role in cardiovascular disorders. Chin. J. Nat. Med. 2013, 11, 345–353. [Google Scholar] [CrossRef]
- Marchese, D. Citrus consumers trend in Europe. new tastes sensation: The blood orange juice case. In Citrus Processing Short Course Proceedings; University of Florida: Gainesville, FL, USA, 1995; pp. 19–39. [Google Scholar]
- Santhakumar, A.B.; Kundur, A.R.; Fanning, K.; Netzel, M.; Stanley, R.; Singh, I. Consumption of anthocyanin-rich queen garnet plum juice reduces platelet activation related thrombogenesis in healthy volunteers. J. Funct. Foods 2015, 12, 11–22. [Google Scholar] [CrossRef]
- Andres, V.; Villanueva, M.-J.; Mateos-Aparicio, I.; Tenorio, M.-D. Colour, bioactive compounds and antioxidant capacity of mixed beverages based on fruit juices with milk or soya. J. Food Nutr. Res. 2014, 53, 71–80. [Google Scholar]
- Peng, B.; Li, H.; Deng, Z. Degradation of anthocyanins in foods during heating process and its mechanism. J. Food Safe Qual. 2016, 7, 3851–3858. [Google Scholar]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125, reprint in J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Turkmen, N.; Velioglu, Y.S.; Sari, F.; Polat, G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules 2007, 12, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chu, Z.; Ren, M.; Jia, R.; Zhao, C.; Fei, D.; Su, H.; Fan, X.; Zhang, X.; Li, Y. Identification of anthocyanin composition and functional analysis of an anthocyanin activator in solanum nigrum fruits. Molecules 2017, 22, 876. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.J.; Tumbas, V.T.; Savatović, S.M.; Đilas, S.M.; Ćetković, G.S.; Čanadanović-Brunet, J.M. Polyphenolic content and antioxidant activity of the four berry fruits pomace extracts. Acta Period. Technol. 2011, 42, 271–279. [Google Scholar] [CrossRef]
- Lang, J.C. Tocopherol stability in controlled release packaging films. Master’s Thesis, The State University of New Jersey, New Brunswick, NJ, USA, January 2009. [Google Scholar]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Sci. Food Agric. 2009, 57, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- De Leonardis, A.; Pizzella, L.; Macciola, V. Evaluation of chlorogenic acid and its metabolites as potential antioxidants for fish oils. Eur. J. Lipid Sci. Technol. 2008, 110, 941–948. [Google Scholar] [CrossRef]
- Tonfack, L.B.; Bernadac, A.; Youmbi, E.; Mbouapouognigni, V.P.; Ngueguim, M.; Akoa, A. Impact of organic and inorganic fertilizers on tomato vigor, yield and fruit composition under tropical andosol soil conditions. Fruits 2009, 64, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Kara, Ş.; Erçelebi, E.A. Thermal degradation kinetics of anthocyanins and visual colour of Urmu mulberry (Morus nigra L.). J. Food Eng. 2013, 116, 541–547. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.B.; Hwang, I.K. Quality attributes of various varieties of Korean red pepper powders (Capsicum annuum L.) and color stability during sunlight exposure. J. Food Sci. 2002, 67, 2957–2961. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Angosto, J.M.; Giménez, P.J.; León, G. Thermal stability of selected natural red extracts used as food colorants. Plant Foods Hum. Nutr. 2013, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Aurelio, D.L.; Edgardo, R.G.; Navarro-Galindo, S. Thermal kinetic degradation of anthocyanins in a roselle (Hibiscus sabdariffa L. cv. ‘Criollo’) infusion. Int. J. Food Sci. Technol. 2008, 43, 322–325. [Google Scholar] [CrossRef]
- Aramwit, P.; Bang, N.; Srichana, T. The properties and stability of anthocyanins in mulberry fruits. Food Res. Int. 2010, 43, 1093–1097. [Google Scholar] [CrossRef]
- Wang, W.-D.; Xu, S.-Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Cemeroglu, B.; Velioglu, S.; Isik, S. Degradation kinetics of anthocyanins in sour cherry juice and concentrate. J. Food Sci. 1994, 59, 1216–1218. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, D.; Song, X.; Deng, Y.; Zhao, Y. Degradation kinetics and antioxidant capacity of anthocyanins in air-impingement jet dried purple potato slices. Food Res. Int. 2018, 105, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V.; Sri Harsha, P.S.C.; Spigno, G. Modelling the stability of maltodextrin-encapsulated grape skin phenolics used as a new ingredient in apple puree. Food Chem. 2016, 209, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: not available. |



| pH 1 | pH 2 | pH 3 | pH 4 | pH 5 | pH 6 | pH 7 | |
|---|---|---|---|---|---|---|---|
| L | 54.34 ± 0.03 | 58.32 ± 0.04 | 57.72 ± 0.03 | 69.36 ± 0.04 | 78.83 ± 0.07 | 75.25 ± 0.09 | 46.43 ± 0.12 |
| a | 57.85 ± 0.14 | 57.72 ± 0.06 | 50.21 ± 0.03 | 35.88 ± 0.04 | 16.52 ± 0.05 | 11.14 ± 0.09 | 19.26 ± 0.06 |
| b | 26.70 ± 0.03 | 24.80 ± 0.09 | 20.18 ± 0.08 | 12.61 ± 0.02 | 10.56 ± 0.01 | 12.46 ± 0.10 | 6.44 ± 0.17 |
| Temperature (°C) | Time (min) | pH 1 | pH 2 | pH 3 | pH 4 | pH 5 | pH 6 | pH 7 |
|---|---|---|---|---|---|---|---|---|
| 70 | 30 | 1.78 | 0.88 | 2.83 | 4.11 | 3.46 | 6.57 | 30.03 |
| 60 | 0.65 | 1.33 | 3.81 | 4.56 | 3.57 | 7.42 | 34.79 | |
| 90 | 0.68 | 1.21 | 4.25 | 5.35 | 4.49 | 8.54 | 36.67 | |
| 120 | 0.69 | 1.97 | 5.31 | 5.73 | 5.01 | 9.32 | 36.47 | |
| 80 | 30 | 1.29 | 1.00 | 3.14 | 2.67 | 4.83 | 7.48 | 24.56 |
| 60 | 1.49 | 2.02 | 5.17 | 3.70 | 6.14 | 9.86 | 27.05 | |
| 90 | 14.46 | 3.32 | 6.71 | 5.53 | 7.02 | 11.75 | 32.84 | |
| 120 | 17.08 | 4.50 | 8.92 | 6.66 | 7.54 | 13.20 | 33.51 | |
| 90 | 30 | 3.51 | 2.19 | 12.09 | 10.07 | 8.10 | 10.26 | 22.76 |
| 60 | 6.45 | 4.96 | 18.02 | 12.13 | 9.10 | 12.45 | 22.05 | |
| 90 | 10.27 | 7.37 | 22.62 | 14.87 | 10.17 | 12.26 | 22.36 | |
| 120 | 13.93 | 13.41 | 26.03 | 15.41 | 11.76 | 16.47 | 25.96 |
| pH | Temperatures (°C) | k (min−1) | t1/2 (min) | Ea (kJ/mol) | Arrhenius Equation (R2) |
|---|---|---|---|---|---|
| 1 | 70 | 6.0 × 10−4 | 1155.2 | 95.7 | y = −11509x + 26.18 (0.991) |
| 80 | 1.8 × 10−3 | 385.1 | |||
| 90 | 3.8 × 10−3 | 182.4 | |||
| 2 | 70 | 1.0 × 10−3 | 693.1 | 75.6 | y = −9090.3x + 19.62 (0.996) |
| 80 | 2.3 × 10−3 | 301.4 | |||
| 90 | 4.3 × 10−3 | 161.2 | |||
| 3 | 70 | 1.4 × 10−3 | 495.1 | 61.6 | y = −7411x + 15.05 (0.998) |
| 80 | 2.7 × 10−3 | 256.7 | |||
| 90 | 4.6 × 10−3 | 150.7 | |||
| 4 | 70 | 1.9 × 10−3 | 364.8 | 55.8 | y = −6709.4x + 13.24 (0.969) |
| 80 | 2.8 × 10−3 | 247.6 | |||
| 90 | 5.6 × 10−3 | 123.8 | |||
| 5 | 70 | 2.2 × 10−3 | 315.1 | 53.6 | y = −6445.4x + 12.66 (0.998) |
| 80 | 3.6 × 10−3 | 192.5 | |||
| 90 | 6.2 × 10−3 | 111.8 | |||
| 6 | 70 | 5.3 × 10−3 | 130.8 | 74.9 | y = −9000.3x + 21.00 (0.999) |
| 80 | 1.2 × 10−2 | 59.2 | |||
| 90 | 2.3 × 10−2 | 30.8 | |||
| 7 | 70 | 2.8 × 10−2 | 24.8 | 31.4 | y = −3773.7x + 7.41 (0.984) |
| 80 | 3.6 × 10−2 | 19.4 | |||
| 90 | 5.1 × 10−2 | 13.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-Y.; Yang, K.-M.; Chiang, P.-Y. Roselle Anthocyanins: Antioxidant Properties and Stability to Heat and pH. Molecules 2018, 23, 1357. https://doi.org/10.3390/molecules23061357
Wu H-Y, Yang K-M, Chiang P-Y. Roselle Anthocyanins: Antioxidant Properties and Stability to Heat and pH. Molecules. 2018; 23(6):1357. https://doi.org/10.3390/molecules23061357
Chicago/Turabian StyleWu, Hai-Yao, Kai-Min Yang, and Po-Yuan Chiang. 2018. "Roselle Anthocyanins: Antioxidant Properties and Stability to Heat and pH" Molecules 23, no. 6: 1357. https://doi.org/10.3390/molecules23061357