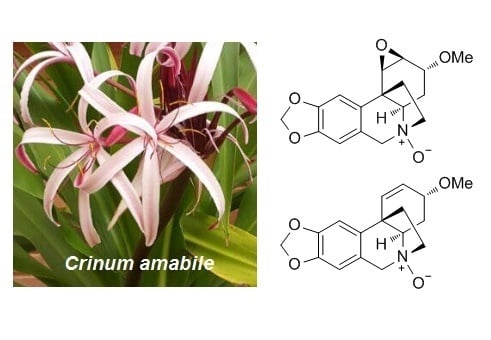
N-oxide alkaloids from Crinum amabile (Amaryllidaceae)
Abstract
:1. Introduction
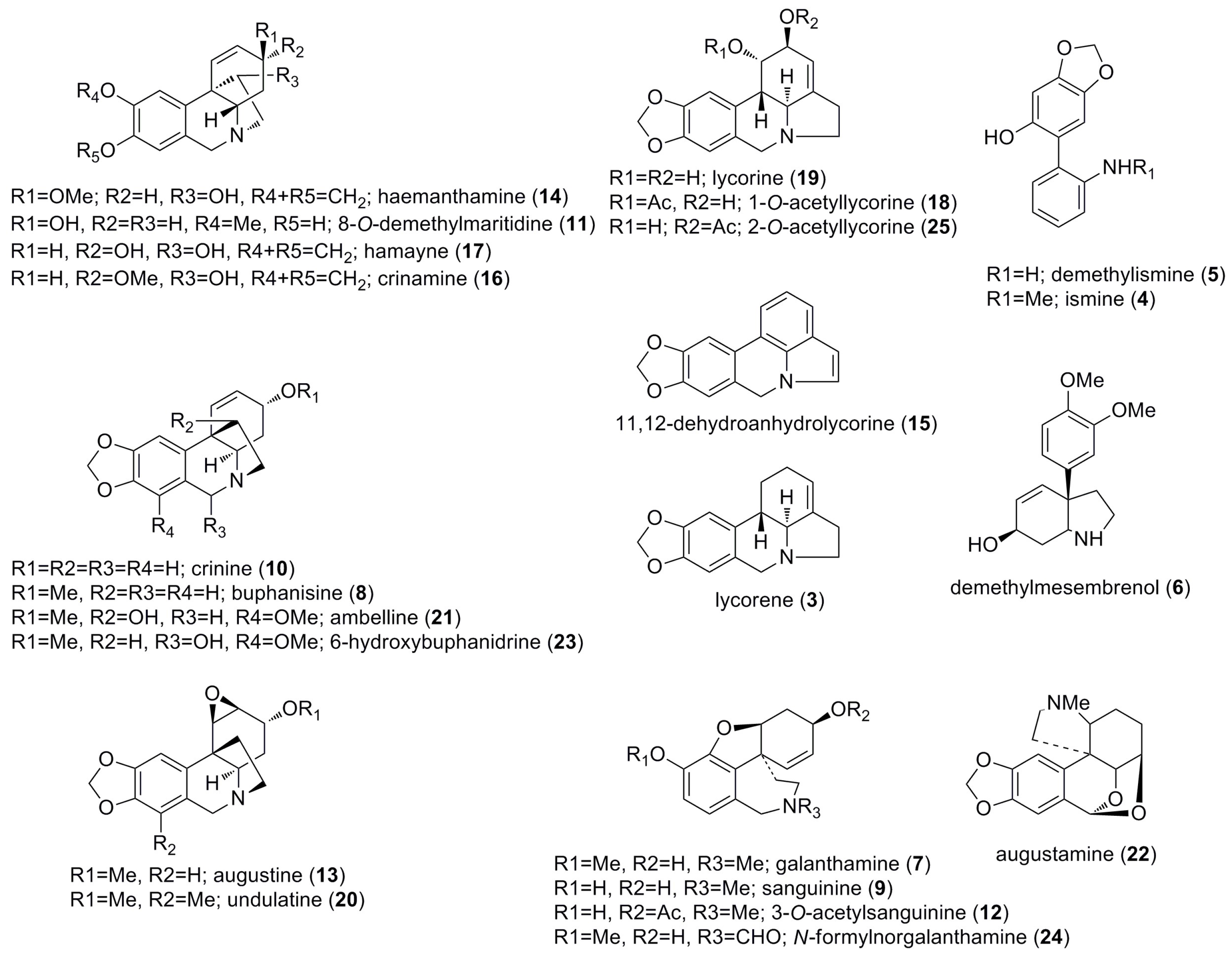
2. Results and Discussion
2.1. Alkaloids Identified by GC-MS
2.2. Structural Elucidation
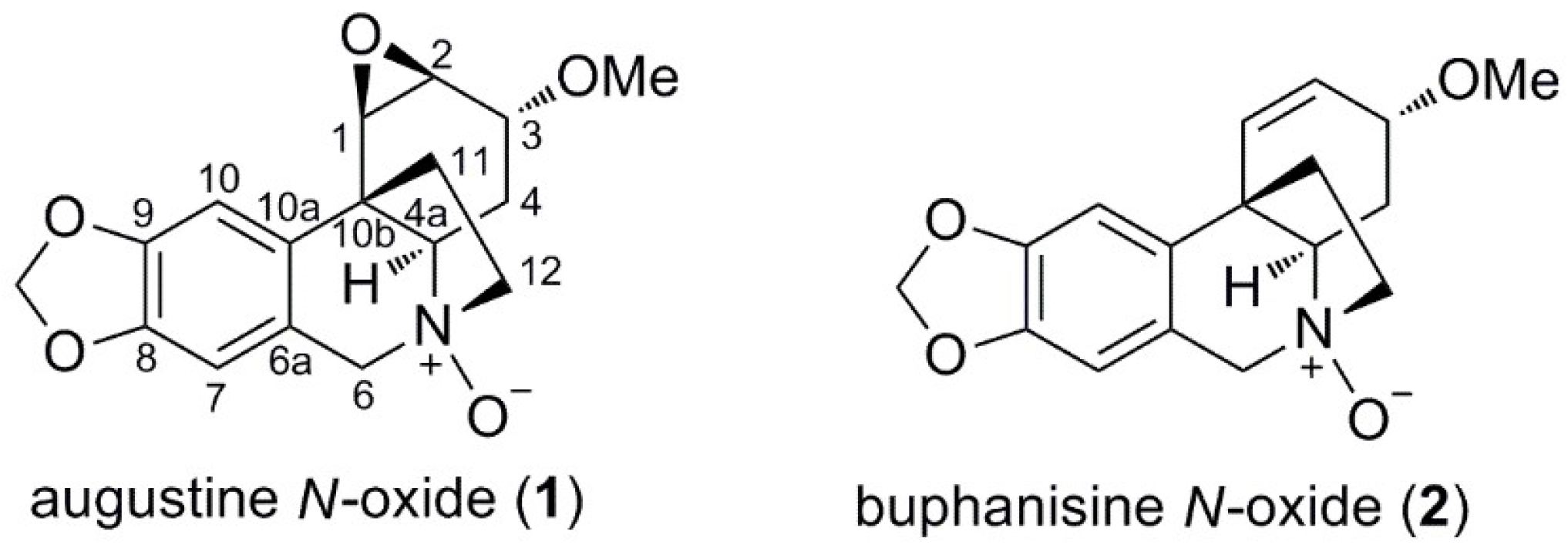
2.2.1. Augustine N-oxide (1)
2.2.2. Buphanisine N-oxide (2)
2.3. Biological Activities
2.3.1. AChE and BuChE Inhibitory Activities
2.3.2. Antiprotozoal Activity
3. Materials and Methods
3.1. Plant Material
3.2. Equipment
3.3. Extraction
3.4. Characterization of Compounds
3.5. Biological Activities
3.5.1. Antiprotozoal Activities
3.5.2. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.; Lavilla, R.; Viladomat, F. Chemical and biological aspects of Narcissus Alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Elsevier: Amsterdam, Netherlands, 2006; Volume 63, pp. 87–179. [Google Scholar]
- Maelicke, A.; Samochocki, M.; Jostock, R.; Fehrenbacher, A.; Ludwig, J.; Albuquerque, E.X.; Zerlin, M. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol. Psychiat. 2001, 49, 279–288. [Google Scholar] [CrossRef]
- APG III. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar]
- Rønsted, N.; Symonds, M.R.E.; Birkholm, T.; Christensen, S.B.; Meerow, A.W.; Molander, M.; Mølgaard, P.; Petersen, G.; Rasmussen, N.; van Staden, J.; et al. Can phylogeny predict chemical diversity and potential medicinal activity of plants? A case study of Amaryllidaceae. BMC Evol. Biol. 2012, 12, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Fennell, C.W.; van Staden, J. Crinum species in traditional and modern medicine. J. Ethnopharmacol. 2001, 78, 15–26. [Google Scholar] [CrossRef]
- Tram, N.T.N.; Titorenkova, T.V.; Bankova, V.S.; Handjieva, N.V.; Popov, S.S. Crinum L. (Amaryllidaceae). Fitoterapia 2002, 73, 183–208. [Google Scholar] [CrossRef]
- Kwembeya, E.G.; Bjora, C.S.; Stedje, B.; Nordal, I. Phylogenetic relationships in the genus Crinum (Amaryllidaceae) with emphasis on tropical African species: Evidence from trnL-F and nuclear ITS DNA sequence data. Taxon 2007, 56, 801–810. [Google Scholar] [CrossRef]
- Maroyi, A. A review of ethnobotany, therapeutic value, phytochemistry and pharmacology of Crinum macowanii Baker: A highly traded bulbous plant in Southern Africa. J. Ethnopharmacol. 2016, 194, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Presley, C.C.; Krai, P.; Dalal, S.; Su, Q.; Cassera, M.; Goetz, M.; Kingston, D.G.I. New potently bioactive alkaloids from Crinum erubescens. Bioorgan. Med. Chem. 2016, 24, 5418–5422. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.H.; Döpke, W.; Wagner, J.; Mügge, C. Alkaloids from Crinum amabile. Phytochemistry 1998, 48, 371–376. [Google Scholar] [CrossRef]
- Murav’eva, D.A.; Popova, O.I. Alkaloid composition of the bulbs of Crinum amabile. Khim. Prir. Soedin. 1982, 2, 263–264. [Google Scholar]
- Likhitwitayawuid, K.; Angerhofer, C.K.; Chai, H.; Pezzuto, J.M.; Cordell, G.A. Cytotoxic and antimalarial alkaloids from the bulbs of Crinum amabile. J. Nat. Prod. 1993, 56, 1331–1338. [Google Scholar] [CrossRef]
- WHOa. World Health Organization Neglected Tropical Diseases. Available online: http://www.who.int/neglected_diseases/diseases/en/ (accessed on 4 May 2018).
- Klug, D.M.; Gelb, M.H.; Pollastri, M.P. Repursposing strategies for tropical disease drug discovery. Bioorg. Med. Chem. Lett. 2016, 26, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- WHOb. World Health Organization Dementia. Available online: http://www.who.int/en/news-room/fact-sheets/detail/dementia (accessed on 4 May 2018).
- Wu, W.Y.; Dai, Y.C.; Li, N.G.; Dong, Z.X.; Gu, T.; Shi, Z.H.; Xue, X.; Tang, Y.P.; Duan, J.A. Novel multitarget-directed tacrine derivatives as potential candidates for the treatment of Alzheimer’s disease. J. Enzym. Inhib. Med. Ch. 2017, 32, 572–587. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, J.P.; Pigni, N.B.; Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Bioactive alkaloid extracts from Narcissus broussonetii: Mass spectral studies. J. Pharmaceut. Biomed. 2012, 70, 13–25. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, J.P.; Guo, Y.; Font-Bardia, M.; Calvet, T.; Dutilh, J.; Viladomat, F.; Codina, C.; Nair, J.J.; Zuanazzi, J.A.S.; Bastida, J. Crinine-type alkaloids from Hippeastrum aulicum and H. calyptratum. Phytochemistry 2014, 103, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Metabolomic analysis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC-MS combined with k-means cluster analysis. Ind. Crop. Prod. 2014, 56, 211–222. [Google Scholar] [CrossRef]
- Tallini, L.R.; de Andrade, J.P.; Kaiser, M.; Viladomat, F.; Nair, J.J.; Zuanazzi, J.A.S.; Bastida, J. Alkaloid constituents of the Amaryllidaceae plant Amaryllis belladonna L. Molecules 2017, 22, 1437. [Google Scholar] [CrossRef] [PubMed]
- Tallini, L.R.; Osorio, E.H.; dos Santos, V.D.; Borges, W.D.S.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. Hippeastrum reticulatum (Amaryllidaceae): Alkaloids profiling, biological activities and molecular docking. Molecules 2017, 22, 2191. [Google Scholar]
- Phillipson, J.D.; Handa, S.S.; El-Dabbas, S.W. N-Oxides of morphine, codeine and thebaine and their occurrence in Papaver species. Phytochemistry 1976, 15, 1297–1301. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring plant isoquinoline N-oxide alkaloids: Their pharmacological and SAR activities. Phytomedicine 2015, 22, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Suau, R.; Gómez, A.I.; Rico, R.; Tato, M.P.V.; Castedo, L.; Riguera, R. Alkaloids N-oxides of Amaryllidaceae. Phytochemistry 1988, 27, 3285–3287. [Google Scholar] [CrossRef]
- Kobayashi, S.; Satoh, K.; Numata, A.; Shingu, T.; Kihara, M. Alkaloid N-oxides from Lycoris sanguinea. Phytochemistry 1991, 675–677. [Google Scholar] [CrossRef]
- Bessa, C.D.P.B.; de Andrade, J.P.; de Oliveira, R.S.; Domingos, E.; Heloa, S.; Romão, W.; Bastida, J.; Borges, W.S. Identification of Alkaloids from Hippeastrum aulicum (Ker Gawl.) Herb. (Amaryllidaceae) Using CGC-MS and Ambient Ionization Mass Spectrometry (PS-MS and LS-MS). J. Braz. Chem. Soc. 2017, 28, 819–830. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Anh, D.H.; Huong, P.T.T.; Thanh, N.V.; Trung, N.Q.; Cuong, T.V.; Mai, N.T.; Cuong, N.T.; Cuong, N.X.; Nam, N.H.; Minh, C.V. Crinane, augustamine, and β-carboline alkaloids from Crinum latifolium. Phytochem. Lett. 2018, 24, 27–30. [Google Scholar] [CrossRef]
- Frahm, A.W.; Ali, A.A.; Kating, H. Relative configuration of the alkaloid augustine. Phytochemistry 1981, 20, 1735–1738. [Google Scholar] [CrossRef]
- Viladomat, F.; Codina, C.; Bastida, J.; Mathee, S.; Campbell, W.E. Further alkaloids from Brunsvigia josephinae. Phytochemistry 1995, 40, 961–965. [Google Scholar] [CrossRef]
- Ali, A.A.; Hambloch, H.; Frahm, A.W. Relative configuration of the alkaloid augustamine. Phytochemistry 1983, 22, 283–287. [Google Scholar] [CrossRef]
- Machocho, A.K.; Bastida, J.; Codina, C.; Viladomat, F.; Brun, R.; Chhabra, S.C. Augustamine type alkaloids from Crinum kirkii. Phytochemistry 2004, 65, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.S.; Pelletier, S.W.; Ali, A.A.; Holt, E.M.; Bowen, J.P.; Ehlers, T. Crystal and molecular structure of augustamine. J. Chem. Crystallogr. 2000, 30, 135–138. [Google Scholar] [CrossRef]
- Coll, J.C.; Bowden, B.F. The application of vacuum liquid chromatography to the separation of terpene mixtures. J. Nat. Prod. 1986, 49, 934–936. [Google Scholar] [CrossRef]
- Targett, N.M.; Kilcoyne, J.P.; Green, B. Vacuum liquid chromatography: An alternative to common chromatographic methods. J. Org. Chem. 1979, 44, 4962–4964. [Google Scholar] [CrossRef]
- Orhan, I.; Şener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs. 2010, 8, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Alkaloid | RI | M+ | MS |
|---|---|---|---|
| Lycorene (3) | 2102.2 | 255 (52) | 254 (100), 227 (17), 226(20), 211 (15), 183(14), 181(10) |
| Ismine (4) | 2124.3 | 257 (28) | 239(16), 238 (100), 196 (10), 168 (10) |
| Demethylismine (5) | 2128.8 | 243 (22) | 225(21), 224 (100), 167 (10), 166 (15), 154 (11), 77 (12) |
| Demethylmesembrenol (6) | 2177.0 | 275 (5) | 206 (9), 205 (76), 115 (6), 70 (100) |
| Galanthamine (7) | 2262.8 | 287 (85) | 286 (100), 244(29), 216 (45), 174(39), 165(16), 141 (14), 128 (21), 115 (31) |
| Buphanisine (8) | 2283.7 | 285 (95) | 273 (54), 272 (43), 254 (40), 215 (100), 157 (39), 129 (35), 128 (55), 115 (64) |
| Sanguinine (9) | 2288.3 | 273 (100) | 272 (85), 202 (40), 165(20), 160 (50), 131 (20), 128 (19),115 (28), 77(20), |
| Crinine (10) | 2326.7 | 271 (100) | 228 (24), 200 (30), 199(81), 187 (76), 173 (28), 129 (34), 128(44), 115 (47), 56 (32) |
| 8-O-Demethylmaritidine (11) | 2373.8 | 273 (100) | 230 (25), 202 (29), 201 (80), 189 (65), 175 (29), 129 (24), 128 (30), 115 (32), 56 (30) |
| 3-O-Acetylsanguinine (12) | 2387.1 | 315 (37) | 256 (100), 255 (42), 254 (37), 212(26), 165 (33), 152 (23), 115 (30), 96 (67) |
| Augustine (13) | 2411.6 | 301 (93) | 228 (36), 187 (30), 175 (300), 173 (24), 159 (38), 143 (57), 128 (259, 115 (75) |
| Buphanisine N-oxide (2) | 2429.8 | 301 (nv) | 285 (100), 270 (33), 254 (35), 216 (21), 215 (82), 201 (24), 157 (20), 128 (22) |
| Haemanthamine (14) | 2436.9 | 301 (55) | 257 (54), 227 (80), 225 (98), 224(80), 181 (100), 153 (46), 152 (46), 115 (64) |
| 11,12-Dehydroanhydrolycorine (15) | 2448.5 | 249 (55) | 248 (100), 191 (13), 190 (31), 189 (11), 95 (14) |
| Crinamine (16) | 2497.6 | 273 (17) | 272 (100), 242 (12), 214 (11), 186 (12), 128 (15) |
| Hamayne (17) | 2551.7 | 259 (14) | 258 (100), 242 (11), 214 (10), 211 (12), 181 (14), 128 (19) |
| 1-O-Acetyllycorine (18) | 2563.1 | 329 (20) | 299(15), 268 (28), 250 (17), 244 (20), 227 (56), 226 (100), 240 (11) |
| Augustine N-oxide (1) | 2571.8 | 317 (nv) | 301 (100), 228 (34), 187 (22), 175 (77), 173 (17), 159 (27), 143 (37), 115 (37) |
| Lycorine (19) | 2592.2 | 287 (19) | 286 (13), 268 (18), 250 (10), 227 (60), 226 (100), 147 (11) |
| Undulatine (20) | 2594.4 | 331 (100) | 258 (37), 219 (22), 217 (36), 205 (71), 203 (37), 189 (43), 173 (39), 115 (35) |
| Ambelline (21) | 2621.1 | 331 (69) | 287 (100), 260 (81), 257 (62), 255 (74), 254 (52), 241 (51), 239 (61), 211 (69) |
| Augustamine (22) | 2628.7 | 301 (76) | 300 (100), 245(16), 244(84), 215(33), 201 (32), 188 (14), 115 (22), 70 (21) |
| 6-Hydroxybuphanidrine (23) | 2631.3 | 331 (35) | 277 (16), 276 (100), 261 (30), 218 (17), 217 (23), 216 (24), 115 (18), 56 (25) |
| N-Formylnorgalanthamine (24) | 2649.1 | 301 (100) | 225 (26), 211 (29), 181 (19), 165 (14), 129 (18), 128 (22), 115 (30), 77 (15) |
| 2-O-Acetyllycorine (25) | 2676.2 | 329 (21) | 328 (17), 270 (40), 269 (72), 268 (100), 252 (43), 250 (73), 227 (27), 226 (67) |
| 1 | 2 | |||
|---|---|---|---|---|
| No. | δC, type | δH (J in Hz) | δC, type | δH (J in Hz) |
| 1 | 52.06 | 3.68, d (3.5) | 129.59 | 6.39, d (10.0) |
| 2 | 55.06 | 3.42, ddd (3.2, 2.4, 0.7) | 127.18 | 6.08, ddd (10.0, 5.3, 1.0) |
| 3 | 73.43 | 4.12, dd (2.7, 2.5) | 71.38 | 3.95, ddd (5.6, 3.6, 2.0) |
| 4α | 19.72 | 2.91, dt (14.1, 3.1) | 23.46 | 3.13 ddt (13.6, 4.2, 2.4) |
| 4β | 19.72 | 1.54, ddd (13.8, 13.8, 2.9) | 23.46 | 1.72 ddd (13.7, 13.7, 4.0) |
| 4a | 72.59 | 3.51, dd (13.4, 3.6) | 74.14 | 3.74, dd (13.2, 4.3) |
| 6α | 67.56 | 4.83, dd (15.7, 1.8) | 76.55 | 4.84, d (15.6) |
| 6β | 67.56 | 4.68, d (15.7) | 76.55 | 4.72, d (15.6) |
| 6a | 122.33 | - | 121.71 | - |
| 7 | 106.40 | 6.57, s | 106.44 | 6.54, s |
| 8 | 147.13 | - | 147.20 | - |
| 9 | 147.79 | - | 147.79 | - |
| 10 | 102.49 | 6.90, s | 102.98 | 6.81, s |
| 10a | 133.92 | - | 134.75 | - |
| 10b | 43.95 | - | 46.60 | - |
| 11endo | 35.64 | 1.99, ddd (12.4, 9.4, 5.1) | 40.02 | 2.11, ddd (12.5, 8.0, 6.0) |
| 11exo | 35.64 | 2.79, ddd (12.4, 12.4, 6.9) | 40.02 | 2.26, ddd (12.2, 10.8, 8.6) |
| 12endo | 67.56 | 3.81, ddd (12.8, 9.4, 7.0) | 68.97 | 3.88, m |
| 12exo | 67.56 | 3.73, dddd (12.5,12.5, 5.0, 2.2) | 68.97 | 3.85, m |
| OCH2O | 101.58 | 5.99, d (1.3), 5.98 d (1.3) | 101.51 | 5.95, d (1.3), 5.93 d (1.3) |
| OMe | 57.92 | 3.47, s | 57.23 | 3.39, s |
| Alkaloid | AchE * | BuChE * |
|---|---|---|
| Augustine N-oxide (1) | 79.64 ± 5.26 | >200 |
| Buphanisine N-oxide (2) | >200 | >200 |
| Agustamine (22) | >200 | >200 |
| Augustine (13) | 45.26 ± 2.11 | >200 |
| Buphanisine (8) | 183.31 ± 36.64 | >200 |
| Crinine (10) | 163.89 ± 15.69 | >200 |
| Galanthamine (7) | 0.45 ± 0.03 | 3.88 ± 0.19 |
| Parasite | T. brucei rhodesiense | T. cruzi | L. donovani | P. falciparum | Cytotoxicity |
|---|---|---|---|---|---|
| Reference drug | 0.003 ± 0.001 a | 0.865 ± 0.08 b | 0.515 ± 0.06 c | 0.004 ± 0.0007 d | 0.004 ± 0.0007 e |
| Augustine (13) | 15.05 ± 1.06 | 56.00 ± 0.71 | >100 | 14.20 ± 0.14 | >100 |
| Augustine N-oxide (1) | 58.85 ± 11.53 | 66.25 ± 11.81 | >100 | 36.65 ± 4.74 | >100 |
| Buphanisine (8) | 16.5 ± 0.57 | 55.55 ± 4.60 | >100 | 4.28 ± 0.18 | 72.85 ± 5.02 |
| Buphanisine N-oxide (2) | 55.25 ± 4.31 | 64.05 ± 1.34 | >100 | 32.55 ± 0.07 | >100 |
| Crinine (10) | 18.95 ± 0.78 | 57.45 ± 6.86 | >100 | 30.95 ± 2.19 | >100 |
| Augustamine (22) | 19.20 ± 2.97 | 54.00 ± 4.53 | >100 | 20.35 ± 0.21 | 81.55 ± 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallini, L.R.; Torras-Claveria, L.; Borges, W.D.S.; Kaiser, M.; Viladomat, F.; Zuanazzi, J.A.S.; Bastida, J. N-oxide alkaloids from Crinum amabile (Amaryllidaceae). Molecules 2018, 23, 1277. https://doi.org/10.3390/molecules23061277
Tallini LR, Torras-Claveria L, Borges WDS, Kaiser M, Viladomat F, Zuanazzi JAS, Bastida J. N-oxide alkaloids from Crinum amabile (Amaryllidaceae). Molecules. 2018; 23(6):1277. https://doi.org/10.3390/molecules23061277
Chicago/Turabian StyleTallini, Luciana R., Laura Torras-Claveria, Warley De Souza Borges, Marcel Kaiser, Francesc Viladomat, José Angelo S. Zuanazzi, and Jaume Bastida. 2018. "N-oxide alkaloids from Crinum amabile (Amaryllidaceae)" Molecules 23, no. 6: 1277. https://doi.org/10.3390/molecules23061277