d-Amino Acid Peptide Residualizing Agents for Protein Radioiodination: Effect of Aspartate for Glutamate Substitution
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Single Domain Antibody Fragment, Cells, and Culture Conditions
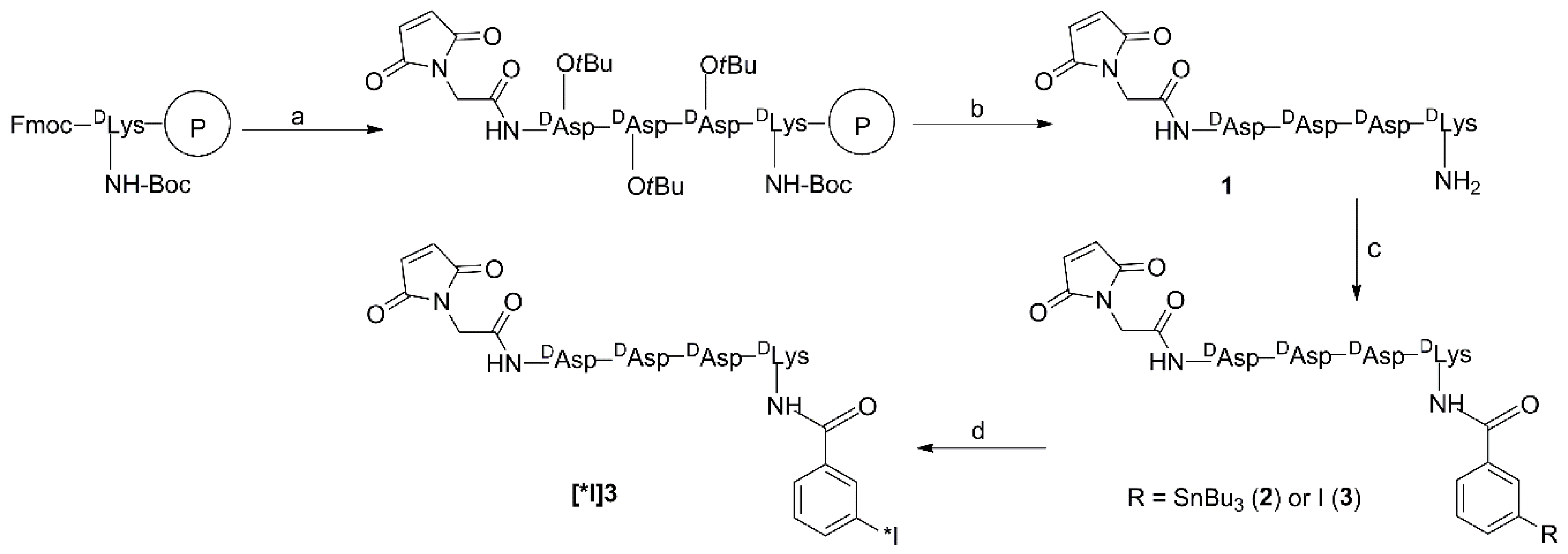
3.3. Synthesis
3.3.1. Mal-d-GDDDK (1)
3.3.2. TB-Mal-d-GDDDK (2)
3.3.3. IB-Mal-d-GDDDK (3)
3.4. Radiochemistry
3.5. Labeling of sdAb
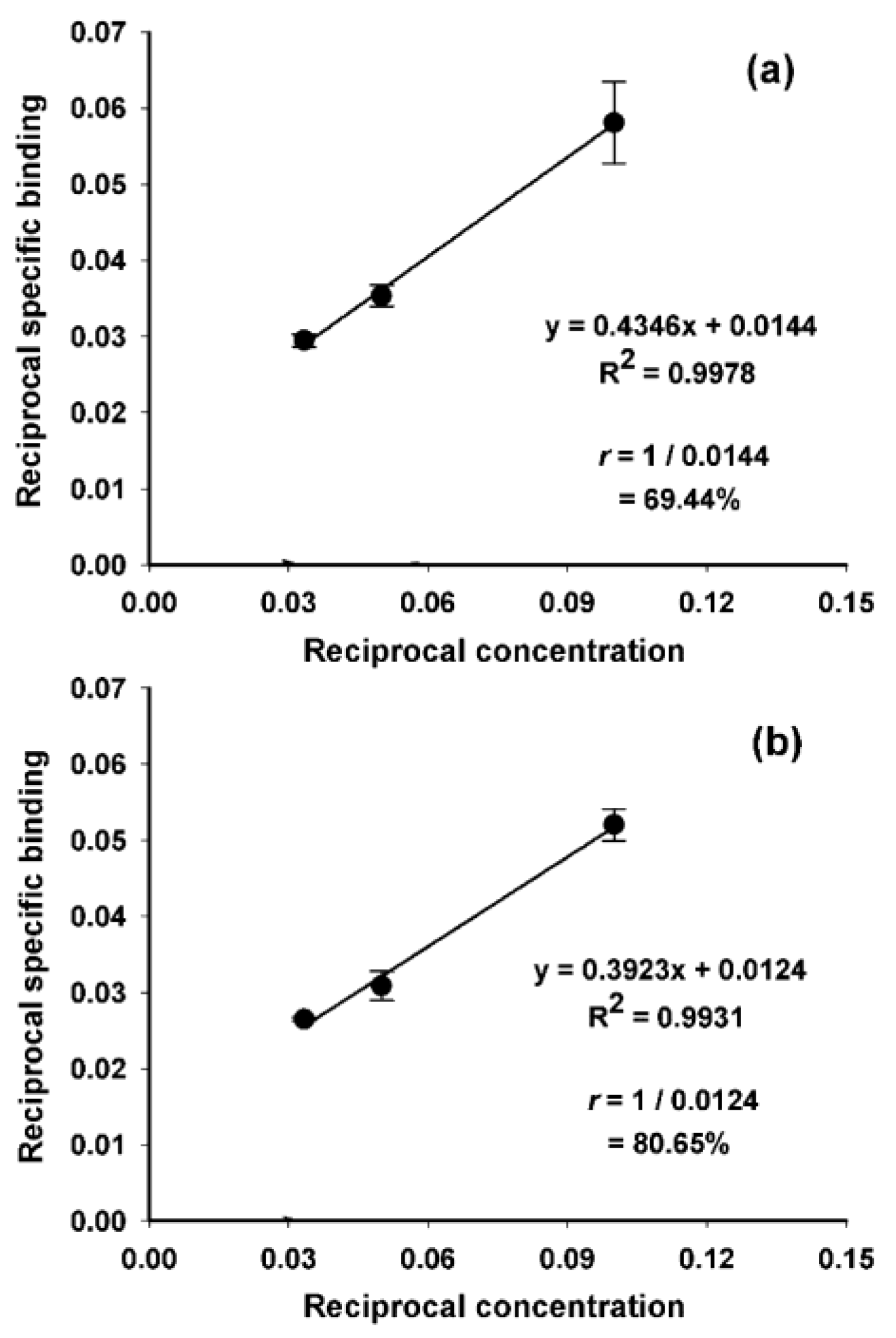
3.6. Evaluation of Protein-Associated Activity and Immunoreactivity
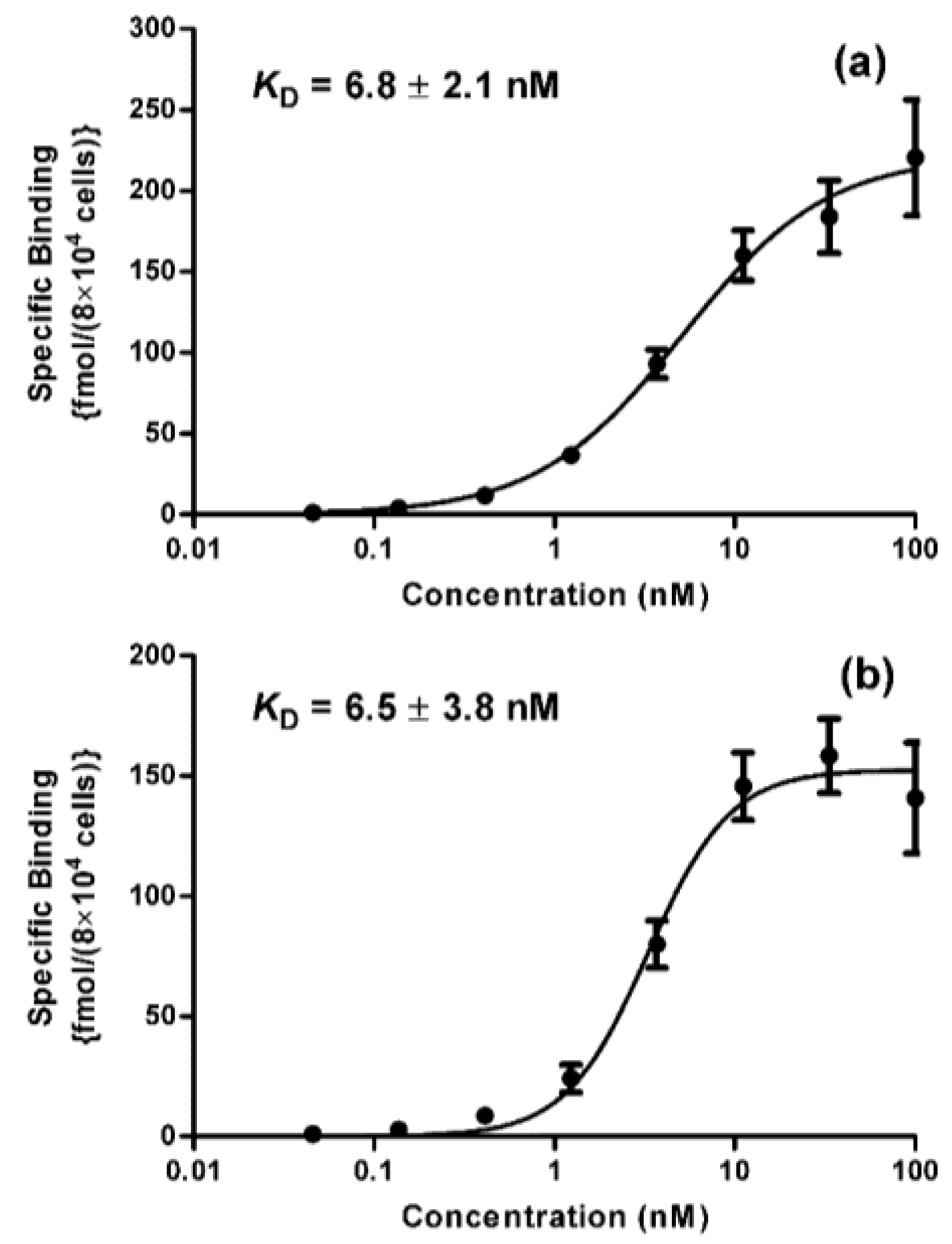
3.7. Determination of Binding Affinity (KD)
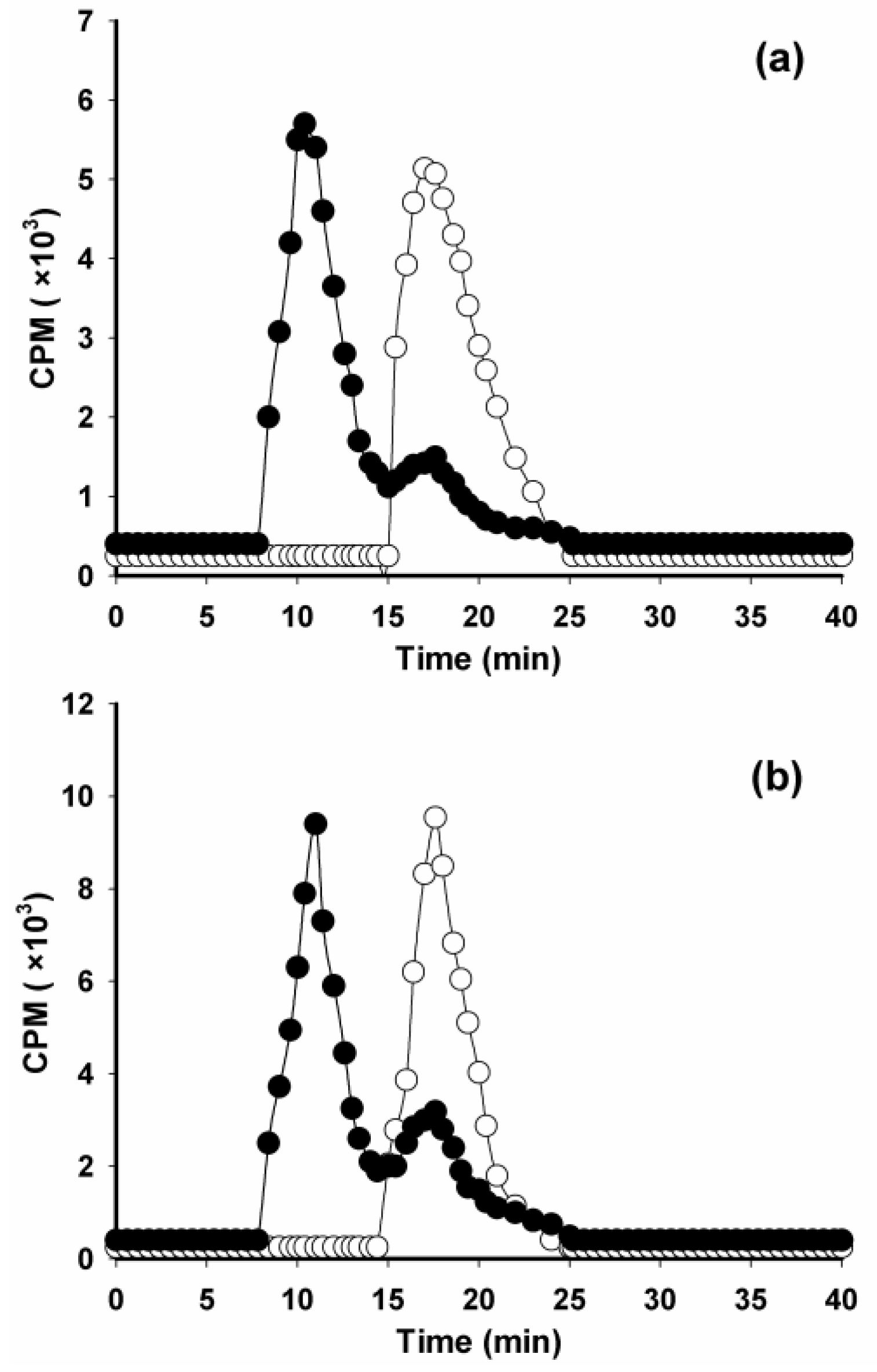
3.8. Paired-Label Internalization Assay
3.9. Paired-Label Biodistribution
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Imam, S.K. Molecular nuclear imaging: The radiopharmaceuticals (review). Cancer Biother. Radiopharm. 2005, 20, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Dixit, M. Metallic radionuclides in the development of diagnostic and therapeutic radiopharmaceuticals. Dalton Trans. 2011, 40, 6112–6128. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Stone-Elander, S. Radiolabelled proteins for positron emission tomography: Pros and cons of labelling methods. Biochim. Biophys. Acta 2010, 1800, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Garmestani, K.; Milenic, D.E.; Plascjak, P.S.; Brechbiel, M.W. A new and convenient method for purification of 86Y using a Sr(II) selective resin and comparison of biodistribution of 86Y and 111In labeled Herceptin. Nucl. Med. Biol. 2002, 29, 599–606. [Google Scholar] [CrossRef]
- Orlova, A.; Höglund, J.; Lubberink, M.; Lebeda, O.; Gedda, L.; Lundqvist, H.; Tolmachev, V.; Sundin, A. Comparative biodistribution of the radiohalogenated (Br, I and At) antibody A33. Implication for in vivo dosimetry. Cancer Biother. Radiopharm. 2002, 17, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Helisch, A.; Förster, G.J.; Reber, H.; Buchholz, H.G.; Arnold, R.; Göke, B.; Weber, M.M.; Wiedenmann, B.; Pauwels, S.; Haus, U.; et al. Pre-therapeutic dosimetry and biodistribution of 86Y-DOTA-Phe1-Tyr3-octreotide versus 111In-pentetreotide in patients with advanced neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.K.; Harrison, C.L.; Zalutsky, M.R. Comparative tissue distribution in mice of the α-emitter 211At and 131I as labels of a monoclonal antibody and F(ab′)2 fragment. Cancer Res. 1990, 50, 3514–3520. [Google Scholar] [PubMed]
- Foulon, C.F.; Reist, C.J.; Bigner, D.D.; Zalutsky, M.R. Radioiodination via d-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing anti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Res. 2000, 60, 4453–4460. [Google Scholar] [PubMed]
- Tolmachev, V.; Orlova, A.; Lundqvist, H. Approaches to improve cellular retention of radiohalogen labels delivered by internalising tumour-targeting proteins and peptides. Curr. Med. Chem. 2003, 10, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.; Govindan, S.V.; Mattes, M.J.; Chen, S.; Reed, L.; Newsome, G.; McBride, B.J.; Griffiths, G.L.; Hansen, H.J.; Goldenberg, D.M. Improved iodine radiolabels for monoclonal antibody therapy. Cancer Res. 2003, 63, 111–118. [Google Scholar] [PubMed]
- Vaidyanathan, G.; White, B.J.; Affleck, D.J.; Zhao, X.G.; Welsh, P.C.; McDougald, D.; Choi, J.; Zalutsky, M.R. SIB-DOTA: A trifunctional prosthetic group potentially amenable for multi-modal labeling that enhances tumor uptake of internalizing monoclonal antibodies. Bioorg. Med. Chem. 2012, 20, 6929–6939. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Marik, J.; Elowson, M.J.; Reyes, N.A.; Ulufatu, S.; Bumbaca, D.; Yip, V.; Mundo, E.E.; Majidy, N.; Van Hoy, M.; et al. Enhanced tumor retention of a radiohalogen label for site-specific modification of antibodies. J. Med. Chem. 2013, 56, 9418–9426. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; McDougald, D.; Pruszynski, M.; Osada, T.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. N-succinimidyl guanidinomethyl iodobenzoate protein radiohalogenation agents: Influence of isomeric substitution on radiolabeling and target cell residualization. Nucl. Med. Biol. 2014, 41, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Alston, K.L.; Bigner, D.D.; Zalutsky, M.R. Nϵ-(3-[*I]iodobenzoyl)-Lys5-Nα-maleimido-Gly1-GEEEK ([*I]IB-Mal-d-GEEEK): A radioiodinated prosthetic group containing negatively charged d-glutamates for labeling internalizing monoclonal antibodies. Bioconjugate Chem. 2006, 17, 1085–1892. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; Jestin, E.; Olafsen, T.; Wu, A.M.; Zalutsky, M.R. Evaluation of an anti-p185HER2 (scFv-CH2-CH3)2 fragment following radioiodination using two different residualizing labels: SGMIB and IB-Mal-d-GEEEK. Nucl. Med. Biol. 2009, 36, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sapienza, G.; Rossotti, M.A.; Tabares-da Rosa, S. Single-domain antibodies as versatile affinity reagents for analytical and diagnostic applications. Front. Immunol. 2017, 8, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Zalutsky, M.R. Targeting breast carcinoma with radioiodinated anti-HER2 nanobody. Nucl. Med. Biol. 2013, 40, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med. 2014, 55, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Maack, T.; Park, C.H.; Camargo, M.J.F. Renal filtration, transport, and metabolism of proteins. In The Kidney: Physiology and Pathophysiology, 2nd ed.; Seldin, D.W., Giebisch, G., Eds.; Raven Press: New York, NY, USA, 1992; pp. 3005–3038. [Google Scholar]
- Miao, Y.; Fisher, D.R.; Quinn, T.P. Reducing renal uptake of 90Y- and 177Lu-labeled alpha-melanocyte stimulating hormone peptide analogues. Nucl. Med. Biol. 2006, 33, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Örlefors, H.; Bergström, M.; Antoni, G.; Omura, H.; Eriksson, B.; Watanabe, Y.; Långström, B. Uptake of 14C- and 11C-labeled glutamate, glutamine and aspartate in vitro and in vivo. Anticancer Res. 2000, 20, 251–256. [Google Scholar] [PubMed]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Chitneni, S.; Zalutsky, M.R. d-amino acid peptide residualizing agents bearing N-hydroxysuccinimido- and maleimido-functional groups and their application for trastuzumab radioiodination. Nucl. Med. Biol. 2015, 42, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; McDougald, D.; Choi, J.; Koumarianou, E.; Weitzel, D.; Osada, T.; Lyerly, H.K.; Zalutsky, M.R. Preclinical evaluation of 18F-labeled anti-HER2 nanobody conjugates for imaging HER2 receptor expression by immunoPET. J. Nucl. Med. 2016, 57, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Vaidyanathan, G.; McDougald, D.; Kang, C.M.; Balyasnikova, I.; Devoogdt, N.; Ta, An.; McNaughton, B.R.; Zalutsky, M.R. Fluorine-18 labeling of the HER2-targeting single-domain antibody 2Rs15d using a residualizing label and preclinical evaluation. Mol. Imaging Biol. 2017, 19, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkgraaf, I.; Liu, S.; Kruijtzer, J.A.W.; Soede, A.C.; Oyen, W.J.G.; Liskamp, R.M.J.; Corstens, F.H.M.; Boerman, O.C. Effects of linker variation on the in vitro and in vivo characteristics of an 111In-labeled RGD peptide. Nucl. Med. Biol. 2007, 34, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, M.; van Eerd-Vismale, J.; Oyen, W.J.G.; de Jong, M.; Zhang, H.; Rolleman, E.; Maecke, H.R.; Béhé, M.; Boerman, O. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J. Nucl. Med. 2007, 48, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Vegt, E.; de Jong, M.; Wetzels, J.F.M.; Masereeuw, R.; Melis, M.; Oyen, W.J.G.; Gotthardt, M.; Boerman, O.C. Renal toxicity of radiolabeled peptides and antibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Mume, E.; Orlova, A.; Larsson, B.; Nilsson, A.S.; Nilsson, F.Y.; Sjöberg, S.; Tolmachev, V. Evaluation of ((4-hydroxyphenyl)ethyl)maleimide for site-specific radiobromination of anti-HER2 affibody. Bioconjugate Chem. 2005, 16, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, G.; McDougald, D.; Choi, J.; Pruszynski, M.; Koumarianou, E.; Zhou, Z.; Zalutsky, M.R. N-succinimidyl 3-((4-(4-[18F]fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl) benzoate ([18F]SFBTMGMB): A residualizing label for 18F-labeling of internalizing biomolecules. Org. Biomol. Chem. 2016, 14, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl. Med. Biol. 2018, 56, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.R.; Khalil, F.L.; Lea, M.A. Decreased uptake of 14C-labeled dicarboxylic amino acids in rapidly growing hepatomas. Cancer Res. 1980, 40, 4053–4058. [Google Scholar] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. 131I-Labeled anti-HER2 camelid sdAb as a theranostic tool in cancer treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef] [PubMed]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an anti-HER2 nanobody labeled with 225Ac for targeted α-particle therapy of cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Marek, G.; Shenoy, N.; Seidel, J.; Griffiths, G.L.; Choyke, P.; Capala, J. 68Ga-DOTA-affibody molecule for in vivo assessment of HER2/neu expression with PET. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Gainkam, L.O.T.; Caveliers, V.; Devoogdt, N.; Vanhove, C.; Xavier, C.; Boerman, O.; Muyldermans, S.; Bossuyt, A.; Lahoutte, T. Localization, mechanism and reduction of renal retention of technetium-99m labeled epidermal growth factor receptor-specific nanobody in mice. Contrast Media Mol. Imaging 2011, 6, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F.; Ristic, Z.; Romeo, E.; Ramadan, T.; Makrides, V.; Dave, M.H.; Wagner, C.A.; Camargo, S.M.R. Novel renal amino acid transporters. Annu. Rev. Physiol. 2005, 67, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.J.; Heacock, A.M.; Fisher, S.K. Muscarinic receptor stimulation of d-aspartate uptake into human SH-SY5Y neuroblastoma cells is attenuated by hypoosmolarity. J. Pharmacol. Exp. Ther. 2010, 333, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, S.; Carter, P.; Welbourne, T. Glutamate transport asymmetry and metabolism in the functioning kidney. Am. J. Physiol. 1999, 277, E439–E446. [Google Scholar] [CrossRef] [PubMed]
- Béhé, M.; Kluge, G.; Becker, W.; Gotthardt, M.; Behr, T.M. Use of polyglutamic acids to reduce uptake of radiometal-labeled minigastrin in the kidneys. J. Nucl. Med. 2005, 46, 1012–1015. [Google Scholar] [PubMed]
- Tran, T.; Engfeldt, T.; Orlova, A.; Sandström, M.; Feldwisch, J.; Abrahmsén, L.; Wennborg, A.; Tolmachev, V.; Karlström, A.E. 99mTc-maEEE-ZHER2:342, an affibody molecule-based tracer for the detection of HER2 expression in malignant tumors. Bioconjugate Chem. 2007, 18, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, T.; Tran, T.; Orlova, A.; Widström, C.; Feldwisch, J.; Abrahmsén, L.; Wennborg, A.; Karlström, A.E.; Tolmachev, V. Development and preclinical characterisation of 99mTc-labelled affibody molecules with reduced renal uptake. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, D.S.; Chyan, M.K.; Nakamae, H.; Chen, Y.; Hamlin, D.K.; Santos, E.B.; Kornblit, B.T.; Sandmaier, B.M. Reagents for astatination of biomolecules. 6. An intact antibody conjugated with a maleimido-closo-decaborate(2-) reagent via sulfhydryl groups had considerably higher kidney concentrations than the same antibody conjugated with an isothiocyanato-closo-decaborate(2-) reagent via lysine amines. Bioconjugate Chem. 2012, 23, 409–420. [Google Scholar]
- Keller, O.; Rudinger, J. Preparation and some properties of maleimido acids and maleoyl derivatives of peptides. Helv. Chim. Acta 1975, 58, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Zalutsky, M.R.; Narula, A.S. A method for the radiohalogenation of proteins resulting in decreased thyroid uptake of radioiodine. Int. J. Rad. Appl. Instrum. A 1987, 38, 1051–1055. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, W.; Wang, H.Y.; Wang, S.C.; Pan, Y.; Kwong, K.Y.; Hortobagyi, G.N.; Hung, M.C. Antitumor activity of an Ets protein, PEA3, in breast cancer cell lines MDA-MB-361YT2 and BT474M1. Mol. Carcinog. 2006, 45, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chitneni, S.K.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Fluorine-18 labeling of an anti-HER2 VHH using a residualizing prosthetic group via a strain-promoted click reaction: Chemistry and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the d-amino acids standards and their precursors are available from the authors. |

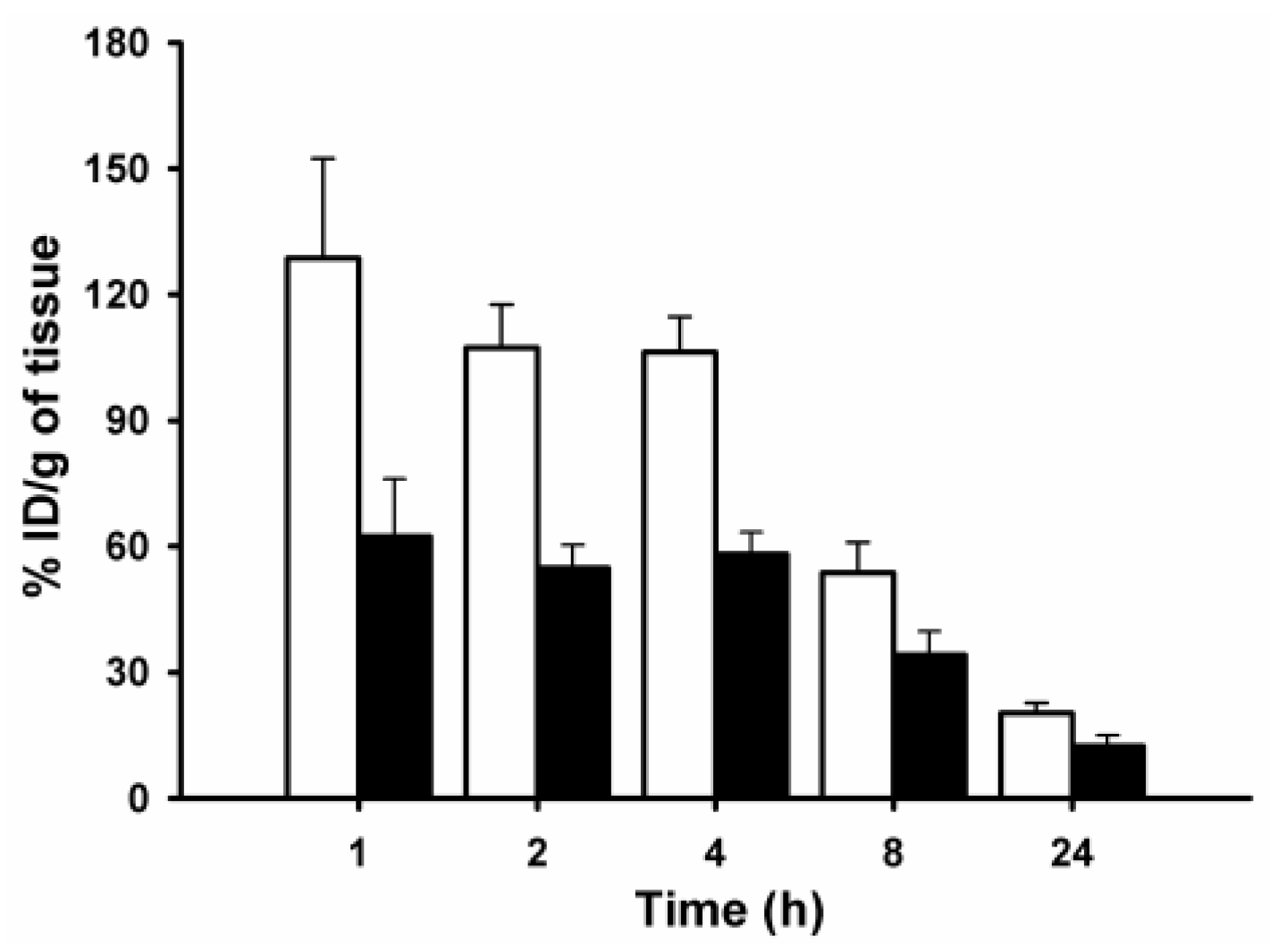
| Organ/Tissue | %ID/g 1 | |||||
|---|---|---|---|---|---|---|
| 1 h | 4 h | 24 h | ||||
| I-125 | I-131 | I-125 | I-131 | I-125 | I-131 | |
| Liver | 4.15 ± 1.12 | 1.99 ± 0.50 | 4.59 ± 0.53 | 1.73 ± 0.09 | 2.52 ± 0.71 | 0.56 ± 0.17 |
| Spleen | 2.28 ± 1.00 | 1.05 ± 0.34 | 1.67 ± 0.69 | 0.73 ± 0.17 | 1.68 ± 0.55 | 0.36 ± 0.12 |
| Lungs | 10.34 ± 2.37 | 4.81 ± 0.93 | 7.00 ± 2.13 | 1.86 ± 0.29 | 2.30 ± 0.68 | 0.52 ± 0.35 |
| Heart | 1.34 ± 0.37 | 0.99 ± 0.34 | 0.67 ± 0.12 | 0.35 ± 0.01 | 0.25 ± <0.01 | 0.05 ± 0.01 |
| Kidneys | 127.5 ± 18.7 | 271.4 ± 66.6 | 132.2 ± 31.1 | 201.1 ± 41.1 | 94.6 ± 22.9 2 | 81.4 ± 28.8 |
| Stomach | 0.80 ± 0.46 2 | 0.83 ± 0.49 | 0.79 ± 0.30 2 | 0.80 ± 0.31 | 0.14 ± 0.08 2 | 0.06 ± 0.04 |
| Small intestine | 1.20 ± 0.59 2 | 1.18 ± 0.60 | 0.83 ± 0.05 2 | 0.77 ± 0.06 | 0.13 ± 0.03 | 0.05 ± 0.01 |
| Large intestine | 0.47 ± 0.12 2 | 0.43 ± 0.12 | 1.89 ± 1.14 2 | 1.84 ± 1.13 | 0.33 ± 0.20 | 0.15 ± 0.11 |
| Thyroid | 0.06 ± 0.05 2 | 0.07 ± 0.07 | 0.05 ± 0.02 2 | 0.05 ± 0.02 | 0.08 ± 0.02 2 | 0.09 ± 0.02 |
| Muscle | 0.82 ± 0.12 | 0.58 ± 0.05 | 0.26 ± 0.09 2 | 0.22 ± 0.05 | 0.13 ± 0.02 | 0.03 ± 0.01 |
| Blood | 2.20 ± 0.65 | 2.00 ± 0.68 | 0.49 ± 0.14 2 | 0.45 ± 0.22 | 0.07 ± 0.01 | 0.05 ± 0.01 |
| Bone | 0.68 ± 0.13 2 | 0.64 ± 0.11 | 0.27 ± 0.04 2 | 0.28 ± 0.09 | 0.17 ± 0.02 2 | 0.10 ± 0.04 |
| Tumor | 4.36 ± 0.77 2 | 4.82 ± 1.00 | 3.28 ± 1.03 2 | 3.49 ± 0.87 | 1.91 ± 0.32 2 | 1.88 ± 0.39 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruszynski, M.; Kang, C.M.; Koumarianou, E.; Vaidyanathan, G.; Zalutsky, M.R. d-Amino Acid Peptide Residualizing Agents for Protein Radioiodination: Effect of Aspartate for Glutamate Substitution. Molecules 2018, 23, 1223. https://doi.org/10.3390/molecules23051223
Pruszynski M, Kang CM, Koumarianou E, Vaidyanathan G, Zalutsky MR. d-Amino Acid Peptide Residualizing Agents for Protein Radioiodination: Effect of Aspartate for Glutamate Substitution. Molecules. 2018; 23(5):1223. https://doi.org/10.3390/molecules23051223
Chicago/Turabian StylePruszynski, Marek, Choong Mo Kang, Eftychia Koumarianou, Ganesan Vaidyanathan, and Michael R. Zalutsky. 2018. "d-Amino Acid Peptide Residualizing Agents for Protein Radioiodination: Effect of Aspartate for Glutamate Substitution" Molecules 23, no. 5: 1223. https://doi.org/10.3390/molecules23051223





