Antioxidants from the Brown Alga Dictyopteris undulata
Abstract
:1. Introduction
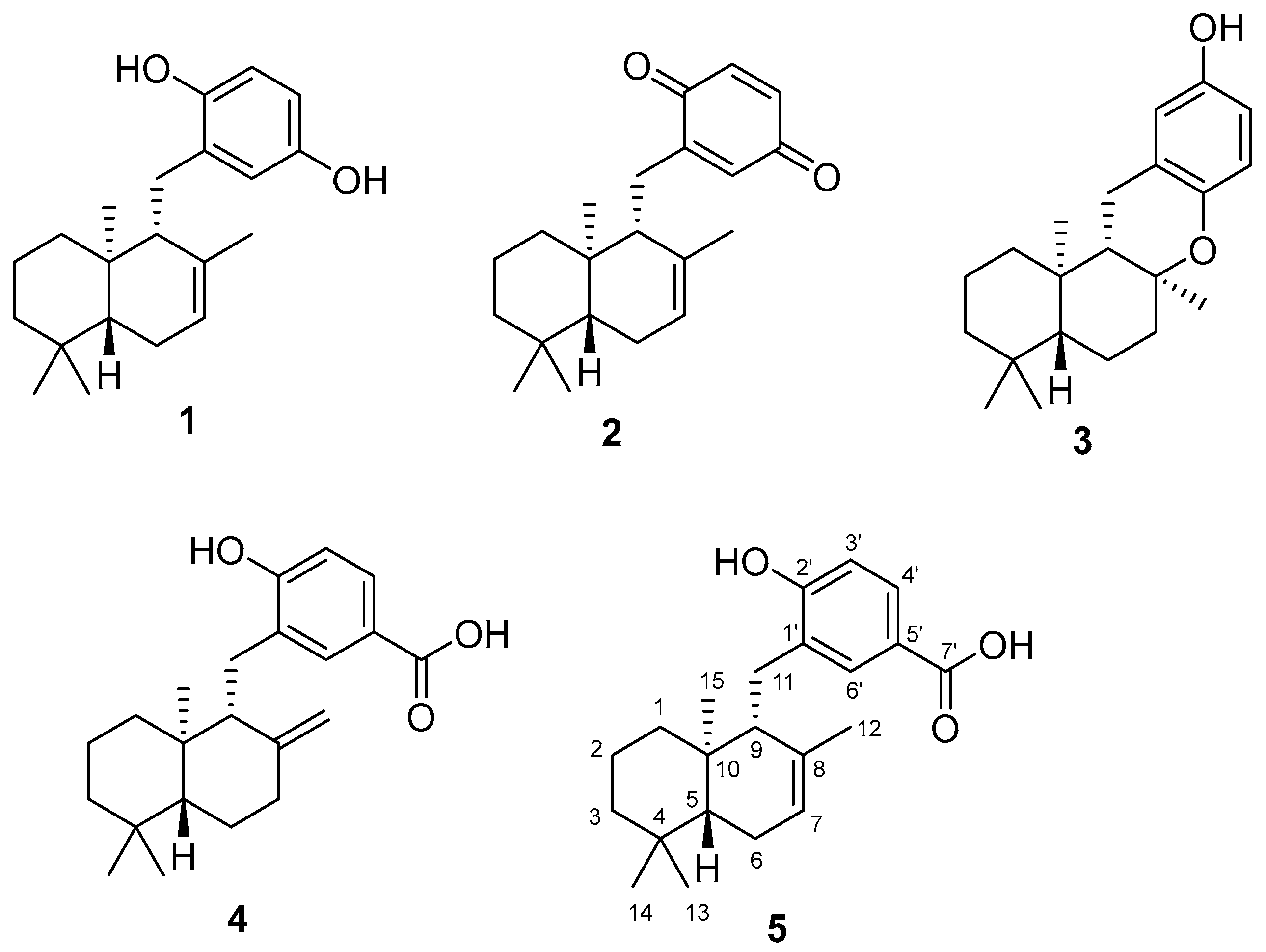
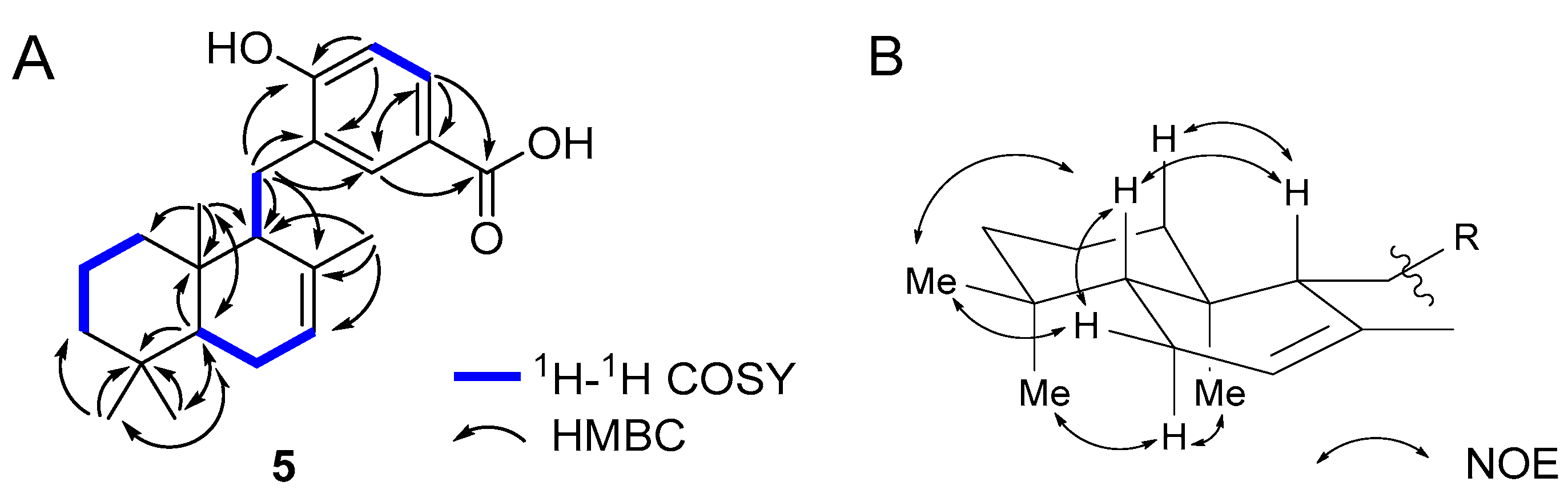
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Isolation of Compounds
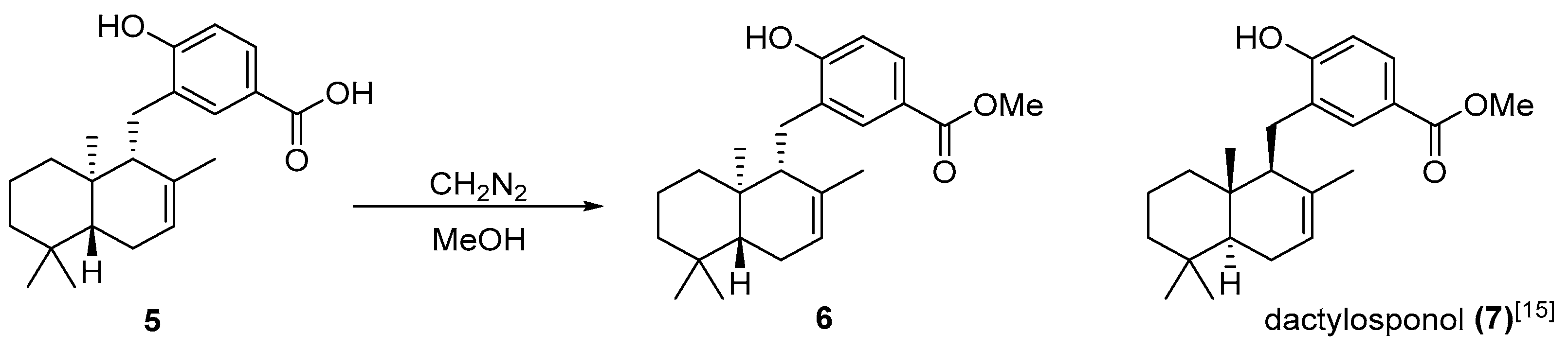
3.4. Methylation of Isozonaroic Acid (5)
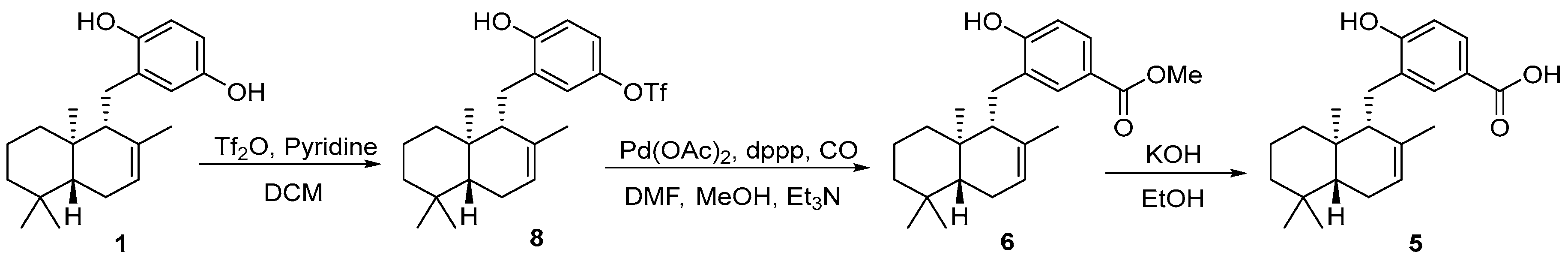
3.5. Semisynthesis of Isozonaroic Acid from Isozonarol
3.6. DPPH Radical Scavenge Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables the millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food. Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Dong, L.M.; Jia, X.C.; Luo, Q.W.; Zhang, Q.; Luo, B.; Liu, W.B.; Zhang, X.; Xu, Q.L.; Tan, J.W. Phenolics from Mikania micrantha and their antioxidant activity. Molecules 2017, 22, 1140. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Jin, D.-Q.; Sung, J.-Y.; Lee, J.H.; Choi, H.G.; Ha, I.; Han, J.S. Antioxidant and anti-inflammatory activities of the methanolic extract of Neorhodomela aculeate in hippocampal and microglial cells. Biol. Pharm. Bull. 2006, 29, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Magg, C.; Seifert, K. Total synthesis of the marine sesquiterpene hydroquinones zonarol and isozonarol and sesquiterpene quinones zonarone and isozonarone. Tetrahedron Lett. 2000, 41, 5469–5473. [Google Scholar] [CrossRef]
- Laube, T.; Schroder, J.; Stehle, R.; Seifert, K. Total synthesis of yahazunol, zonarone and isozonarone. Tetrahedron 2002, 58, 4299–4309. [Google Scholar] [CrossRef]
- Taniguchi, K.; Yamaga, J.; Kurata, K.; Suzuki, M. Feeding-deterrents from brown alga Dictyopteris undulata against the abalone Haliotis discus hannai. Nippon Suisan Gakkaishi 1993, 59, 339–343. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Suzuki, M. Cyclozonarone, a sesquiterpene-substituted benzoquinone derivative from the brown alga Dictyopteris undulata. Phytochemistry 1996, 41, 749–752. [Google Scholar] [CrossRef]
- Fenical, W.; Sims, J.J.; Squatrito, D.; Wing, R.M.; Radlick, P. Zonarol and isozonarol, fungitoxic hydroquinones from the brown seaweed Dictyopteris zonaroides. J. Org. Chem. 1973, 38, 2383–2386. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Ishihara, K.; Yamamoto, H. A new artificial cyclase for polyprenoids: Enantioselective total synthesis of (−)-chromazonarol, (+)-8-epi-puupehedione, and (−)-11’-deoxytaondiol methyl ether. J. Am. Chem. Soc. 2004, 126, 11122–11123. [Google Scholar] [CrossRef] [PubMed]
- Cimio, G.; Stefano, S.D.; Fenical, W.; Minale, L.; Sims, J.J. Zonaroic acid from the brown seaweed Dictyopteris undulata (= zonarioides). Experimentia 1975, 31, 1250–1251. [Google Scholar] [CrossRef]
- Ochi, M.; Kotsuki, H.; Muraoka, K.; Tokoroyama, T. The structure of yahazunol, a new sesquiterpene-substituted hydroquinone from the brown seaweed Dictyopteris undulata Okamura. Bull. Chem. Soc. Jpn. 1979, 52, 629–630. [Google Scholar] [CrossRef]
- Koker, M.E.S. Bioactive compounds from the alga Dictyopteris undulata. J. Pharm. Bioresour. 2010, 7, 77–92. [Google Scholar] [CrossRef]
- Rodrigues, J.; Quinoa, E.; Riguera, R.; Peters, B.M.; Abrell, L.M.; Crews, P. The structures and stereochemistry of cytotoxic sesquiterpene quinones from dactylospongia elegans. Tetrahedron 1992, 48, 6667–6680. [Google Scholar] [CrossRef]
- Talpir, R.; Rudia, A.; Kashman, Y.; Loya, Y.; Hizi, A. Three new sesquiterpene hydroquinones from marine origin. Tetrahedron 1994, 50, 4179–4184. [Google Scholar] [CrossRef]
- Bernet, A.; Schroder, J.; Seifert, K. Total synthesis of the marine sesquiterpene quinones hyatellaquinone and spongiaquinone. Helv. Chem. Acta 2003, 86, 2009–2020. [Google Scholar]
- Rubio, B.K.; van Soest, R.W.M.; Crews, P. Extending the record of meroditerpenes from Cacospongia marine sponges. J. Nat. Prod. 2007, 70, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Cimio, G.; Stefano, S.D.; Minale, L. ent-Chromazonarol, a chroman-sesquiterpenoid from the sponge Disidea pallescens. Experientia 1975, 31, 1117–1118. [Google Scholar] [CrossRef]
- Prez-Garcia, E.; Zubia, E.; Ortega, M.J.; Carballo, J.L. Merosesquiterpenes from two sponges of the genus Dysidea. J. Nat. Prod. 2005, 68, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Ohkubo, K.; Ogawa, Y.; Matsumoto, K.; Ozawa, T.; Fukuzumi, S. Aluminium ion-promoted radical-scavenging reaction of methylated hydroquinone derivatives. Org. Biomol. Chem. 2016, 14, 7956–7961. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellan, C.; Mercader-Ros, M.T.; Zafrilla, M.P.; Gabaldon, J.A.; Nunez-Delicado, E. Comparative study of different methods to measure antioxidant activity of resveratorol in the presence of cyclodextrins. Food Chem. Toxicol. 2011, 49, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.W. Hydroquinone for skin lightening: Safety profile, duration of use and when should we stop. J. Dermatol. Treat. 2010, 21, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Koyama, T.; Noguchi, H.; Ueda, Y.; Kitsuyama, R.; Shimizu, H.; Tanimoto, A.; Wang, K.-Y.; Nawata, A.; Nakayama, T.; et al. Marine hydroquinone zonarol prevents inflammation and apoptosis in dextran sulfate sodium-induced mice ulcerative colitis. PLoS ONE 2014, 9, e113509. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Jana, R.; Tunge, J.A. A Homogeneous, recyclable rhodium(I) catalyst for the hydroarylation of Michael acceptors. Org. Lett. 2009, 11, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Dolle, R.E.; Schmidt, S.J.; Kruse, L.I. Palladium catalysed alkoxycarbonylation of phenols to benzoate esters. J. Chem. Soc. Chem. Commun. 1987, 904–905. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound Name | DPPH Radical |
|---|---|
| Scavenging Activity | |
| EC50 Value (µM) | |
| Isozonarol (1) | 71 |
| Isozonarone (2) | 145 |
| Chromazonarol (3) | 121 |
| Zonaroic acid (4) | >1000 |
| Isozonaroic acid (5) | >1000 |
| α-Tocopherol | 74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumagai, M.; Nishikawa, K.; Matsuura, H.; Umezawa, T.; Matsuda, F.; Okino, T. Antioxidants from the Brown Alga Dictyopteris undulata. Molecules 2018, 23, 1214. https://doi.org/10.3390/molecules23051214
Kumagai M, Nishikawa K, Matsuura H, Umezawa T, Matsuda F, Okino T. Antioxidants from the Brown Alga Dictyopteris undulata. Molecules. 2018; 23(5):1214. https://doi.org/10.3390/molecules23051214
Chicago/Turabian StyleKumagai, Momochika, Keisuke Nishikawa, Hiroshi Matsuura, Taiki Umezawa, Fuyuhiko Matsuda, and Tatsufumi Okino. 2018. "Antioxidants from the Brown Alga Dictyopteris undulata" Molecules 23, no. 5: 1214. https://doi.org/10.3390/molecules23051214
APA StyleKumagai, M., Nishikawa, K., Matsuura, H., Umezawa, T., Matsuda, F., & Okino, T. (2018). Antioxidants from the Brown Alga Dictyopteris undulata. Molecules, 23(5), 1214. https://doi.org/10.3390/molecules23051214






