Tissue-Specific Analysis of Secondary Metabolites Creates a Reliable Morphological Criterion for Quality Grading of Polygoni Multiflori Radix
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Extraction
2.2. Optimization of Analytical Conditions
2.3. Method Validation
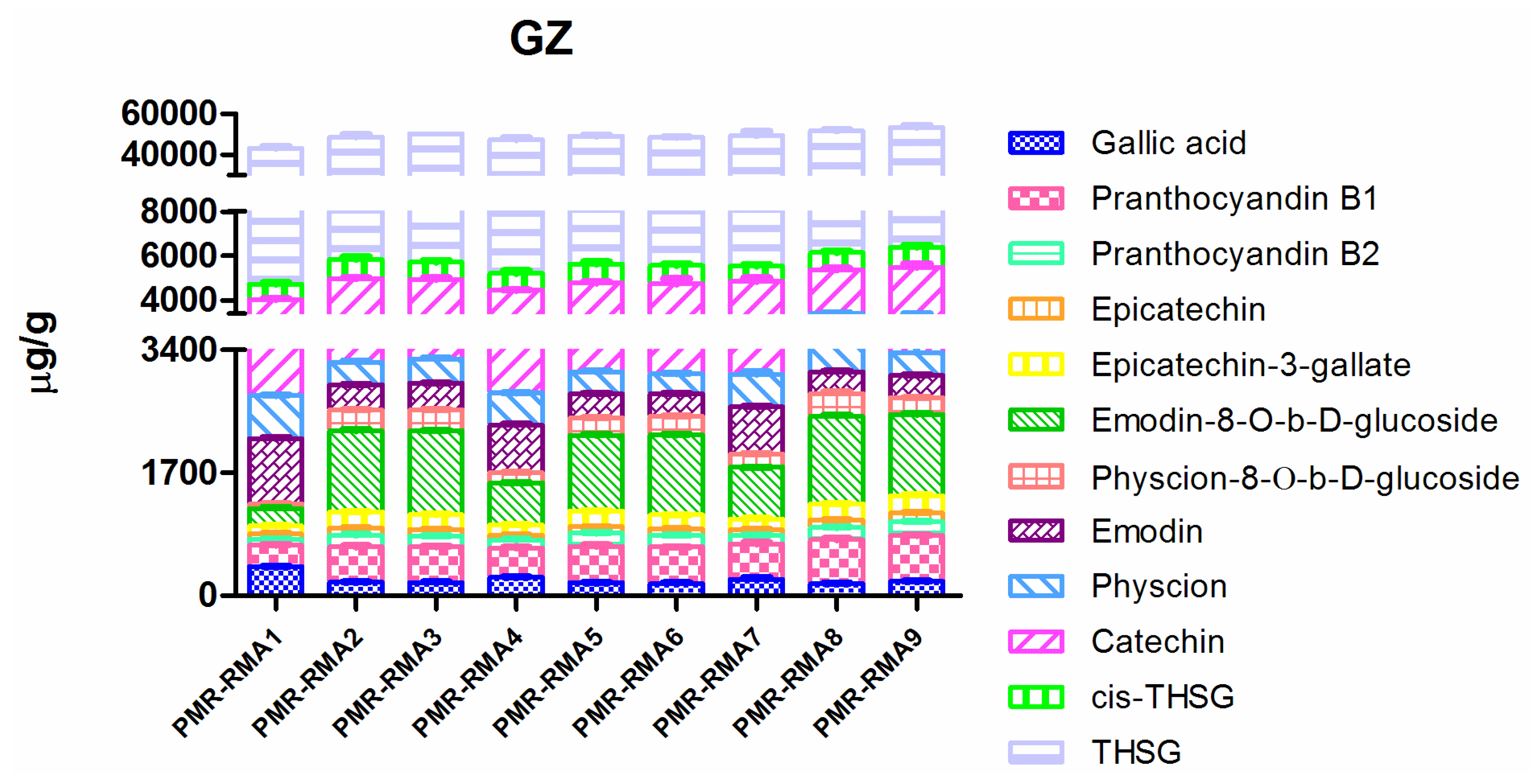
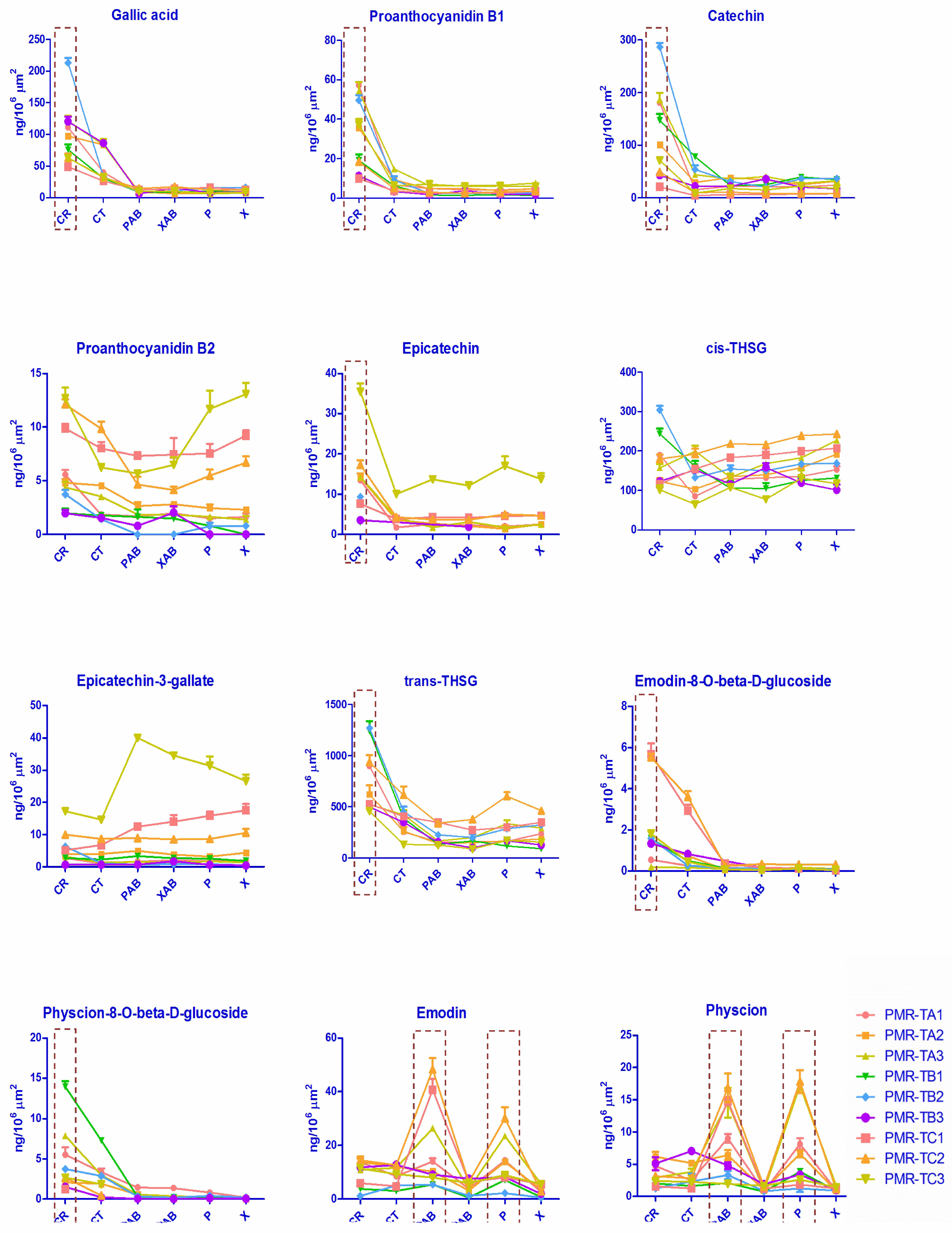
2.4. Quantitative Results and Discussion
2.4.1. Raw Materials
2.4.2. Micro-Dissected Tissues
3. Materials and Methods
3.1. Plant Materials, Chemicals and Reagents
3.2. Sample Preparation
3.2.1. Raw Material Extraction
3.2.2. Tissue Extraction
Laser Microdissection
Micro-Dissected Tissue Extraction
3.3. Ultra-Performance Liquid Chromatography Triple-Quadrupole Mass Spectrometry (UPLC-QqQ-MS/MS Analysis)
3.3.1. Conditions
3.3.2. Quantitative Method Validation
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhao, Z.Z.; Liang, Z.T.; Guo, P. Macroscopic Identification of Chinese Medicinal Materials: Traditional Experiences and Modern Understanding. J. Ethnopharmacol. 2011, 134, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.H.; Wang, J.B.; Zhou, C.P.; Xia, X.H.; Zhang, P.; Xiao, X.H.; Yuan, H.L.; Zhao, H.P.; Zhang, X.R. Comparison of Antioxidative Activities of Fructus Lycii with Different Commercial Grades by Bioassay Detection. Chin. J. New Drugs 2011, 20, 599–603. [Google Scholar]
- Wang, J.B.; Zhang, X.R.; Xiao, X.H.; Chu, X.H.; Zhou, C.P.; Jin, C.; Yan, D. Rationality of Commercial Specification of Rhubarb Based on Chemical Analysis. Zhongguo Zhong Yao Za Zhi 2010, 35, 470–476. [Google Scholar] [PubMed]
- Al-Saidi, S.; Rameshkumar, K.B.; Hisham, A.; Sivakumar, N.; Al-Kindy, S. Composition and Antibacterial Activity of the Essential Oils of Four Commercial Grades of Omani Luban, the Oleo-Gum Resin of Boswellia Sacra Flueck. Chem. Biodivers. 2012, 9, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, D.M.; Huang, L.F. Analysis of the Correlation between Commodity Grade and Quality of Angelica Sinensis by Determination of Active Compounds Using Ultraperformance Liquid Chromatography Coupled with Chemometrics. Evid.-Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Jin, L.; Ma, Y.; Cui, Z.; Li, Q.; Huang, D.D. NAnalysis of Commercial Grades of Schisandrae Sphenantherae Fructus Based on Schisantherin. Zhongguo Zhong Yao Za Zhi 2017, 42, 3728–2732. [Google Scholar] [PubMed]
- Ng, K.M.; Liang, Z.T.; Lu, W.; Tang, H.W.; Zhao, Z.Z.; Che, C.M.; Cheng, Y.C. In Vivo Analysis and Spatial Profiling of Phytochemicals in Herbal Tissue by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry. Anal. Chem. 2007, 79, 2745–2755. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Qiao, C.F.; Liang, Z.T.; Xu, H.X.; Zhao, Z.Z.; Cai, Z.W. Alkaloid Profiling in Crude and Processed Strychnos Nux-Vomica Seeds by Matrix-Assisted Laser Desorption/ionization-Time of Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2007, 45, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liang, Z.T.; Zhao, Z.Z.; Cai, Z.W. Direct Analysis of Alkaloid Profiling in Plant Tissue by Using Matrix-Assisted Laser Desorption/ionization Mass Spectrometry. J. Mass Spectrom. 2007, 42, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Liang, Z.T.; Peng, Y.; Yao, X.; Chen, H.B.; Zhao, Z.Z. Tissue-Specific Metabolite Profiling of Alkaloids in Sinomenii Caulis Using Laser Microdissection and Liquid Chromatography-Quadrupole/time of Flight-Mass Spectrometry. J. Chromatogr. A 2012, 1248, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.T.; Sham, T.; Yang, G.Y.; Yi, L.; Chen, H.B.; Zhao, Z.Z. Profiling of Secondary Metabolites in Tissues from Rheum Palmatum L. Using Laser Microdissection and Liquid Chromatography Mass Spectrometry. Anal. Bioanal. Chem. 2013, 405, 4199–4212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Liang, Z.T.; Peng, Y.; Hou, J.L.; Wei, S.L.; Zhao, Z.Z.; Wang, W.Q. Whole Transverse Section and Specific-Tissue Analysis of Secondary Metabolites in Seven Different Grades of Root of Paeonia Lactiflora Using Laser Microdissection and Liquid Chromatography-Quadrupole/time of Flight-Mass Spectrometry. J. Pharm. Biomed. Anal. 2015, 103, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Krauss, M.; Singer, H.; Hollender, J. LC-High Resolution MS in Environmental Analysis: From Target Screening to the Identification of Unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Hunter, C.L. Quantitative Mass Spectrometric Multiple Reaction Monitoring Assays for Major Plasma Proteins. Mol. Cell. Proteom. 2006, 5, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Engström, M.T.; Pälijärvi, M.; Salminen, J.P. Rapid Fingerprint Analysis of Plant Extracts for Ellagitannins, Gallic Acid, and Quinic Acid Derivatives and Quercetin-, Kaempferol- and Myricetin-Based Flavonol Glycosides by UPLC-QqQ-MS/MS. J. Agric. Food Chem. 2015, 63, 4068–4079. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, B.; Liu, A.; Zhu, W.; Yao, S. Liquid Chromatography-Mass Spectrometric Multiple Reaction Monitoring-Based Strategies for Expanding Targeted Profiling towards Quantitative Metabolomics. Curr. Drug Metab. 2012, 13, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.C.; Russell, J.D.; Bailey, D.J.; Westphall, M.S.; Coon, J.J. Parallel Reaction Monitoring for High Resolution and High Mass Accuracy Quantitative, Targeted Proteomics. Mol. Cell. Proteom. 2012, 11, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Outlaw, W.H.; Zhang, S. Single-Cell Dissection and Microdroplet Chemistry. J. Exp. Bot. 2001, 52, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Moco, S.; Schneider, B.; Vervoort, J. Plant Micrometabolomics: The Analysis of Endogenous Metabolites Present in a Plant Cell or Tissue. J. Proteome Res. 2009, 8, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Runions, J.; Moore, I. Quantitative Fluorescence Microscopy: From Art to Science. Annu. Rev. Plant Biol. 2006, 57, 79–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Liang, Z.T.; Zhu, Y.; Xie, G.Y.; Tian, M.; Zhao, Z.Z.; Qin, M.J. Tissue-Specific Metabolites Profiling and Quantitative Analyses of Flavonoids in the Rhizome of Belamcanda Chinensis by Combining Laser-Microdissection with UHPLC-Q/TOF-MS and UHPLC-QqQ-MS. Talanta 2014, 130, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China, Version 2015; China Medical Science Publisher: Beijing, China, 2015; Volume 1. [Google Scholar]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional Usages, Botany, Phytochemistry, Pharmacology and Toxicology of Polygonum Multiflorum Thunb.: A Review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z. Compendium of Materia Medica (Ben Cao Gang Mu); Shanghai Science and Technology Publisher: Shanghai, China, 2008. [Google Scholar]
- Bai, S.Q.; Zhang, X.Q. Chinese Material Medica Commoditology; Tianjin Science and Technology Press: Tianjin, China, 1999. [Google Scholar]
- Liang, Z.T.; Shi, Y.X.; Chen, H.B.; Zhao, Z.Z. Histochemical Analysis of the Root Tuber of Polygonum Multiflorum Thunb. (Fam. Polygonaceae). Microsc. Res. Tech. 2011, 74, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.H.; Choi, J.S.; Choi, S.U.; Kim, Y.S.; Lee, K.R.; Kim, Y.K.; Ryu, S.Y. A New Stilbene Glucoside Gallate from the Roots of Polygonum Multiflorum. Arch. Pharm. Res. 2008, 31, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.G.; Kim, J.; Sung, N.D.; Son, K.H.; Cheon, H.G.; Kim, K.R.; Kwon, B.M. Anthraquinones, Cdc25B Phosphatase Inhibitors, Isolated from the Roots of Polygonum Multiflorum Thunb. Nat. Prod. Res. 2007, 21, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Han, D.Q.; Zhao, J.; Xu, J.; Peng, H.S.; Chen, X.J.; Li, S.P. Quality Evaluation of Polygonum Multiflorum in China Based on HPLC Analysis of Hydrophilic Bioactive Compounds and Chemometrics. J. Pharm. Biomed. Anal. 2013, 72, 223–230. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the trans-2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucopyranoside (trans-THSG), cis-THSG, emodin, physcion, emodin-8-O-β-D-glucosides and physcion-8-O-β-D-glucosides, gallic acid, proanthocyanidin B1, proanthocyanidin B2, epicatechin, catechin and epicatechin-3-gallate are available from the authors. |

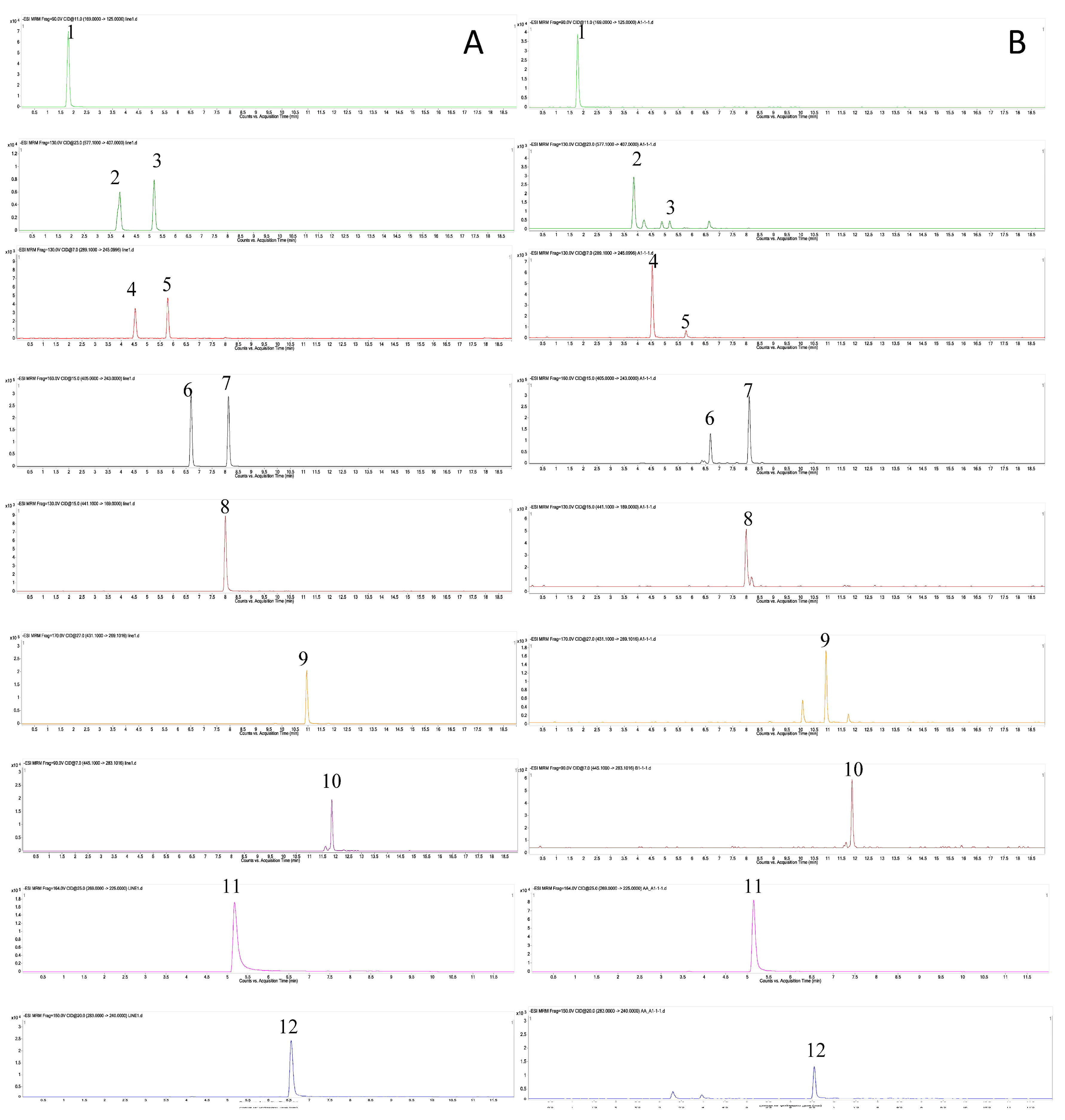
| Analyte | MRM | Collision Voltage (eV) | Calibration Curve | Sensitivity (ng/mL) | |||
|---|---|---|---|---|---|---|---|
| Range (ng/mL) | Equation | R2 | LODs | LOQs | |||
| Gallic acid | 169.0→125.0 | 11 | 80–4000 | y = 95.80x − 605.88 | 0.9972 | 4.12 | 14.85 |
| Proanthocyanidin B1 | 577.1→407.0 | 23 | 40–2000 | y = 28.09x − 105.54 | 0.9994 | 2.51 | 7.59 |
| Catechin | 289.1→245.1 | 7 | 20–1000 | y = 25.33x + 256.56 | 0.9974 | 17.95 | 48.25 |
| Proanthocyanidin B2 | 577.1→407.0 | 23 | 40–2000 | y = 25.93x − 48.90 | 0.9991 | 3.17 | 15.92 |
| Epicatechin | 289.1→245.1 | 7 | 10–1000 | y = 31.33x − 78.60 | 0.9939 | 15.52 | 40.66 |
| cis-THSG | 405.0→243.0 | 15 | 5–1000 | y = 213.54x − 348.14 | 0.9948 | 1.04 | 1.62 |
| Epcatechini-3-gallate | 441.1→169.0 | 15 | 5–1000 | y = 55.85x − 82.32 | 0.9936 | 0.99 | 6.62 |
| trans-THSG | 405.0→243.1 | 15 | 40–8000 | y = 196.23x − 357.59 | 0.9992 | 9.64 | 11.02 |
| Emodin-8-O-β-D-glucoside | 431.1→269.1 | 27 | 40–2000 | y = 801.51x + 29.72 | 0.9911 | 4.36 | 4.58 |
| Physcion-8-O-β-D-glucoside | 445.1→283.1 | 7 | 40–2000 | y = 58.92x − 103.32 | 0.9929 | 4.84 | 7.14 |
| Emodin | 269.0→225.0 | 25 | 80–800 | y = 2422.72 + 16798.78 | 0.9907 | 0.22 | 1.15 |
| Physcion | 283.0→240.0 | 20 | 40–2000 | y = 117.20x − 1082.86 | 0.9945 | 1.73 | 4.03 |
| Repeatability (n = 6, RSD, %) | Precision (n = 6) | Spike Recovery (n = 3, Mean (RSD), %) | Stability (48 h, n = 7) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-Day | Low | Middle | High | ||||||
| Mean (ng/mL) | RSD, % | Mean (ng/mL) | RSD, % | Mean (ng/mL) | RSD, % | |||||
| Gallic acid | 10.81 | 394.15 | 3.01 | 471.86 | 7.69 | 96.71 (4.58) | 97.23 (4.15) | 94.94 (1.53) | 445.58 | 8.73 |
| Proanthocyanidin B1 | 7.43 | 959.60 | 2.47 | 999.00 | 4.08 | 90.73 (6.29) | 91.72 (5.46) | 90.85 (4.85) | 1042.45 | 7.81 |
| Catechin | 9.35 | 293.40 | 4.43 | 329.87 | 9.01 | 101.89(7.75) | 95.14(6.51) | 98.52(1.86) | 315.646 | 7.38 |
| Proanthocyanidin B2 | 8.31 | 341.51 | 2.21 | 357.12 | 4.16 | 96.17(4.73) | 93.72(1.52) | 96.08(3.70) | 369.96 | 5.54 |
| Epicatechin | 3.48 | 205.35 | 5.58 | 221.47 | 4.09 | 102.69(4.13) | 107.37(1.31) | 101.70(4.44) | 203.26 | 5.68 |
| cis-THSG | 4.22 | 2012.47 | 1.21 | 2198.07 | 1.79 | 82.51(10.18) | 102.87(9.21) | 87.96(10.49) | 2107.79 | 4.76 |
| Epcatechini-3-gallate | 7.27 | 440.67 | 7.25 | 448.75 | 5.69 | 103.50(5.13) | 95.52(8.18) | 100.89(2.06) | 456.16 | 4.23 |
| trans-THSG | 2.42 | 7396.96 | 2.28 | 7932.62 | 6.72 | 95.15(6.80) | 92.82(5.34) | 85.98(8.56) | 7865.36 | 5.95 |
| Emodin-8-O-β-D-glucoside | 3.19 | 2124.69 | 0.48 | 2365.74 | 0.78 | 94.72(1.47) | 100.49(1.99) | 97.93(1.58) | 2272.80 | 4.48 |
| Physcion-8-O-β-D-glucoside | 3.58 | 503.18 | 1.54 | 558.48 | 1.59 | 94.11(2.09) | 95.36(4.40) | 85.81(0.38) | 529.89 | 4.66 |
| Emodin | 2.02 | 719.23 | 8.32 | 669.05 | 9.95 | 99.05(2.92) | 106.78(2.56) | 101.18(2.33) | 682.54 | 5.77 |
| Physcion | 3.40 | 605.38 | 4.49 | 569.70 | 6.19 | 97.94(9.05) | 92.87(6.61) | 99.27(8.78) | 578.33 | 3.97 |
| Repeatability (n = 6, RSD, %) | Precision (n = 6) | Spike Recovery (n = 3, mean (RSD), %) | Stability (48 h, n = 7) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day | Inter-day | Low | Middle | High | ||||||
| Mean (ng/mL) | RSD, % | Mean (ng/mL) | RSD, % | Mean (ng/mL) | RSD, % | |||||
| Gallic acid | 2.85 | 144.60 | 3.08 | 147.10 | 8.14 | 108.78(5.00) | 101.82(15.80) | 120.95(10.18) | 142.90 | 3.82 |
| Proanthocyanidin B1 | 9.55 | 54.92 | 5.65 | 60.05 | 4.27 | 111.34(11.31) | 109.33(10.52) | 111.09(4.81) | 51.82 | 5.19 |
| Catechin | 6.11 | 113.16 | 7.96 | 141.29 | 6.68 | 109.47(5.94) | 102.63(11.29) | 98.44(5.67) | 135.61 | 5.60 |
| Proanthocyanidin B2 | 6.25 | 96.75 | 6.14 | 103.44 | 6.50 | 100.39(11.61) | 99.32(3.13) | 100.73(0.33) | 100.70 | 7.46 |
| Epicatechin | 3.68 | 63.25 | 7.48 | 72.78 | 4.76 | 100.98(7.78) | 100.80(9.17) | 109.27(9.68) | 78.55 | 8.64 |
| cis-THSG | 4.25 | 3040.38 | 2.09 | 3682.49 | 3.91 | 113.93(9.18) | 103.54(6.08) | 99.46(5.65) | 3378.57 | 5.82 |
| Epcatechini-3-gallate | 6.33 | 162.27 | 6.03 | 178.00 | 4.79 | 93.66(8.48) | 97.62(6.72) | 90.98(10.54) | 131.54 | 4.90 |
| trans-THSG | 5.91 | 4296.71 | 0.56 | 4921.06 | 5.25 | 109.77(6.74) | 112.86(4.00) | 102.28(9.76) | 7249.63 | 7.37 |
| Emodin-8-O-β-D-glucoside | 4.92 | 193.50 | 2.44 | 241.92 | 3.34 | 113.83(1.50) | 109.74(15.73) | 101.83(2.13) | 359.83 | 6.05 |
| Physcion-8-O-β-D-glucoside | 11.17 | 25.00 | 6.44 | 31.79 | 9.84 | 109.61(6.19) | 103.38(8.95) | 115.32(8.42) | 28.27 | 9.00 |
| Emodin | 6.23 | 183.42 | 1.66 | 192.12 | 4.33 | 102.25(8.27) | 107.03(11.56) | 109.24(1.84) | 218.22 | 8.14 |
| Physcion | 11.22 | 90.94 | 1.92 | 85.36 | 5.69 | 94.96(4.87) | 90.12(9.48) | 92.84(6.01) | 68.27 | 3.54 |
| Sample No. | Grade | Type (a) | Cultivated Location | Collection Date |
|---|---|---|---|---|
| PMR-RMA1 | 1 (b) | RM | Guizhou Province, Shibing Country | 2017-1-18 |
| PMR-RMA2 | 1 | RM | Guizhou Province, Shibing Country | 2017-1-16 |
| PMR-RMA3 | 1 | RM | Guizhou Province, Shibing Country | 2017-2-18 |
| PMR-RMA4 | 2 | RM | Guizhou Province, Shibing Country | 2017-1-18 |
| PMR-RMA5 | 2 | RM | Guizhou Province, Shibing Country | 2017-2-16 |
| PMR-RMA6 | 2 | RM | Guizhou Province, Shibing Country | 2017-2-18 |
| PMR-RMA7 | 3 | RM | Guizhou Province, Shibing Country | 2017-1-18 |
| PMR-RMA8 | 3 | RM | Guizhou Province, Shibing Country | 2017-2-16 |
| PMR-RMA9 | 3 | RM | Guizhou Province, Shibing Country | 2017-2-18 |
| PMR-TA1 | 1 | T | Guizhou Province, Shibing Country | 2017-2-16 |
| PMR-TA2 | 2 | T | Guizhou Province, Shibing Country | 2017-2-16 |
| PMR-TA3 | 3 | T | Guizhou Province, Shibing Country | 2017-2-16 |
| PMR-TB1 | 1 | T | Guangdong Province, Xinxing Country | 2017-2-8 |
| PMR-TB2 | 2 | T | Guangdong Province, Xinxing Country | 2017-2-8 |
| PMR-TB3 | 3 | T | Guangdong Province, Xinxing Country | 2017-2-8 |
| PMR-TC1 | 1 | T | Hubei Province, Lizhou City | 2017-1-16 |
| PMR-TC2 | 2 | T | Hubei Province, Lizhou City | 2017-1-16 |
| PMR-TC3 | 3 | T | Hubei Province, Lizhou City | 2017-1-16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Xu, J.; Liang, Z.-T.; Dong, X.-P.; Chen, H.-B.; Zhao, Z.-Z. Tissue-Specific Analysis of Secondary Metabolites Creates a Reliable Morphological Criterion for Quality Grading of Polygoni Multiflori Radix. Molecules 2018, 23, 1115. https://doi.org/10.3390/molecules23051115
Liang L, Xu J, Liang Z-T, Dong X-P, Chen H-B, Zhao Z-Z. Tissue-Specific Analysis of Secondary Metabolites Creates a Reliable Morphological Criterion for Quality Grading of Polygoni Multiflori Radix. Molecules. 2018; 23(5):1115. https://doi.org/10.3390/molecules23051115
Chicago/Turabian StyleLiang, Li, Jun Xu, Zhi-Tao Liang, Xiao-Ping Dong, Hu-Biao Chen, and Zhong-Zhen Zhao. 2018. "Tissue-Specific Analysis of Secondary Metabolites Creates a Reliable Morphological Criterion for Quality Grading of Polygoni Multiflori Radix" Molecules 23, no. 5: 1115. https://doi.org/10.3390/molecules23051115
APA StyleLiang, L., Xu, J., Liang, Z.-T., Dong, X.-P., Chen, H.-B., & Zhao, Z.-Z. (2018). Tissue-Specific Analysis of Secondary Metabolites Creates a Reliable Morphological Criterion for Quality Grading of Polygoni Multiflori Radix. Molecules, 23(5), 1115. https://doi.org/10.3390/molecules23051115





