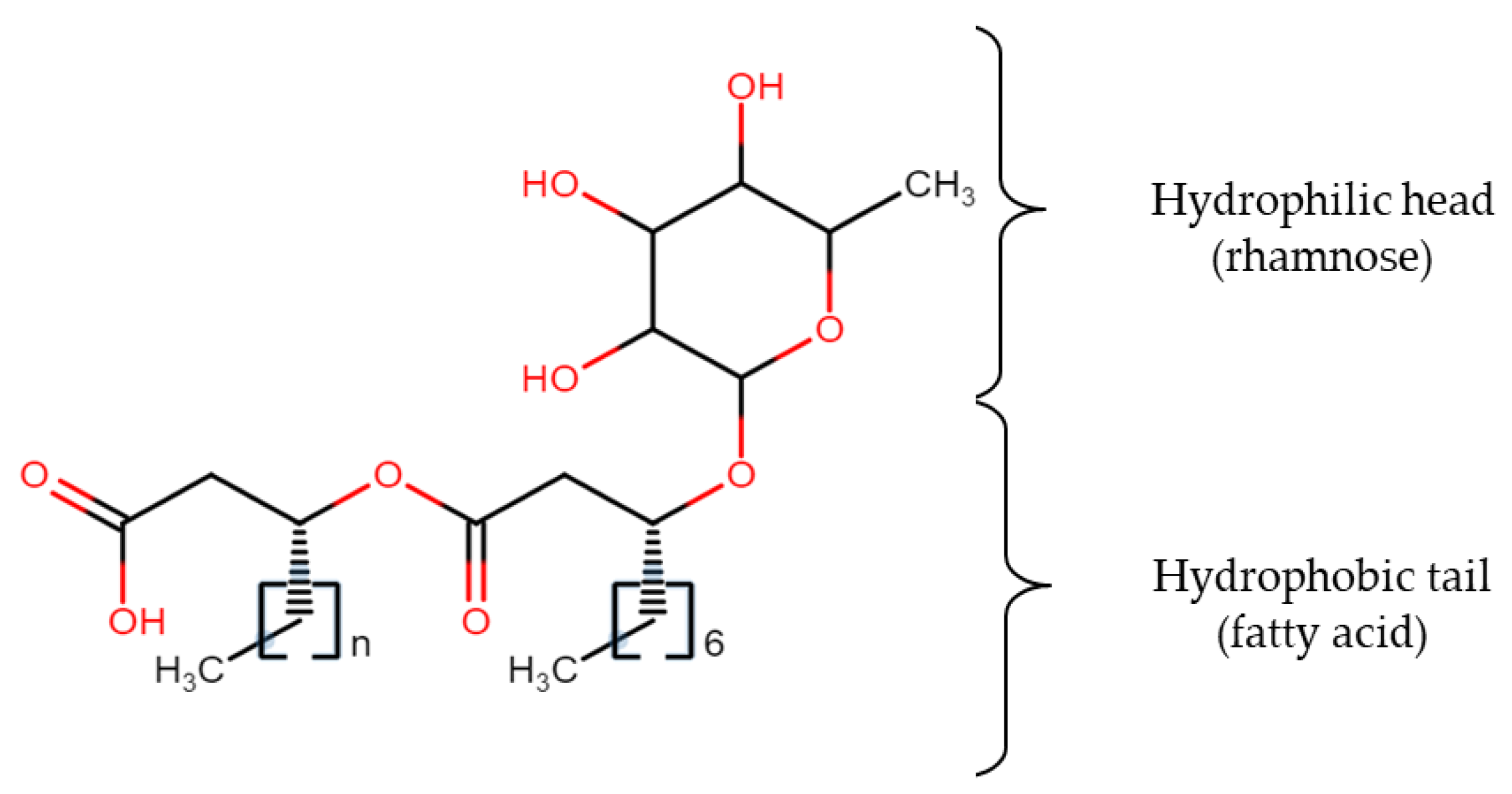
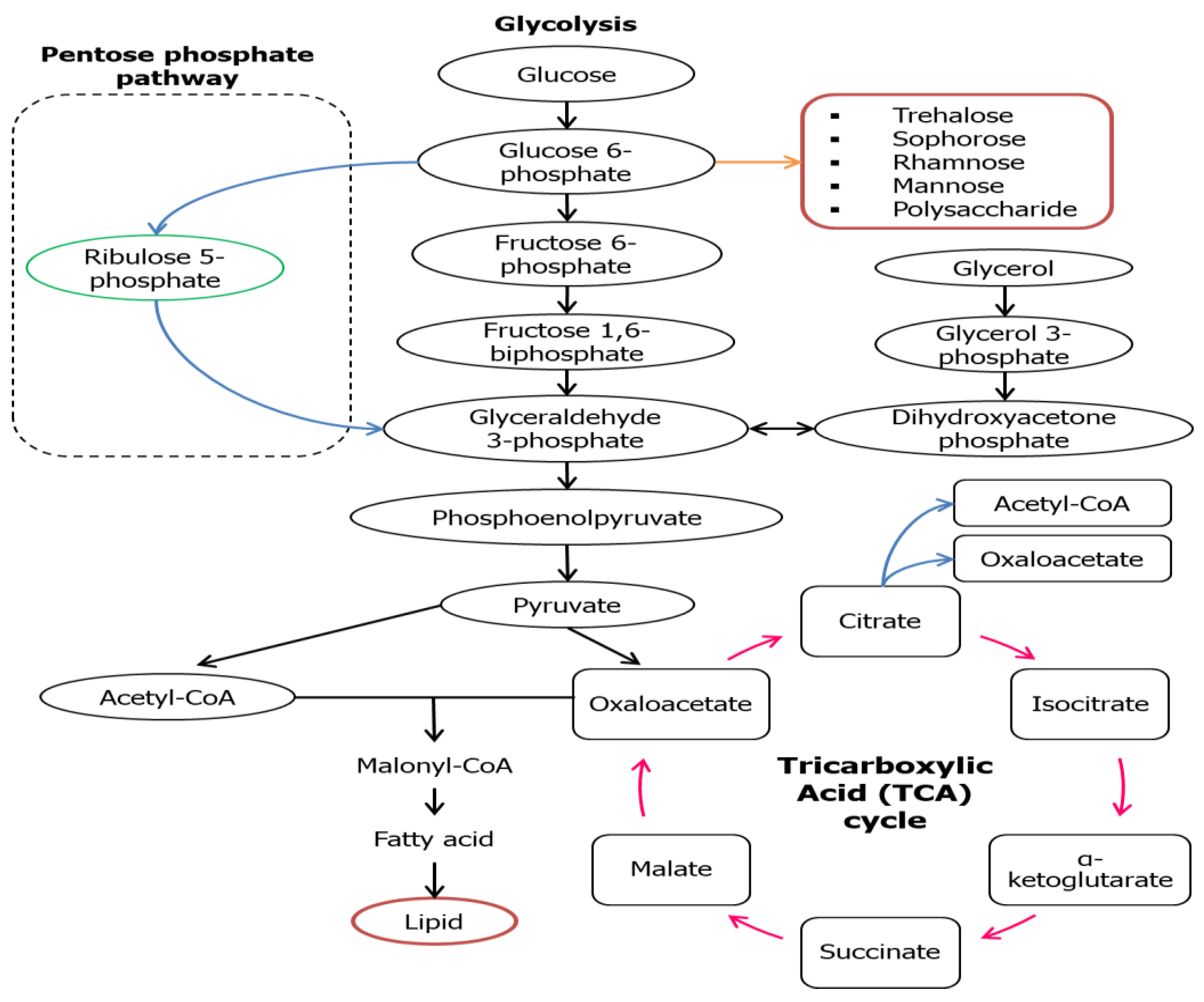
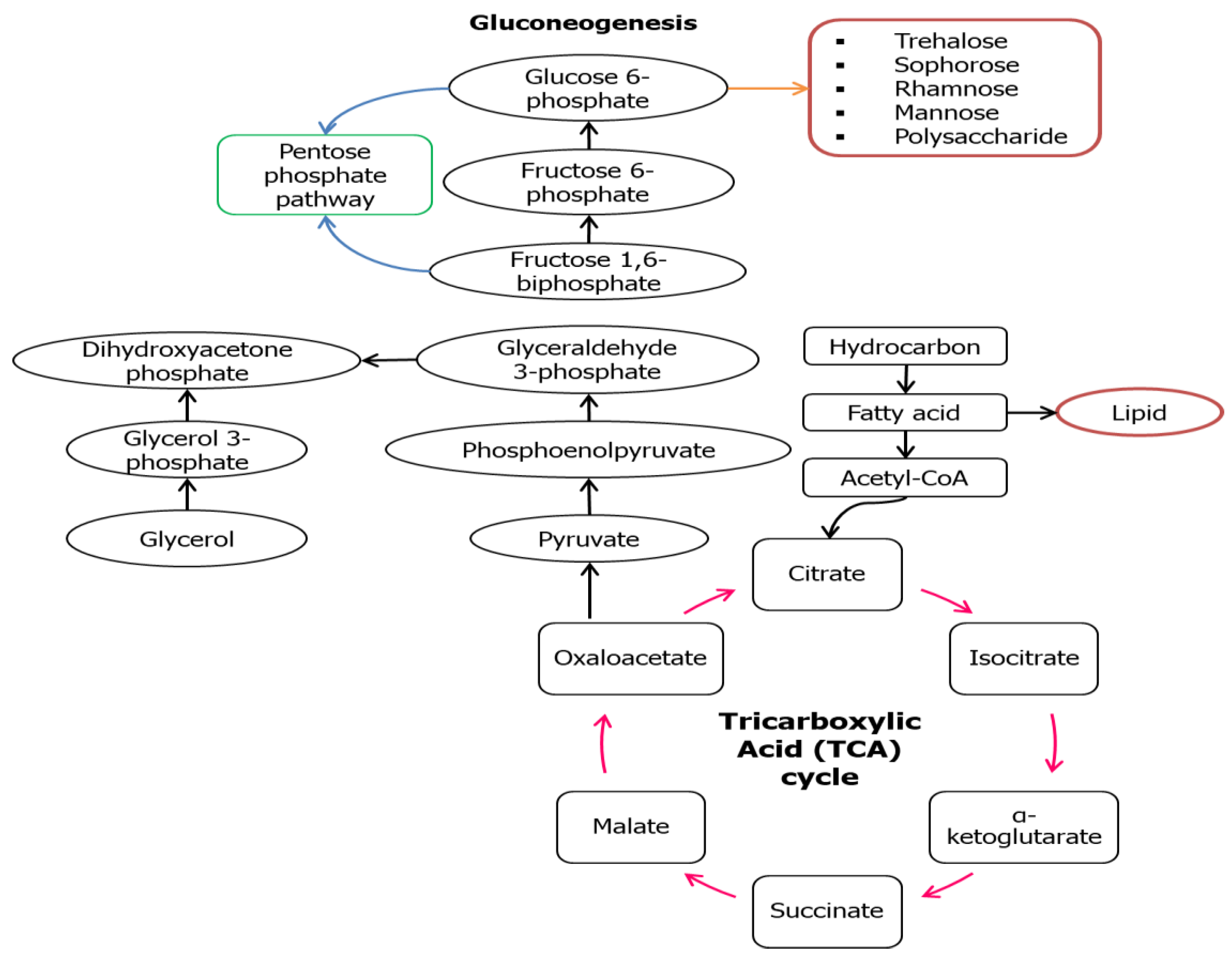
4.1. Carbon Sources
Biosurfactants producing microorganisms are usually heterotrophs whereby they consume organic constituent of carbon source to grow and produce their metabolites. About 30–40% of the total cost comes solely from the preparation of growth and production medium [
17] for biosurfactant production, thus warranting the need for cheaper type of feedstocks. The biomass and product formation are usually being controlled by the carbon consumption rate of microorganisms during cultivation [
47]. Generally, there are three types of carbon sources being commonly used in biosurfactant productions; carbohydrate, oils and fats, and hydrocarbon groups.
Under the carbohydrate group, simple sugar, starch, and plant sugar-based carbohydrates are the major carbon sources used in biosurfactant production. Glucose is the typical example of the carbon source which can easily be metabolized by microorganisms through the glycolysis pathway for the generation of energy and is commonly reported to give higher yield of product. Previous investigations on the carbohydrate group as the carbon source for biosurfactant production by various microorganisms is tabulated in
Table 1.
P. aeruginosa MTCC 7815 utilized glucose much better than other carbon sources (glycerol, fructose, and starch) to yield higher amounts of biosurfactant, biomass, emulsification index, E24 (76.77%), and the lowest surface tension (34.53 mN/m) [
51]. For exceptional biosurfactant producers like
Klebsiella sp. RJ-03, production of biosurfactant was found to be the highest with starch followed by sucrose, xylose, galactose, glucose, and fructose [
60]. In certain cases, different raw materials containing a variety of carbohydrate groups were tested on several microorganisms to produce biosurfactants. For example, a mineral medium containing clarified cashew apple juice (MM-CCAJ) which contained about 12.05 g/L of sugar [
61] was utilized by
B. subtilis LAMI005 to produce two-fold less than the amount produced using mineral medium (MM) supplemented with 10 g/L of glucose and 8.7 g/L of fructose (MM-GF) [
62]. However, critical micelle concentration (CMC) of the biosurfactants produced using MM-CCAJ was 2.5-fold lower than the one produced using MM-GF, which indicates it is a more efficient biosurfactant and indicate that it is feasible to produce surfactin from clarified cashew apple juice.
In normal cases, microorganisms will metabolize sugar substrate through the glycolysis pathway. Substrates like glycerol feed into the central carbon metabolism at the level of glyceraldehyde-3-phosphate and thus, do not employ the pentose phosphate (PP) pathway, which wastes the carbon via CO
2 production. For glucose and sucrose, they will enter the central carbon metabolism via the Entner-Doudoroff (ED) pathway. The PP pathway will be activated when energy for cell maintenance is required (via redox cofactor synthesis) resulting in the loss of carbon through CO
2 generation. When high amount of energy is required (i.e., high growth rates), full oxidation through acetyl-CoA and tricarboxylic acid (TCA) cycle happened and cause the CO
2 formation which contributes to the lower biosurfactant (rhamnolipid) yield [
63].
Utilization of various types of oils and fats as the carbon source in biosurfactant production has been well documented. This is due to the fact that the bioprocess pathway to manufacture biosurfactant itself undergoes four possibilities. First, the hydrophilic and hydrophobic parts of biosurfactants are developed de novo along independent pathways. Second, both of the biosurfactant moiety productions are influenced by the carbon source. Third, the hydrophilic part is synthesized de novo and the carbon substrate will promote the production of the hydrophobic part or lastly, the hydrophobic part is synthesized de novo and the carbon source will induce the production of the hydrophilic part.
Oil substrates have been sourced from fresh feedstock or waste by-product of a particular process. For example,
P. aeruginosa D strain utilized 2% waste frying coconut oil effectively to produce 3.55 g/L of biosurfactant [
17]. Other than that,
P. aeruginosa F23 also utilized fresh coconut oil (2%) as carbon source to produce 2.8 g/L of biosurfactant and reduce the surface tension of medium from 45 mN/m to 31 mN/m when grown in an optimized SM medium [
64].
P. aeruginosa achieved higher biosurfactant production when using waste frying coconut oil compare to fresh coconut oil in complex production medium due to higher free fatty acid content in waste frying coconut oil which supports the growth of microorganisms [
65].
In the case of the fat substrate, the cultivation of
C. glabrata UCP1002 yeast supplied with 5% vegetable fat waste in 100 mL aqueous medium ultimately produced a maximum value of 7 g/L biosurfactant after 144 h of incubation time [
66]. This substrate yielded two times the biosurfactant compared to the run which used waste frying coconut oil. Alternatively, Santos et al. [
67] evaluation on
C. lipolytica UCP0988 ability to produce glycolipid in medium formulated with 5% of animal fat and 2.5% corn steep liquor yielded the highest reduction in surface tension (from 50 to 28 mN/m). Their result suggested that animal fat alone did not support high microbial growth and hence affecting the biosurfactant production as compared to the addition of corn steep liquor into production medium. The fatty acid composition of the animal fat substrate sourced from a bovine processing plant had been reported to contain higher composition of palmitic acid (26.40%) and oleic acid (24.16%) which could be the main reason affecting the biosurfactant production. On the contrary,
P. aeruginosa was capable to consume 80% of the initial amount of oleic acid used and left a final residual concentration of about 8% to produce a maximum growth of 2.3 × 10
8 of cells/mL after 3 days of incubation period [
68].
As for fat containing a much higher fatty acid content, a comparative study on biosurfactant production by
P. aeruginosa PAO1 was carried out on palm fatty acid distillate (PFAD), which is a by-product from a crude palm oil refinery plant having more than 70% of free fatty acids against glucose as the carbon components formulated into peptone-glucose ammonium salt (PPGas) medium [
69]. The biosurfactant produced from 20–100 g/L of PFAD was in the range of 0.38–0.43 g/L, which was almost similar to the PPGas with glucose-containing medium (0.36 g/L). At higher concentrations of PFAD, biosurfactant production did not show any significant difference. This was mainly due to the low solubility of PFAD in the culture medium and as such, causing gross heterogeneity in the cultivation system. Therefore, regardless of the concentrations of PFAD being added, inadvertently there was a threshold amount of this substance able to dissolve in the medium which were indifferent in all runs. One of the advantages of using PFAD was that foam formation could be hampered during cultivation process due to the presence of free fatty acids which acts as an antifoaming agent [
70]. PFAD can be a promising substrate for biosurfactant production and as to our present knowledge, it was the first published work on utilizing PFAD as substrate in biosurfactant production by
Pseudomonas sp.
The third type of carbon source refers to hydrocarbon. Hydrocarbon is usually supplied in liquid form to biosurfactant-producing microorganism. For instance, isolated
P. aeruginosa was produced biosurfactant which reduced the surface tension of culture to 30 mN/m from 65 mN/m within 3 days of utilizing hexadecane [
71]. About 2.1 g/L of biomass was obtained after 11 days of incubation and nearly 70% of hexadecane was degraded after a 7 day incubation period which complemented a concurrent increase of biomass and biosurfactant produced. On the other hand, various strains of
P. aeruginosa isolated from petroleum-contaminated soil in Assam, India were grown in various polycyclic aromatic hydrocarbon (PAH) substrates like phenantrene, fluorine, and pyrene [
72]. The medium with mixtures of fluorine and phenantrene as carbon sources showed higher biosurfactant production by
P. aeruginosa MTCC7815 (0.45 g/L) and MTCC7814 (0.38 g/L). The biosurfactant produced can actually increase the solubility of the hydrocarbon to be consumed by the microorganisms with the purpose of reinforcing their metabolism. Biosurfactants produced were identified as lipopeptide and protein-starch-lipid complex which are able to reduce surface tension from 72 mN/m to 35 mN/m of pure water. Last, but not least, is the utilization of diesel oil as the substrate, whereby
Aeromonas sp. strain A2 could produce 0.067 g/L of biosurfactants after 7 days of incubation with 75% of the supplemented diesel oil had apparently been consumed [
73]. Evidently, most of the biosurfactant can be produced by using all three types of carbon sources listed above. However performance-wise, cultivating microorganism which fed on oil and fat may be more favored over the rest. This might be due to the existence of a hydrophobic component in the substrate that will induce the production of hydrophobic moiety of biosurfactant though this is highly dependent on the behavior and metabolism of microorganism itself.
Pure substrates like glucose (Sigma, USD 64.75/kg), sucrose (Sigma, USD 91.68/kg), and glycerol (Sigma, USD 163.16/kg) are good candidates as carbon sources for various biosurfactant producers. However, wastes generated from industries and processing plants could also be potential candidates as carbon source for biosurfactant production (
Table 2) as it is more economic and abundantly available compared to the pure substrates mentioned above, which may lead to food competition concerns. However, the variation of complex substrate’s composition between batches should be expected as its characteristic is fully dependent on the process and the raw material used. In particular, biosurfactant produced by
P. fluorescens grown in media containing a mixture of natural manipueira (cassava flour wastewater) and nutrient broth reduced the surface tension of water to 59 mN/m from 80 mN/m compared to the media containing decanted manipueira [
74]. Other agricultural waste like cashew apple juice (CAJ) was also utilized in biosurfactant production by
Acinetobacter calcoaceticus. CAJ is rich in carbohydrates, fibers, vitamins, and minerals salt, that turn it into an interesting and inexpensive (USD 0.30/kg) substrate in the biosurfactant field [
75]. Molasses that contains high amounts of sugar has been seen as one of the economical carbon sources (USD 0.10/kg) [
76] for
P. aeruginosa GS3 to grow and to produce biosurfactant.
In term of performance, biosurfactants produced by
Deinococcus caeni PO5 reduced the surface tension of the culture supernatant from 67.0 to 25.0 mN/m after 87 h of cultivation when 40 g/L of jackfruit seed powder and 1 g/L of commercial monosodium glutamate were used as the carbon and nitrogen sources, respectively [
92]. Other than that, a newly discovered bacterium,
Lysinibacillus chungkukjangi produced biosurfactant which reduced the surface tension of the media to 27.9 from 72 mN/m when the bacterium was grown on rice bran (by-product of rice milling) [
93]. Rice bran contained high amounts of carbohydrates with 5% of bran which contained 12–18.5% oil [
94]. In terms of price, the agricultural and industrial processing of waste like corn steep liquor (USD 0.46/kg), baggase (USD 0.04/kg), rice husk (USD 0.08/kg) are much cheaper compared to the pure substrate and more economical to be used as feedstocks. Besides that, utilization of novel substrates like vineyard pruning waste allowed
L. paracasei to produce a biosurfactant with the highest surface tension reduction of water (27.3 mN/m) when grown in lactose-based medium [
95].
4.2. Nitrogen Sources
Nitrogen is also required for microbial growth and production of certain primary and secondary metabolites [
1]. The type of nitrogen existing in the production medium will affect the biosurfactant by microorganisms [
96]. There are two types of nitrogen sources; organic and inorganic nitrogen. The two can be differentiated clearly based on the unit structure present in them. The unit structure for organic nitrogen will be in molecules such as yeast extract, meat extract, tryptone, or peptone, while inorganic nitrogen will have unit structures that consist of positive and negative ions like ammonium nitrate (NH
4NO
3). Organic nitrogen may also contain some carbon component and had been reported to significantly support cell growth and polysaccharides formation as compared to inorganic nitrogen [
97] whereas nitrates, ammonia, and amino acids had been the nitrogen sources of choice for a few strains of
P. aeruginosa [
98].
Table 3 summarizes some of the organic nitrogen sources used in the production medium of biosurfactants by various microorganisms. Apparently, yeast extract has been widely chosen in many studies. It has been reported that a better emulsification index can be achieved when using a complex structure of the nitrogen source in the production medium, but it could become economically irrelevant in the MEOR (Microbial Enhanced Oil Recovery) process [
99]. Nevertheless, Fooladi et al. [
52] suggested that, while it uses can increase the concentration of biomass produced, somehow the substance showed less ability to reduce the surface tension as compared to other complex nitrogen sources. For instance,
L. paracasei ssp. paracasei A20 favored yeast extract as the most important factor for bacterial growth and followed by meat extract, whereas peptone seems to be the least important factor when a medium containing a mixture of two different nitrogen sources was used to produce biosurfactant [
100]. Most investigations on the different type of organic nitrogen sources would have fixed their concentrations used in the cultivation process. As such, an outright performance comparison of the cultivation outcome might be a little inaccurate since the true amount of nitrogen content, even for sources of similar types might differ for each batch or manufacturer, thus requiring the correct referral from the product datasheet.
It is far easier to determine the amount of nitrogen supplied in inorganic form based on their chemical formula and molecular weight.
Table 4 represents some of the inorganic nitrogen sources in tandem with their exact nitrogen count in the culture medium from previously known bodies of work on biosurfactant production. It seems that most microorganisms in the list show preference towards nitrate-based nitrogen sources (NH
4NO
3 and NaNO
3) for biosurfactant production compared to others. Perhaps, this attributes to the high nitrogen content readily available in these chemical solutions even when supplied in lower concentration. Microorganisms will first reduce nitrates to nitrite before turning it into ammonium. Then, ammonium is assimilated to form glutamate by glutamate dehydrogenase or to form glutamine by glutamine synthetase.
l-glutamine 2-oxoglutarate aminotransferase will cause glutamine and α-ketoglutarate to be converted into glutamine. However, the formation of the lipid moiety rather than the sugar moiety in rhamnolipid is the rate-determining factor and various reports have shown that rhamnolipid can be produced more effectively in nitrogen-limiting conditions [
70,
110]. The production of biosurfactants often occurs when the nitrogen source is depleted in the culture medium during the stationary phase of cell growth. There is a possible inhibitory effect on the bacterial metabolism due to a likely nutrient transport deficiency as nitrate first undergoes nitrate reduction simulation to ammonium and then it is incorporated by glutamine-glutamate metabolism [
111].
Others than the above nitrogen sources, there were few previous works utilizing waste materials to replace manufactured nitrogen sources for lowering the production cost. For instance,
P. aeruginosa OG1 make used of chicken feather peptone (CFP) as the nitrogen source to yield maximum biosurfactant concentration (7.2 g/L). CFP contains high amounts of protein, ash, and nitrogen, low fat content, and various amino acids at different concentrations, especially alanine, leucine, glutamate, glycine, serine, and proline which make the most suitable nitrogen sources compared to yeast extracts, tryptone and peptone [
117]. Other than being a substrate, corn steep liquor also had been used as nitrogen source together with NaNO
3 for
P. aeruginosa MR01 to produce 24 g/L of biosurfactant while employing soybean oil as the substrate [
118]. Corn steep liquor is very rich in sugars (mainly starch, but also some glucose), soluble proteins (including peptides and amino acids), minerals (potassium, calcium, and magnesium) which make it a potential nitrogen source in biosurfactant production [
119].
4.3. C/N Ratio
Carbon to nitrogen ratio (C/N) is the term to describe the relationship of carbon and nitrogen proportion needed in the production medium of biosurfactant by particular microorganisms. The C/N ratio required by microbial cultivation depends on the different types of microorganisms used, carbon and nitrogen type, culture conditions, and the desired product [
120]. A previous study proved that nitrogen limitation conditions can cause the microorganisms to yield higher biosurfactant production and allows for alteration of the product’s composition to ensue [
121]. High C/N ratios (i.e., low nitrogen levels) restrict bacterial growth, favoring cell metabolism towards the production of metabolites. For instance, when supplementing glycerol and NaNO
3 as carbon and nitrogen sources on isolated
P. aeruginosa, the optimal yield of biosurfactant (Y
P/S) and bacterial cells (Y
P/X) was found to be 0.13 g/g and 0.70 g/g, respectively, which corresponded to the C/N ratio of 55 [
122]. Likewise, another isolated
P. aeruginosa LBM10 produced an optimum biosurfactant concentration (1.42 g/L) when cultured in a nitrogen limiting condition (C/N ratio of 100) as compared to a C/N ratio of 22 (0.94 g/L) with soybean oil (carbon source) and NaNO
3 (nitrogen source) being used in the production medium [
123]. Both findings were using the same nitrogen source and same species of microorganism which led to maximum biosurfactant production under the nitrogen limiting conditions.
However, in most of the previous works, the common suitable C/N ratio for typical biosurfactant producer,
Pseudomonas sp. is between 6 and 13 (non-nitrogen limiting condition) which seems contradictory to the previous statement [
124,
125,
126,
127]. For example,
P. aeruginosa RS29 produced the highest biosurfactant (0.80 g/L) and maximum surface tension reduction (63.2 to 27.2 mN/m) when a C/N ratio of 12.5 was used. A higher C/N ratio (17.5 and 22.5) and lower C/N ratio (2.5 and 7.5) were tested and had caused a 25–39% reduction of biosurfactant production [
128]. Other than that, higher biosurfactant production with maximum surface tension reduction can be seen when
P. fluorescens Migula 1895-DSMZ was cultivated in a C/N ratio of 10 compared to 30 and 50 when olive oil and NH
4NO
3 were used as the carbon and nitrogen sources, respectively [
129]. Hamzah et al. [
130] suggested that there is a possible inhibitory effect occurred on
P. aeruginosa UKMP14T when a C/N ratio of more than 20 was used. Therefore, the optimal biosurfactant performances which reduce surface tension from 47.43 to 30.6 mN/m at a C/N ratio of 14 were observed with glycerol and (NH
4)
2SO
4 as the carbon and nitrogen sources, respectively. However,
P. nitroreducens achieved the maximum biosurfactant production (5.5 g/L) when a C/N ratio of 22 was used and more than 18% of biosurfactant production was depleted when using other C/N ratios with glucose and NaNO
3 acting as the carbon and nitrogen sources, respectively [
111].
For other genus of microorganisms,
B. subtilis strain showed that the increment of surface tension reduction could be observed at a lower C/N ratio condition (3 and 9) inversely proportional with the agitation speed used. At a C/N ratio of 15, it caused slight reduction in their performance in term of surface tension reduction even with the increased agitation rate where crystal sugar and NH
4NO
3 act as carbon and nitrogen sources, respectively [
131]. This is further being supported when
B. subtilis SPB1 produced the maximum amount of biosurfactant using 5 g/L of urea as organic nitrogen source and applying a C/N ratio of 7 with ammonium chloride as the inorganic nitrogen source [
49]. In addition,
B. pumilus 2IR yielded the maximum amount of biosurfactant (0.72 g/L) when potassium nitrate and glucose were used as a nitrogen and carbon sources, respectively, with a C/N ratio of 12.
Bacillus sp. BMN 14 worked the best under a C/N ratio of 12.4, with a decrease in surface tension of up to 27 mN/m compared to other C/N ratios (10.6 and 17.51) with glucose and NH
4NO
3 utilized as the substrate and nitrogen source, respectively [
132]. From the above study, most cases for
Bacillus sp. preferred to have a lower C/N ratio to yield higher biosurfactant production.
In most cases, it seems like
Yarrowia lipolytica showed an almost similar performance as
Bacillus sp. as it achieved maximum surface tension reduction (19.0 mN/m) when a C/N ratio of 12 was used compared to lower or higher C/N ratios [
133]. Others findings involved
Aureobasidium pullulans YTP6-14 which acquired maximum surface tension reduction of up to 38.4 mN/m when using a C/N ratio of 300 compared to a C/N ratio of 100 and 200 with glucose and glycerol as carbon sources and NH
4NO
3 as the nitrogen source, respectively [
134]. Higher amounts of carbon source are required as NH
4NO
3 provides two times the nitrogen source more than NaNO
3 which can explain this outcome.
Virgibacillus salarius attained maximum emulsifying activity (85%), and minimal surface tension (29 mN/m) at a C/N ratio of 30 (frying oil as carbon source; urea as nitrogen source) compared to other C/N ratios (10, 20, 40, 50, 60, and 70) [
135].
4.4. Minerals
Minerals can be classified into two groups: macronutrient minerals and micronutrient minerals (trace elements). Potassium (K), calcium (Ca), magnesium (Mg), and iron (Fe) serve as macronutrient minerals in medium formulation which are important to balance the cell wall communication and aid in the protein synthesizing mechanism [
136]. However, these metal ions can become an intracellular threat when present in excess. In the growth process, a metal ion acts as regulator for the production of physiologically active materials like a biosurfactant. Previous work proved iron, manganese, and magnesium to be cofactors of enzymes involved in the synthesis of surfactin by
B. subtilis [
27]. In this work, corn steep liquor (CSL) was utilized as the carbon source and the surfactin concentration was increased up to 4.8 g/L at optimum concentrations of these metal ions. They were supplemented simultaneously into the production medium.
Potassium dihydrogen phosphate, KH
2PO
4 and dipotassium hydrogen phosphate, K
2HPO
4 are usually added into the production medium to maintain the desired pH throughout the cultivation process. For the buffering system, only phosphate ion serves as a pH regulator while potassium ion will be a source of energy as it is the major intracellular cation in bacteria [
137]. Another study opted for potassium chloride (KCl) as the source of potassium in the medium containing glucose and alkane as the carbon sources for
Sphingobacterium detergens [
138]. It was suggested that potassium ion might be substantial for regulating ribosomal structures so that high amount of potassium ion should be present in growing bacteria [
139].
Calcium works as a common mediator in signal delivering processes from the cell surface into the intracellular of microorganisms [
140]. In biosurfactant production, calcium is usually supplied in the form of chloride or hydrated chloride salts, mostly in a concentration of 0.1 g/L for biosurfactant production from
P. aeruginosa [
112] and less than 0.02 g/L when producing glycolipid from
B. megatarium [
115] which goes to show that a tiny amount of Ca
2+ ions are still required in the production medium. Both potassium and calcium ions play a crucial role in balancing the osmotic pressure and controlling the cell’s membrane potential which can prevent the lysis of the cell in the medium [
141].
In order for ATP, which is the origin of the cell’s energy, to be active it would necessitate a bonding with a magnesium ion. This interaction is called Mg–ATP and the amount to be used will increase upon higher metabolic activity detected, and thus consequently increases in the rate of ADP and magnesium released [
142]. Magnesium ion as typically supplied in the form of magnesium sulphate (MgSO
4) and is mostly around 50 times higher than the concentration of calcium ion used in the production media of biosurfactants [
124,
128,
143]. A study on the effect of individual metal ions towards growth and biosurfactant production points to significant influence of magnesium over other metals. When
Halobacterium salinarum (HS) medium was supplemented with 5 mM of MgSO
4, MnSO
4, ZnSO
4, CaCl
2, FeSO
4, and CoCl
2, separately,
Halobacterium salinarum J1 was able to obtain three-fold growth against controlled basal medium and doubled against the second best metal supplement (ZnSO
4) and biosurfactant able to reach 68% of the E24 index [
144].
Iron is a very popular cofactor in metabolism of various microorganisms. Most formulations will utilize iron in the form of Fe
2+ or Fe
3+ ions depending on the iron uptake mechanism of the microbe itself. Fe
2+ is usually soluble in water and can be easily used by the microorganism compare to Fe
3+. Most of the formulations in MSM medium will use Fe
2+ supplied by FeSO
4.7H
2O [
120,
145] while Bushnell-Haas medium utilized FeCl
3.6H
2O to contribute Fe
3+ in the production medium [
77]. Works by Guerra-Santos et al. [
146] recorded that the limitation of magnesium, calcium, and potassium might enhance rhamnolipid production by
P. aeruginosa.
On the other hand, addition of some micronutrient minerals or trace elements could have positive effect on biosurfactant production. Trace elements are chemical elements that are necessary for the microorganisms in amounts that are less than 0.1% of the total working volume. The specific requirements of trace elements are depending on the microorganism itself, but the most popular trace elements used by biosurfactant producer are zinc (Zn), copper (Cu), boron (B), molybdenum (Mo), and cobalt (Co). Makkar and Cameotra [
57] added different concentrations of trace elements (0, 1, 2, 4, 6, 8, and 16 mL/L) into the production medium of the biosurfactant for
B. subtilis MTCC 2423. The results demonstrated that the biosurfactant produced declined from a maximum value (1.30 g/L) when either more or less than 1 mL/L of the trace elements were used. However, the biomass had increased to more than 1.5 g/L when higher than 4 mL/L of trace elements were supplemented into the production medium. Based on this finding, the trace element limiting condition might cause overproduction of biosurfactants. This was further supported by Kiran et al. [
147] demonstrating that further increments of FeSO
4 and MgCl
2 used from the original concentration (0.1 mM) drastically inhibit the biosurfactant production. More examples of trace elements used for biosurfactant production can be found in
Table 5 along with their producer.
In some other cases, using the same trace elements with similar concentrations for different microorganisms might lead to wide differences in biosurfactant produced. For example,
B. megaterium produced biosurfactant nearly 10 times higher than
P. aeruginosa RS29 when cultivated in medium containing the same composition of trace elements [
115,
124]. It probably happened due to the different requirement levels by different microorganisms. Besides that, the addition of ethylenediaminetetraacetic acid (EDTA) into the production medium will induce the secretion of both moieties of the active compound in the biosurfactant. With the presence of this active compound, the function of the biosurfactant can be completed either by increasing the production of water soluble substrate or by solubilizing the nonpolar hydrocarbon substrates in production media [
149]. EDTA which is a versatile chelating agent can potentially aid in forming complexes with metal ions like Fe
3+, so that the ion can be gradually utilized by the microorganisms [
25]. It can form four or six bonds with a metal ion, and chelates with both transition-metal ions and main-group ions to prevent them from precipitating in the medium.
4.5. Vitamins
Vitamin is the organic compound required in minute amount but was still necessary for the metabolic process for all organisms. Vitamins are rarely added on purpose since they are readily available in some of the natural medium components. Folic acid is one example of a vitamin that exists in the synthetic form of the water soluble B-vitamins which helps in synthesizing the nucleic acid for the microorganism to build up their DNA and is being added in small quantity into the medium. There is another type of vitamin B-complex, namely thiamine HCl, which is essential for the carbohydrate metabolism to digest the oily substrate in biosurfactant production.
In terms of practicality, it is quite uncommon to add vitamin directly into the medium since the price of pure vitamin is expensive to begin with. Nonetheless, Qazi et al. [
150] tried using vitamin B2 (1.0 g/L) as a nitrogen source to culture
P. putida SOL-10. It was found that the growth performance was actually comparable to using sodium nitrite (NaNO
2) as well as providing similar reduction in surface tension (35 mN/m). Other than that, beetroot waste was considered as a good potential substrate for biosurfactant production by
B. licheniformis STK 01 [
18]. Besides being rich in major nutrients (88% water, 1.2% protein, and 9.3% carbohydrates), it has all the mineral elements and essential trace amounts of carotene, thiamine, riboflavin, niacin, biotin, and vitamins C, E, B1, B2, and B12 [
151].
B. subtilis MZ-7 was capable to grow on readily formulated pharmamedia commercial medium which contained significant amounts of vitamins such as carotene (<0.001 g/L), ascorbic acid (0.032 g/L), thiamine (0.004 g/L), riboflavin (0.005 g/L), niacin (0.083 g/L), choline (3.27 g/L), and pantothenic acid (0.012 g/L) with no carbon source provided and the microbe was capable of generating 0.22 g/L of surfactin [
152]. Bayoumi et al. [
153] managed to quantify the optimum vitamin concentration required by different microorganisms as presented in
Table 6.
4.7. Salinity Level
Salinity of the culture medium could become one of the critical parameters needed to be optimized to show the stability level of the biosurfactants produced by particular microorganisms. Frequently, sodium chloride (NaCl) is used to establish a certain level of salinity that adjusts osmolarity of media for microbial growth. Kiran et al. [
147] showed that marine endosymbiotic fungus
Aspergillus ustus (MSF3) isolated from the marine sponge
Fasciospongia cavernosa exhibited the highest E24 index at 3% of NaCl compared to other NaCl concentrations used in the production medium. In the case of bacteria, Rismani et al. [
163] showed that the cell growth of
B. licheniformis was affected by different concentrations of NaCl with optimal cell growth and maximum surface tension reduction observed at 2% NaCl. For yeast,
Trichosporon asahii demonstrated the highest E24 index when 8–10% of NaCl was added into the production medium [
164].
In most findings, bacteria are capable to assimilate to a certain range of salt concentrations which is generally not more than 5%. For examples,
Aeromonas spp. isolated from the tropical estuarine water required 5% of NaCl in MSM medium containing crude oil as the sole carbon source to achieve the highest E24 index compared to a medium devoid of salt or those with salt concentrations higher than 5.0% [
165].
Bacillus sp. isolated from oil reservoirs was capable to reduce surface tension effectively at 5% of NaCl in comparison with 1–15% NaCl addition [
166]. Moreover,
Bacillus sp. (E24 = 70%) and
Pseudomonas sp. (E24 = 79%) yielded maximum biosurfactant in the presence of 0.2% (
w/
v) until 0.8% (
w/
v) of NaCl [
107]. In the case of yeast, it showed that the production of biosurfactant by
C. albicans No. 13 increased as the NaCl concentration increased to 5% but the biosurfactant production decreased gradually when higher concentrations of NaCl were used [
167]. In addition, Saikia et al. [
168] also showed that at a very low concentration of NaCl or without NaCl supplementation to the mineral medium,
P. aeruginosa RS29 showed good production of biosurfactants.
In contrast, higher concentration of NaCl up to 30% could be compromised by
B. licheniformis BAS50 to reduce surface tension to 35 mN/m [
169]. Further increment of salt concentrations was not done in this work. It had been revealed that the intracellular concentration of rhamnolipid might be directly proportional to the external concentration of NaCl which explained this situation. Therefore, any increment of rhamnolipid intracellular concentration will cause the biosurfactant activity to be enhanced followed by the reduction of fat globulli dissimilation or addition of cytoplasmic membrane retention [
170]. To conclude, cellular efficiency will be reduced and viscosity for both aqueous and oil phase will be increased followed by an increment in osmotic pressure at higher NaCl concentration which promotes higher emulsification activity.
For instance, the function of synthetic surfactant was greatly affected at NaCl concentrations higher than 2% which became their drawbacks. Biosurfactants can overcome this weakness by having the ability to resist a higher salinity level [
32]. For example, rhamnolipid produced by bacteria isolated from the Arabian Sea coast of Karachi had the potential to be used in bioremediation for oil spill as the isolates represent the naturally occurring halophilic bacteria surviving in heavily contaminated regions [
171]. On the other hand, halophilic archaeon
Haloarcula sp. IRU1 surviving in production medium that contained 25% of NaCl (olive oil and yeast extract as carbon source and nitrogen source, respectively) demonstrated the maximum E24 index reading of 42.5%. This biosurfactant could be the promising candidate for oil recovery process [
172].