Determination of Arsenic Species in Ophiocordyceps sinensis from Major Habitats in China by HPLC-ICP-MS and the Edible Hazard Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analytical Performances of the Proposed Method
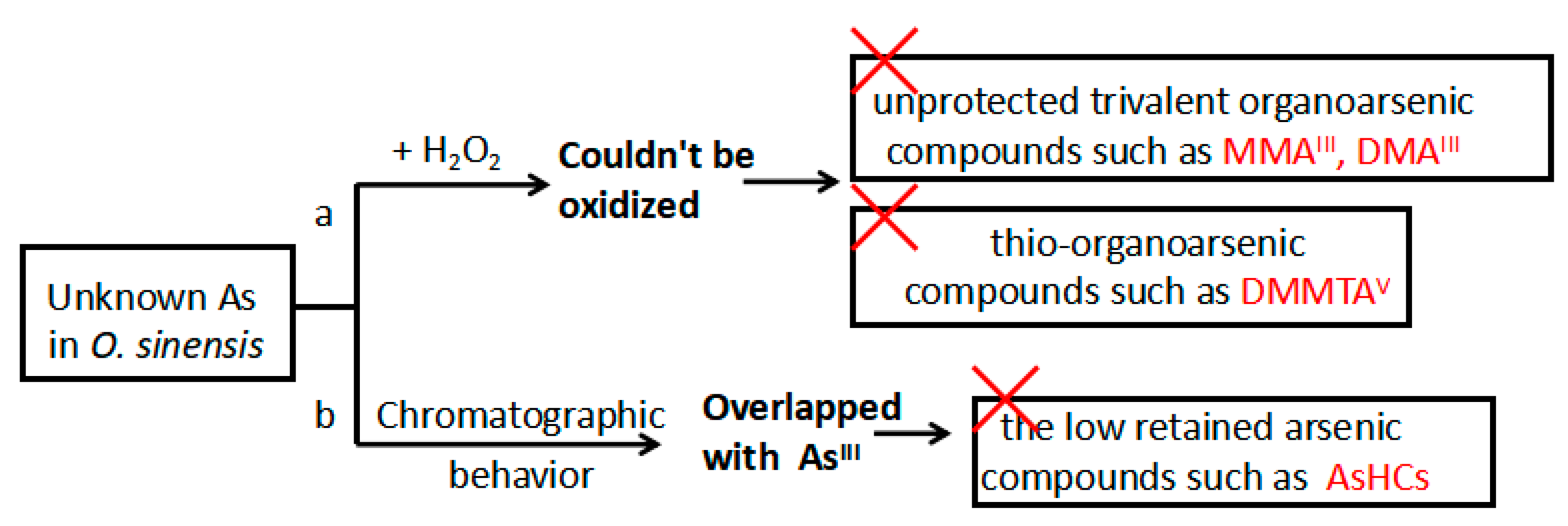
2.2. Total Arsenic Concentration and Arsenic Species in O. sinensis Samples
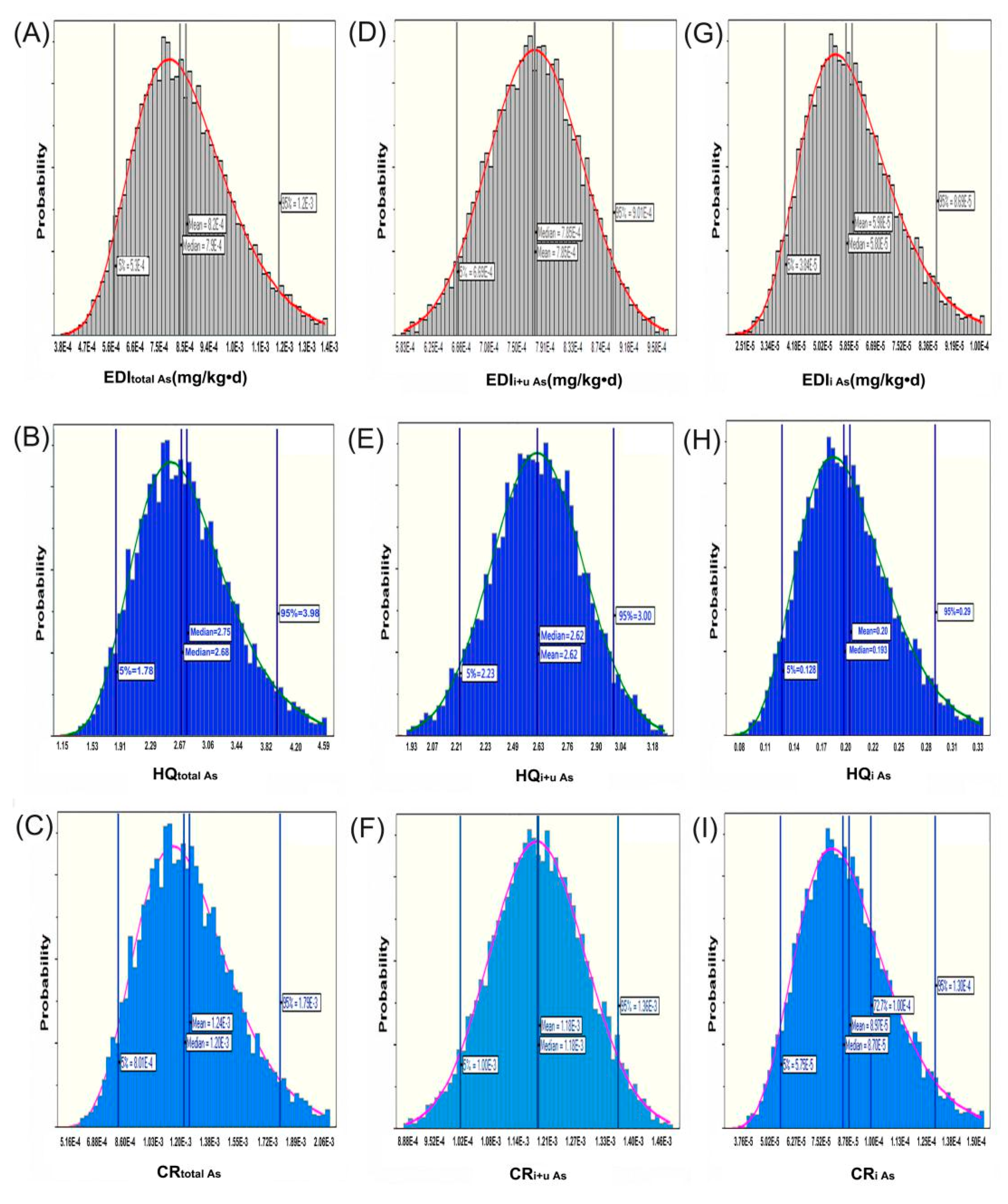
2.3. Hazard Risk Assessment of Long-Term O. sinensis Consumption
3. Materials and Methods
3.1. Reagents
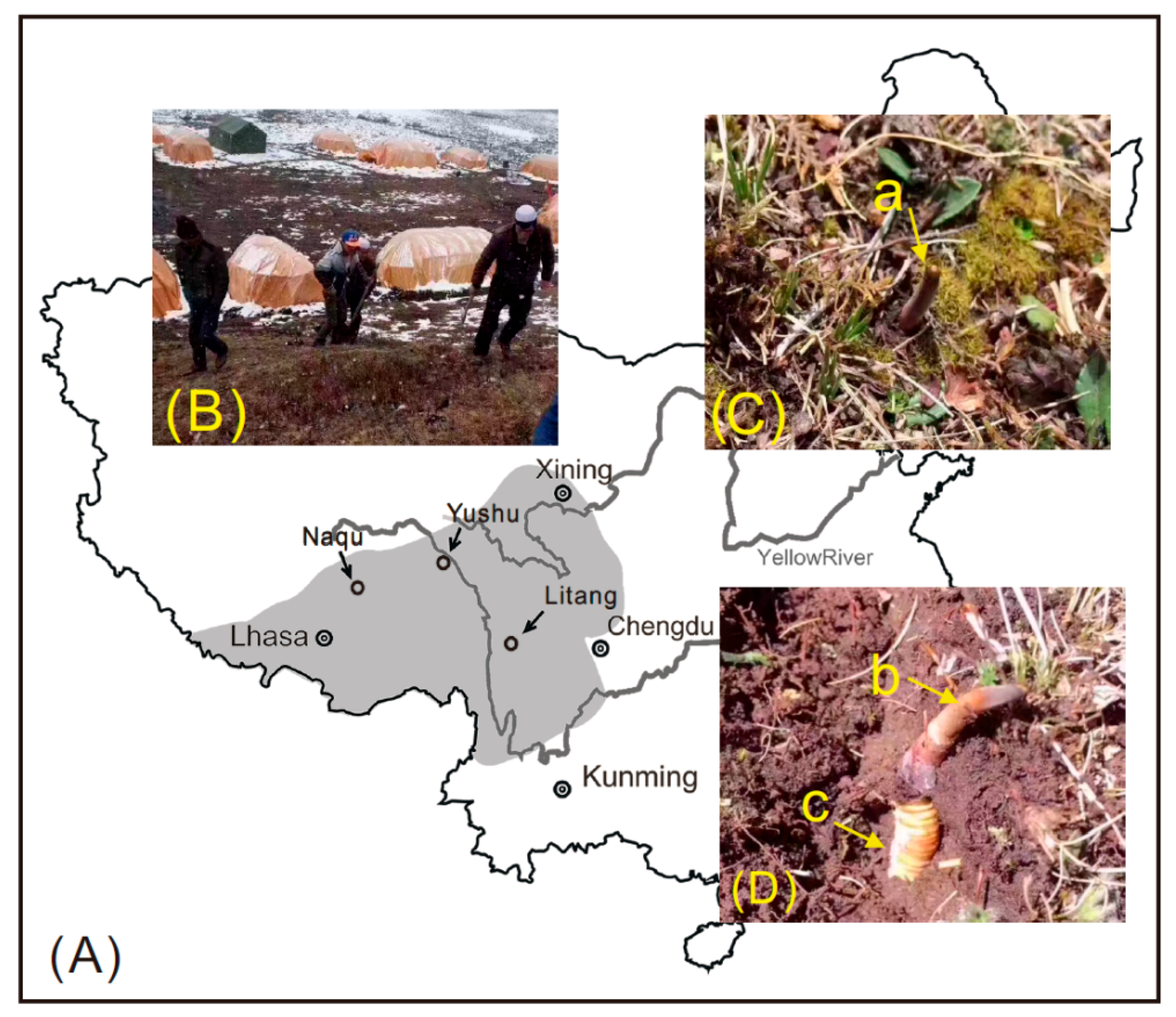
3.2. Sample Collection and Preparation
3.3. Total Arsenic Analysis
3.4. Arsenic Speciation Analysis
3.5. Health Risk Assessment
4. Patents
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| O. sinensis | Ophiocordyceps sinensis |
| As | Arsenic |
| AsШ | Arsenite |
| AsV | Arsenate |
| DMA | Dimethylarsinic acid |
| MMA | Methylarsonic acid |
| AsB | Arsenobetaine |
| DMMTA | Thio-organoarsenic |
| uAs | Unknown organic As |
| oAs | Organic arsenic |
| iAs | Inorganic arsenic |
| i+uAs | Sum of iAs and uAs |
| LOQ | Limits of quantification |
| LOD | Limits of detection |
| EDI | Estimated Daily Intake |
| HQ | Hazard Quotient |
| CR | Cancer Risk |
| HPLC-ICP-MS | High-Performance Liquid Chromatography–Inductively Coupled Plasma Mass Spectrometry |
Appendix A
| Parameters | Units | Distribution |
|---|---|---|
| Adult Body weight, BW | BW kg | Lognormal (Mean = 58.7, SD = 12.0) |
| Daily ingestion rate, IR | kg/day | Lognormal (Mean = 0.01, SD = 0.0143) |
| Total As concentration, Ctotal As | μg/kg | Lognormal (Mean = 4.63, SD = 0.42) |
| i+uAs concentration, Ci+uAs | μg/kg | Lognormal (Mean = 4.53, SD = 0.41) |
| iAs concentration, CiAs | μg/kg | Lognormal (Mean = 0.34, SD = 0.03) |
References
- Dong, C.H.; Wen-Jia, L.I. Cordyceps industry in China: Current status, challenges and perspectives–Jinhu declaration for Cordyceps industry development. Mycosystema 2016, 35, 1–15. [Google Scholar] [CrossRef]
- Guo, L.X.; Hong, Y.H. Fungus-larva relation in the formation of Cordyceps sinensis as revealed by stable carbon isotope analysis. Sci. Rep. 2017, 7, 7789. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ko, Y.F. Myths and realities surrounding the mysterious caterpillar fungus. Trends Biotechnol. 2017, 35, 1017. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Lei, W. Pharmacological study progress of the Cordyceps sinensis. Global Tradit. Chin. Med. 2014, 7, 227–232. [Google Scholar]
- Liu, Y.; Wang, J. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid. Based Complement. Altern. Med. 2015, 2015, 575063. [Google Scholar]
- Caplins, L.; Halvorson, S. Collecting Ophiocordyceps sinensis: An emerging livelihood strategy in the garhwal, indian himalaya. Mt. Sci. 2017, 14, 390–402. [Google Scholar] [CrossRef]
- Winkler, D. Yartsa gunbu (Cordyceps sinensis) and the fungal commodification of Tibet’s rural economy. Econ. Bot. 2008, 62, 291–305. [Google Scholar] [CrossRef]
- Consumption Tips on Cordyceps sinensis Products. Available online: http://samr.cfda.gov.cn/WS01/CL1986/144020.html (accessed on 4 February 2016).
- The Common Standards of Functional Food; GB16740-2014; National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2014.
- Notice on the Suspension of All the Pilot Work on Cordyceps sinensis for Health Food. Available online: http://samr.cfda.gov.cn/WS01/CL0847/146100.html (accessed on 4 March 2016).
- Wang, H.; Shan, Y. The utilization and analysis of present market situation for Cordyceps sinensis. Res. Pract. Chin. Med. 2016, 30, 83–86. [Google Scholar]
- Tsuda, T.; Babazono, A. Inorganic arsenic: A dangerous enigma for mankind. Appl. Organomet. Chem. 2010, 6, 309–322. [Google Scholar] [CrossRef]
- Khairul, I.; Wang, Q.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Arnold, L.L.; Eldan, M.; Lewis, A.S.; Beck, B.D. Methylated arsenicals: The implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit. Rev. Toxicol. 2006, 36, 99–133. [Google Scholar] [CrossRef] [PubMed]
- Styblo, M.; Razo, L.M.D. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch. Toxicol. 2000, 74, 289. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ke, Q. Molecular mechanisms of arsenic carcinogenesis. Mol. Cell. Biochem. 2004, 255, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Hua, N.; Carew, M.W. Comparative toxicity of arsenic metabolites in human bladder cancer EJ-1 cells. Chem. Res. Toxicol. 2011, 24, 1586–1596. [Google Scholar]
- Meyer, S.; Matissek, M.; Müller, S.M.; Taleshi, M.S.; Ebert, F.; Francesconi, K.A.; Schwerdtle, T. In vitro toxicological characterisation of three arsenic-containing hydrocarbons. Metallomics 2014, 6, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wang, G. Arsenic speciation analysis of 15 traditional Chinese medicines. Instrum. Anal. 2009, 28, 918–921. [Google Scholar]
- Cao, X.; Wang, J. Analysis of arsenic speciation in Cordyceps sinensis in Tibet by HPLC-HG-AFS. Chin. Tradit. Patent Med. 2015, 37, 1985–1989. [Google Scholar]
- Gómezariza, J.L.; Sánchezrodas, D. A comparison between ICP-MS and AFS detection for arsenic speciation in environmental samples. Talanta 2000, 51, 257. [Google Scholar] [CrossRef]
- Popp, M.; Hann, S. Environmental application of elemental speciation analysis based on liquid or gas chromatography hyphenated to inductively coupled plasma mass spectrometry—A review. Anal. Chim. Acta 2010, 668, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Sankararamakrishnan, N.; Mishra, S. A Comprehensive Review on Various Analytical Methods for the Determination of Inorganic and Organic Arsenic in Environmental Samples. In Environmental Contaminants; Springer: Singapore, 2018. [Google Scholar]
- U.S. EPA. Risk Assessment Guidance for Superfund (RAGS). In Human Health Evaluation Manual (Part A); EPA Publication: Washington, DC, USA, 1989. [Google Scholar]
- U.S. EPA. Integrated Risk Information System (IRIS). In Arsenic, Inorganic (CASRN 7440-38-2)-748; EPA Publication: Washington, DC, USA, 2009. [Google Scholar]
- Francesconi, K.A.; Goessler, W.; Panutrakul, S.; Irgolic, K.J. A novel arsenic containing riboside (arsenosugar) in three species of gastropod. Sci. Total Environ. 1998, 221, 139–148. [Google Scholar] [CrossRef]
- Sele, V.; Sloth, J.J.; Holmelid, B.; Valdersnes, S.; Skov, K.; Amlund, H. Arsenic-containing fatty acids and hydrocarbons in marine oils—Determination using reversed-phase HPLC-ICP-MS and HPLC-qTOF-MS. Talanta 2014, 121, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Niegel, C.; Matysik, F.M. Analytical methods for the determination of arsenosugars—A review of recent trends and developments. Anal. Chim. Acta 2010, 657, 83. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.; Hansen, M. Speciation and health risk considerations of arsenic in the edible mushroom Laccaria amethystina collected from contaminated and uncontaminated locations. Appl. Organomet. Chem. 1998, 12, 285–291. [Google Scholar] [CrossRef]
- Kuehnelt, D.; Goessler, W. Arsenic compounds in terrestrial organisms I: Collybiamaculata, Collybiabutyracea and Amanita muscaria from arsenic smelter sites in Austria. Appl. Organomet. Chem. 1997, 11, 289–296. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Q. Determination of arsenic species in Edible mushrooms by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry. Food Anal. Methods 2017, 10, 740–748. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Verstovsek, S. Chemical and clinical development of darinaparsin, a novel organic arsenic derivative. Anti-Cancer Agents Med. Chem. 2008, 8, 904. [Google Scholar] [CrossRef]
- Kuntze, A.M.; Braz, O. Development of an organic arsenic derivative as a therapy for leukaemia. Spies Int. Symp. Opt. Sci. 2003, 2003, 347–355. [Google Scholar]
- Tang, L.; Zhang, L.L. Arsenic speciation of dried Lentinus edodes by HPLC-ICP-MS. Food Ind. 2015, 36, 275–277. [Google Scholar]
- Feng-Lei, X.U.; Wen-Ying, H. Clinical efficacy of Cordyceps sinensis for chronic kidney diseases: A systematic review. Chin. J. Evid. Based Med. 2006, 6, 804–808. [Google Scholar]
- Ma, L.; Yang, Z.; Kong, Q.; Wang, L. Extraction and determination of arsenic species in leafy vegetables: Method development and application. Food Chem. 2017, 217, 524–530. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds AsB, DMA, MMA, AsIII, AsV and uAs are available from the authors. |
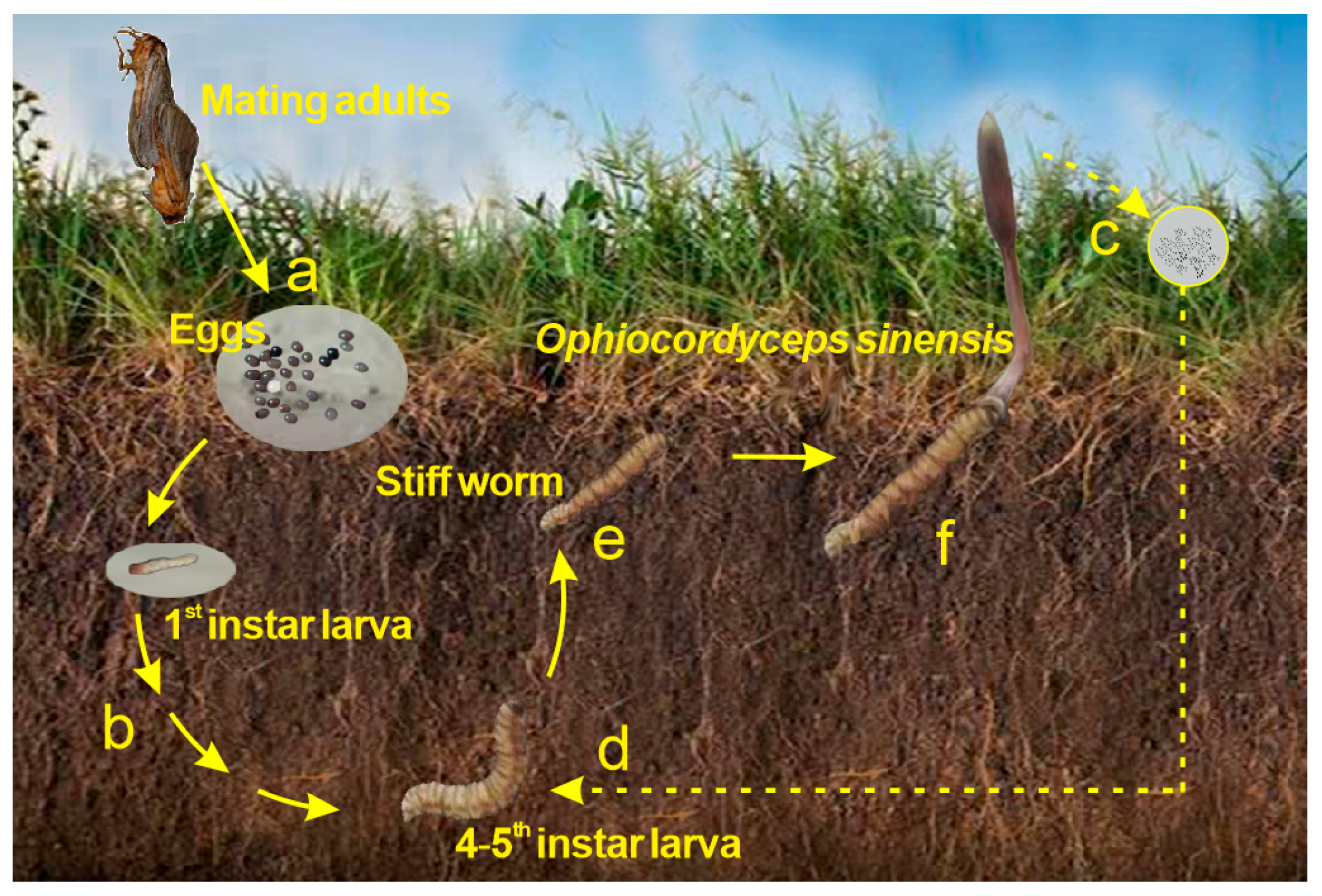
 AsШ and
AsШ and  AsV) are shown in red and yellow sections, and organic As (
AsV) are shown in red and yellow sections, and organic As (  AsB and
AsB and  uAs) is shown in dark and light gray sections.
uAs) is shown in dark and light gray sections.
 AsШ and
AsШ and  AsV) are shown in red and yellow sections, and organic As (
AsV) are shown in red and yellow sections, and organic As (  AsB and
AsB and  uAs) is shown in dark and light gray sections.
uAs) is shown in dark and light gray sections.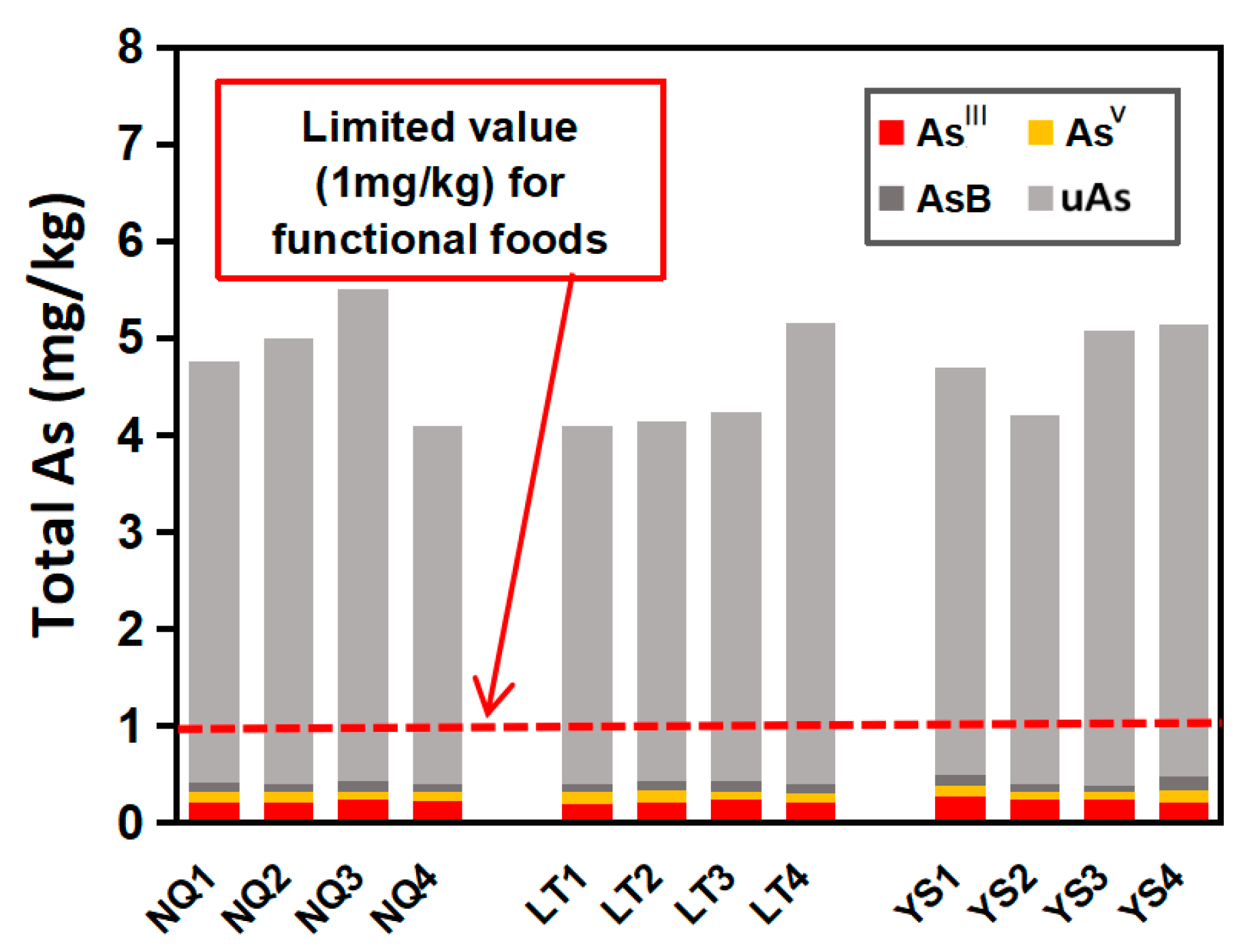
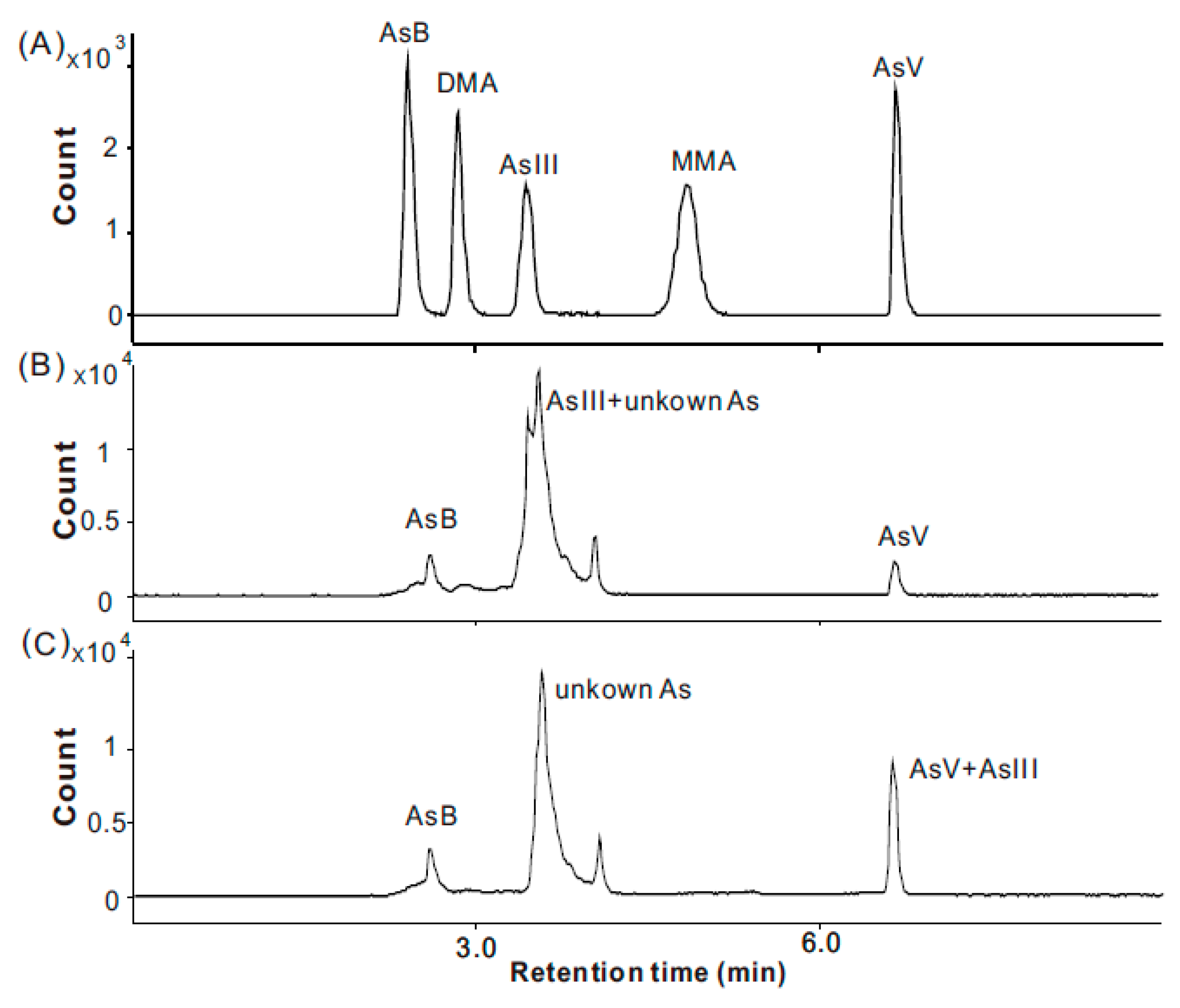
| Analytes | Linear Range (μg/L) | Linear Equation | R | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|
| Total As | 0.5–500 | y = 2731.03x + 5.5567 | 0.9998 | 2.3 | 6.9 |
| AsB | 0.2–300 | y = 18,867.9x + 2319.6 | 0.9999 | 1.1 | 3.3 |
| DMAV | 0.2–300 | y = 19,212.4x + 4065.2 | 0.9999 | 1.3 | 4.0 |
| AsШ | 0.2–300 | y = 17,090.6x + 3289.1 | 0.9997 | 1.0 | 3.0 |
| MMAV | 0.5–300 | y = 18,373.6x + 196.8 | 1.0000 | 2.2 | 6.6 |
| AsV | 0.2–300 | y = 18,504.6x + 8247.2 | 1.0000 | 1.1 | 3.3 |
| Analytes | Background Value (mg/kg) | Added (μg/L) | Measured Value (μg/L) | Recovery (%) | RSD (%, n = 6) |
|---|---|---|---|---|---|
| Total As | 0.51 | 5.00 | 14.8~15.3 | 92.3~99.4 | 4.6 |
| 10.0 | 20.4~21.5 | 94.7~102.3 | 3.8 | ||
| 50.0 | 60.7~64.3 | 95.8~106.6 | 2.4 | ||
| AsB | 0.010 | 2.00 | 2.09~2.26 | 91.7~100.1 | 4.2 |
| 10.0 | 9.45~9.87 | 91.9~96.2 | 1.9 | ||
| 50.0 | 42.0~47.0 | 83.6~93.4 | 5.0 | ||
| DMAV | ND | 2.00 | 1.73~1.98 | 86.5~99.0 | 5.7 |
| 10.0 | 9.20~9.55 | 92.0~95.5 | 1.5 | ||
| 50.0 | 50.4~52.6 | 100.9~105.2 | 1.7 | ||
| AsШ | 1.70 | 2.00 | 45.3~45.6 | 86.3~99.2 | 5.5 |
| 10.0 | 52.8~53.7 | 88.8~98.0 | 4.3 | ||
| 50.0 | 96.8~99.2 | 106.9~111.7 | 2.0 | ||
| MMAV | 0.0031 | 2.00 | 2.07~2.19 | 99.4~105.7 | 2.7 |
| 10.0 | 10.6~10.8 | 105.0~107.2 | 0.85 | ||
| 50.0 | 47.2~48.6 | 94.2~97.1 | 1.2 | ||
| AsV | 0.12 | 2.00 | 4.81~5.08 | 86.6~100.0 | 6.6 |
| 10.0 | 12.8~13.0 | 97.4~99.4 | 0.80 | ||
| 50.0 | 49.0~50.2 | 91.9~94.4 | 1.1 |
| Sample Type | Reference Materials | Certified Value (mg/kg) | Determined Value (mg/kg) | Recovery (%) |
|---|---|---|---|---|
| Green Chinese onion | GBW10049 | 0.52 ± 0.11 | 0.507 ± 0.08 | 97.5 |
| Pork liver | GBW10051 | 1.4 ± 0.3 | 1.42 ± 0.15 | 101.4 |
| Yellow-fin tuna | GBW08573 | 5.08 ± 0.39 | 4.98 ± 0.11 | 98.0 |
| Rice | GBW100358 | 0.16 ± 0.02 (total As) | 0.165 ± 0.012 | 103.1 |
| 0.13 ± 0.02 (iAs) | 0.144 ± 0.006 | 110.8 |
| Sample Name | AsB b mg/kg (%) | DMAV | MMAV | uAs mg/kg (%) | AsШ mg/kg (%) | AsV mg/kg (%) | iAs mg/kg (%) | oAs mg/kg (%) | Total As mg/kg |
|---|---|---|---|---|---|---|---|---|---|
| NQ1 | 0.10 (2.1%) | nd c | nd | 4.34 (91.2%) | 0.22 (4.6%) | 0.10 (2.1%) | 0.32 (6.7%) | 4.44 (93.3%) | 4.76 |
| NQ2 | 0.09 (1.8%) | nd | nd | 4.59 (91.8%) | 0.21 (4.2%) | 0.11 (2.2%) | 0.32 (6.4%) | 4.68 (93.6%) | 5.00 |
| NQ3 | 0.12 (2.3%) | nd | nd | 4.81(91.6%) | 0.25 (4.8%) | 0.07 (1.3%) | 0.32 (6.1%) | 4.93 (93.9%) | 5.25 |
| NQ4 | 0.08 (2.0%) | nd | nd | 3.69 (90.0%) | 0.23 (5.6%) | 0.10 (2.4%) | 0.33 (8.0%) | 3.77 (92.0%) | 4.10 |
| LT1 | 0.07 (1.7%) | nd | nd | 3.69 (90.2%) | 0.20 (4.9%) | 0.13 (3.2%) | 0.33 (8.1%) | 3.76 (91.9%) | 4.09 |
| LT2 | 0.09 (2.2%) | nd | nd | 3.72 (89.6%) | 0.22 (5.3%) | 0.12 (2.9%) | 0.34 (8.2%) | 3.81 (91.8%) | 4.15 |
| LT3 | 0.11 (2.8%) | nd | nd | 3.56 (89.0%) | 0.24 (6.0%) | 0.09 (2.3%) | 0.33 (8.3%) | 3.67 (91.7%) | 4.00 |
| LT4 | 0.09 (1.7%) | nd | nd | 4.75 (92.2%) | 0.21 (4.1%) | 0.10 (1.9%) | 0.31 (6.0%) | 4.84 (94.0%) | 5.15 |
| YS1 | 0.12 (2.6%) | nd | nd | 4.19 (89.3%) | 0.27 (5.8%) | 0.11 (2.3%) | 0.38 (8.1%) | 4.31 (91.9%) | 4.69 |
| YS2 | 0.08 (1.9%) | nd | nd | 3.80 (90.5%) | 0.24 (5.7%) | 0.08 (1.9%) | 0.32 (7.6%) | 3.88 (92.4%) | 4.20 |
| YS3 | 0.07 (1.4%) | nd | nd | 4.69 (92.3%) | 0.25 (4.9%) | 0.07 (1.4%) | 0.32 (6.3%) | 4.76 (93.7%) | 5.08 |
| YS4 | 0.15 (2.9%) | nd | nd | 4.65 (90.5%) | 0.22 (4.3%) | 0.12 (2.3%) | 0.34 (6.6%) | 4.8 (93.4%) | 5.14 |
| AVR d | 0.09 (1.9%) | nd | nd | 4.21 (90.9%) | 0.23 (5.0%) | 0.10 (2.2%) | 0.33 (7.1%) | 4.3 (92.9%) | 4.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.-X.; Zhang, G.-W.; Wang, J.-T.; Zhong, Y.-P.; Huang, Z.-G. Determination of Arsenic Species in Ophiocordyceps sinensis from Major Habitats in China by HPLC-ICP-MS and the Edible Hazard Assessment. Molecules 2018, 23, 1012. https://doi.org/10.3390/molecules23051012
Guo L-X, Zhang G-W, Wang J-T, Zhong Y-P, Huang Z-G. Determination of Arsenic Species in Ophiocordyceps sinensis from Major Habitats in China by HPLC-ICP-MS and the Edible Hazard Assessment. Molecules. 2018; 23(5):1012. https://doi.org/10.3390/molecules23051012
Chicago/Turabian StyleGuo, Lian-Xian, Gui-Wei Zhang, Jia-Ting Wang, Yue-Ping Zhong, and Zhi-Gang Huang. 2018. "Determination of Arsenic Species in Ophiocordyceps sinensis from Major Habitats in China by HPLC-ICP-MS and the Edible Hazard Assessment" Molecules 23, no. 5: 1012. https://doi.org/10.3390/molecules23051012
APA StyleGuo, L.-X., Zhang, G.-W., Wang, J.-T., Zhong, Y.-P., & Huang, Z.-G. (2018). Determination of Arsenic Species in Ophiocordyceps sinensis from Major Habitats in China by HPLC-ICP-MS and the Edible Hazard Assessment. Molecules, 23(5), 1012. https://doi.org/10.3390/molecules23051012




