Highly Stable Zr(IV)-Based Porphyrinic Metal−Organic Frameworks as an Adsorbent for the Effective Removal of Gatifloxacin from Aqueous Solution
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instruments
2.2. PCN-224 NP Synthesis
2.3. Adsorption and Removal of GTF by PCN-224 NPs
3. Results and Discussion
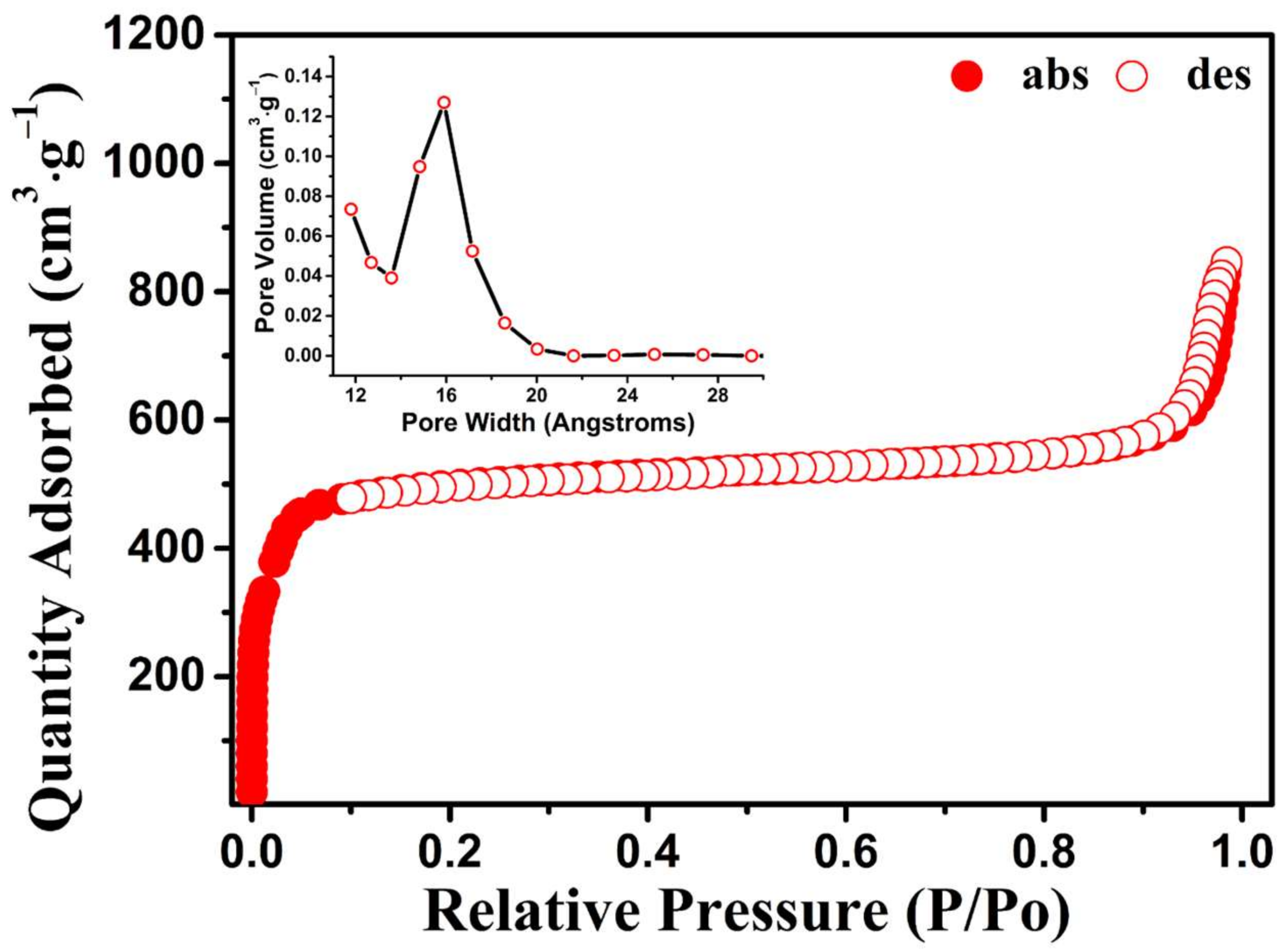
3.1. Characterization of PCN-224 NPs
3.2. Adsorption Capacity and Removal Percentage
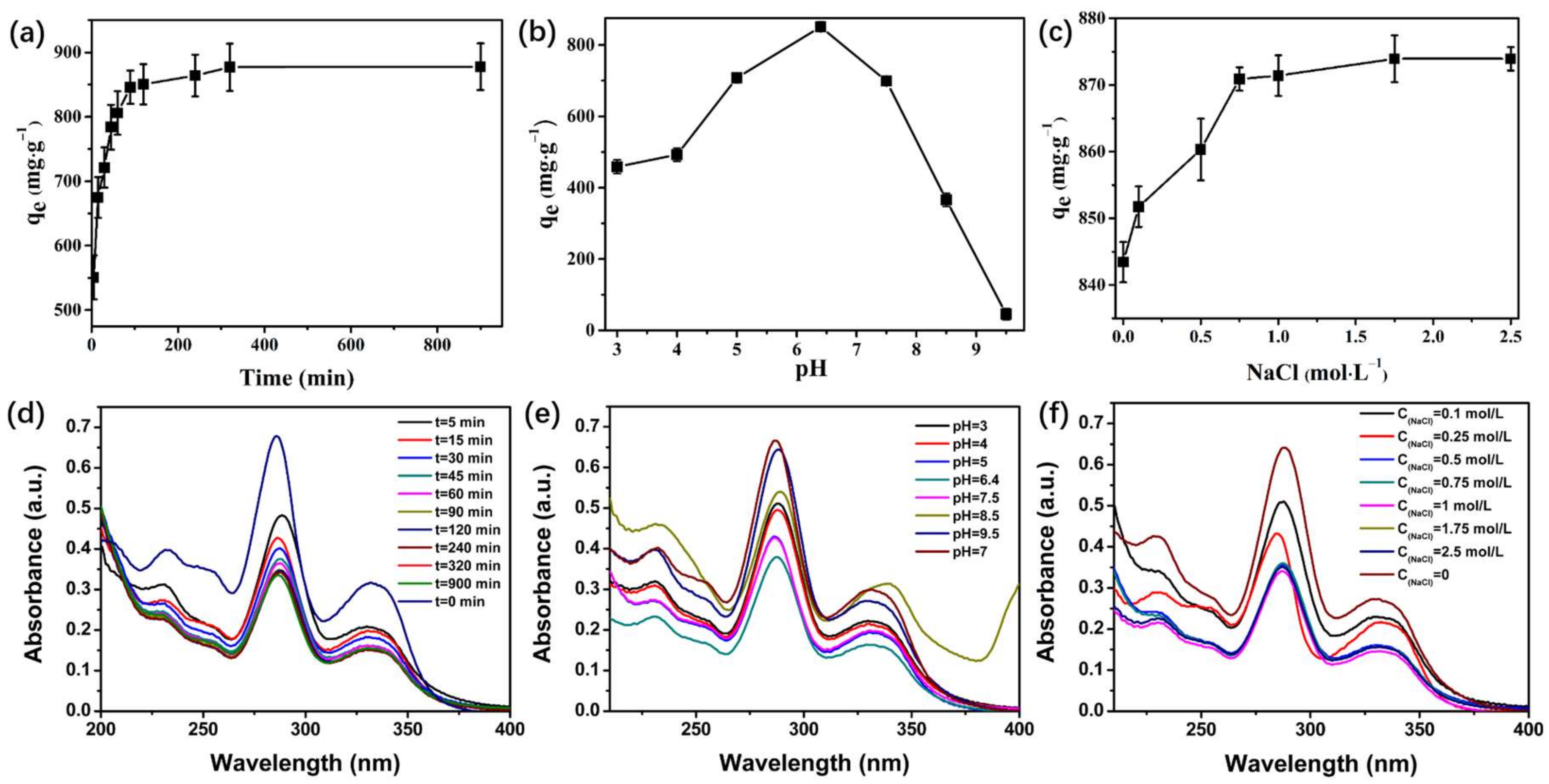
3.3. Effect of Contact Time
3.4. Effect of pH
3.5. Effect of Ionic Strength
3.6. Adsorption Kinetics
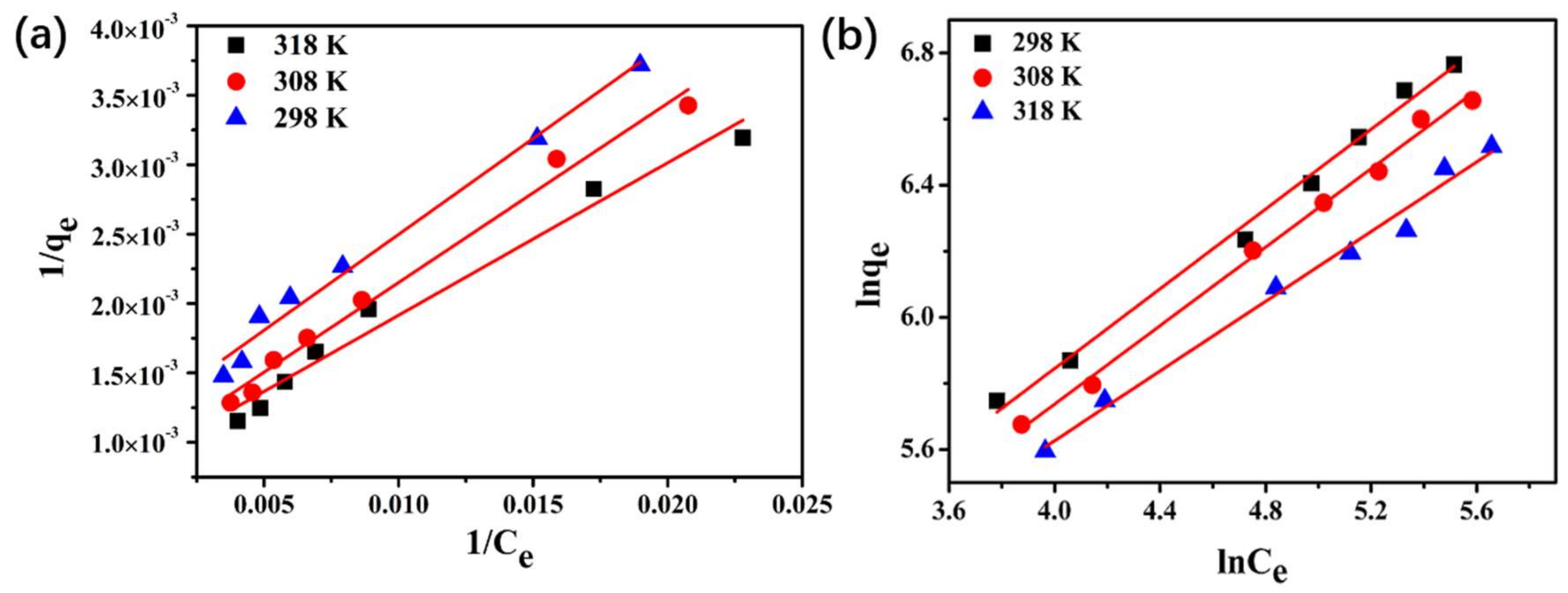
3.7. GTF Adsorption Isotherms
3.8. GTF Adsorption Thermodynamics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, L.J.; Ying, G.G.; Liu, S.; Zhao, J.L.; Yang, B.; Chen, Z.F.; Lai, H.J. Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci. Total Environ. 2013, 452, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Shi, Y.L.; Gao, L.H.; Liu, J.M.; Cai, Y.Q. Occurrence and removal of antibiotics in a municipal wastewater reclamation plant in Beijing, China. Chemosphere 2013, 92, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.L. Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere 2014, 95, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Li, M.X.; Guo, C.S.; An, D.; Xu, J.; Zhang, Y.; Xi, B.D. Distribution and ecological risk of antibiotics in a typical effluent receiving river (Wangyang River) in north China. Chemosphere 2014, 112, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Kolpine, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.R.; Wang, Q.J.; Mo, C.H.; Li, Y.W.; Gao, P.; Tai, Y.P.; Zhang, Y.; Ruan, Z.L.; Xu, J.W. Determination of four fluoroquinolone antibiotics in tap water in Guangzhou and Macao. Environ. Pollut. 2010, 158, 2350–2358. [Google Scholar]
- Oberle, K.; Capdeville, M.J.; Berthe, T.; Budzinski, H.; Petit, F. Evidence for a Complex Relationship between Antibiotics and Antibiotic-Resistant Escherichia coli: From Medical Center Patients to a Receiving Environment. Environ. Sci. Technol. 2012, 46, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Yi, Y.J.; Yang, Z.F.; Cheng, X. Water pollution risk simulation and prediction in the main canal of the South-to-North Water Transfer Project. J. Hydrol. 2014, 519, 2111–2120. [Google Scholar] [CrossRef]
- Holmstrom, K.; Graslund, S.; Wahlstrom, A.; Poungshompoo, S.; Bengtsson, B.E.; Kautsky, N. Antibiotic use in shrimp farming and implications for environmental impacts and human health. Int. J. Food Sci. Technol. 2003, 38, 255–266. [Google Scholar] [CrossRef]
- Kim, S.; Aga, D.S. Potential Ecological and Human Health Impacts of Antibiotics and Antibiotic-Resistant Bacteria from Wastewater Treatment Plants. J. Toxicol. Environ. Health 2007, 10, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Qiu, X.Y.; Chen, B.; Yu, X.G.; Liu, Z.H.; Zhong, G.P.; Li, H.Y.; Chen, M.; Sun, G.D.; Huang, H.; et al. Antibiotics pollution in Jiulong River estuary: Source, distribution and bacterial resistance. Chemosphere 2011, 84, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Zhang, G.; Li, X.D.; Zou, S.C.; Li, P.; Hu, Z.H.; Li, J. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Hua, Y.Q.; Zhu, F.F.; Gu, W.; Jiang, J.H.; Shen, H.Q.; Shi, W.D. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 p-n junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B Environ. 2017, 202, 518–527. [Google Scholar] [CrossRef]
- Pan, S.F.; Zhu, M.P.; Chen, J.P.; Yuan, Z.H.; Zhong, L.B.; Zheng, Y.M. Separation of tetracycline from wastewater using forward osmosis process with thin film composite membrane-Implications for antibiotics recovery. Sep. Purif. Technol. 2015, 153, 76–83. [Google Scholar] [CrossRef]
- Choi, K.J.; Son, H.J.; Kim, S.H. Ionic treatment for removal of sulfonamide and tetracycline classes of antibiotic. Sci. Total Environ. 2007, 387, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.X.; Zhang, H.M.; Feng, Y.J.; Yang, F.L.; Zhang, J.P. Removal of trace antibiotics from wastewater: A systematic study of nano filtration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Azimi, S.; Nezamzadeh-Ejhieh, A. Enhanced activity of clinoptilolite-supported hybridized PbS-CdS semiconductors for the photocatalytic degradation of a mixture of tetracycline and cephalexin aqueous solution. J. Catal. A Chem. 2015, 408, 152–160. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Torrellas, S.Á.; Ribeiro, R.S.; Gomes, H.T.; Ovejero, G.; García, J. Removal of antibiotic compounds by adsorption using glycerol-based carbon materials. Chem. Eng. J. 2016, 296, 277–288. [Google Scholar] [CrossRef]
- Genc, N.; Dogan, E.C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin. Water Treat. 2013, 53, 785–793. [Google Scholar] [CrossRef]
- Chao, Y.H.; Zhu, W.S.; Yan, B.; Lin, Y.B.; Xun, S.H.; Ji, H.Y.; Wu, X.Y.; Li, H.M.; Han, C.R. Macroporous Polystyrene Resins as Adsorbents for the Removal of Tetracycline Antibiotics from an Aquatic Environment. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Gao, J.S.; Zhang, X.Y.; Xu, S.T.; Tan, F.; Li, X.Y.; Zhang, Y.B.; Qu, Z.P.; Quan, X.; Liu, J. Clickable Periodic Mesoporous Organosilicas: Synthesis, Click Reactions, and Adsorption of Antibiotics. Chem. Eur. J. 2014, 20, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Sun, D.M.; Gao, J.S.; Zhao, Q.; Wang, X.C.; Teng, F.; Quan, X.; Chen, J.W. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J. Hazard. Mater. 2013, 244, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.S.; Medeiros, T.P.V.; Ardisson, J.D.; Lago, R.M. Role of [FeOx(OH)y] surface sites on the adsorption of β-lactamic antibiotics on Al2O3 supported Fe oxide. J. Hazard. Mater. 2016, 317, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Wang, X.; Chen, X.F.; Wang, M.L.; Zhao, R.S. In situ hydrothermal growth of ytterbium-based metal-organic framework on stainless steel wire for solid-phase microextraction of polycyclic aromatic hydrocarbons from environmental samples. J. Chromatogr. A 2015, 1415, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tokalıoglu, S.; Yavuz, E.; Selçuk, D.; Patat, S. Zirconium-based highly porous metal-organic framework (MOF-545) as an efficient adsorbent for vortex assisted-solid phase extraction of lead from cereal, beverage and water samples. Food Chem. 2017, 237, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.D.; Lv, H.T.; Shan, S.Y.; Jia, Q.M.; Su, H.Y.; Tian, N.; He, S.C. Porous structured MIL-101 synthesized with different mineralizers for adsorptive removal of oxytetracycline from aqueous solution. RSC Adv. 2016, 6, 73741–73747. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.L.; Feng, D.W.; Xie, L.H.; Zhang, J.; Li, M.; Xie, Y.B.; Li, J.R.; Zhou, H.C. Highly Stable Zr(IV)-Based Metal-Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jiang, Q.; Feng, D.W.; Mao, L.Q.; Zhou, H.C. Size-Controlled Synthesis of Porphyrinic Metal−Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


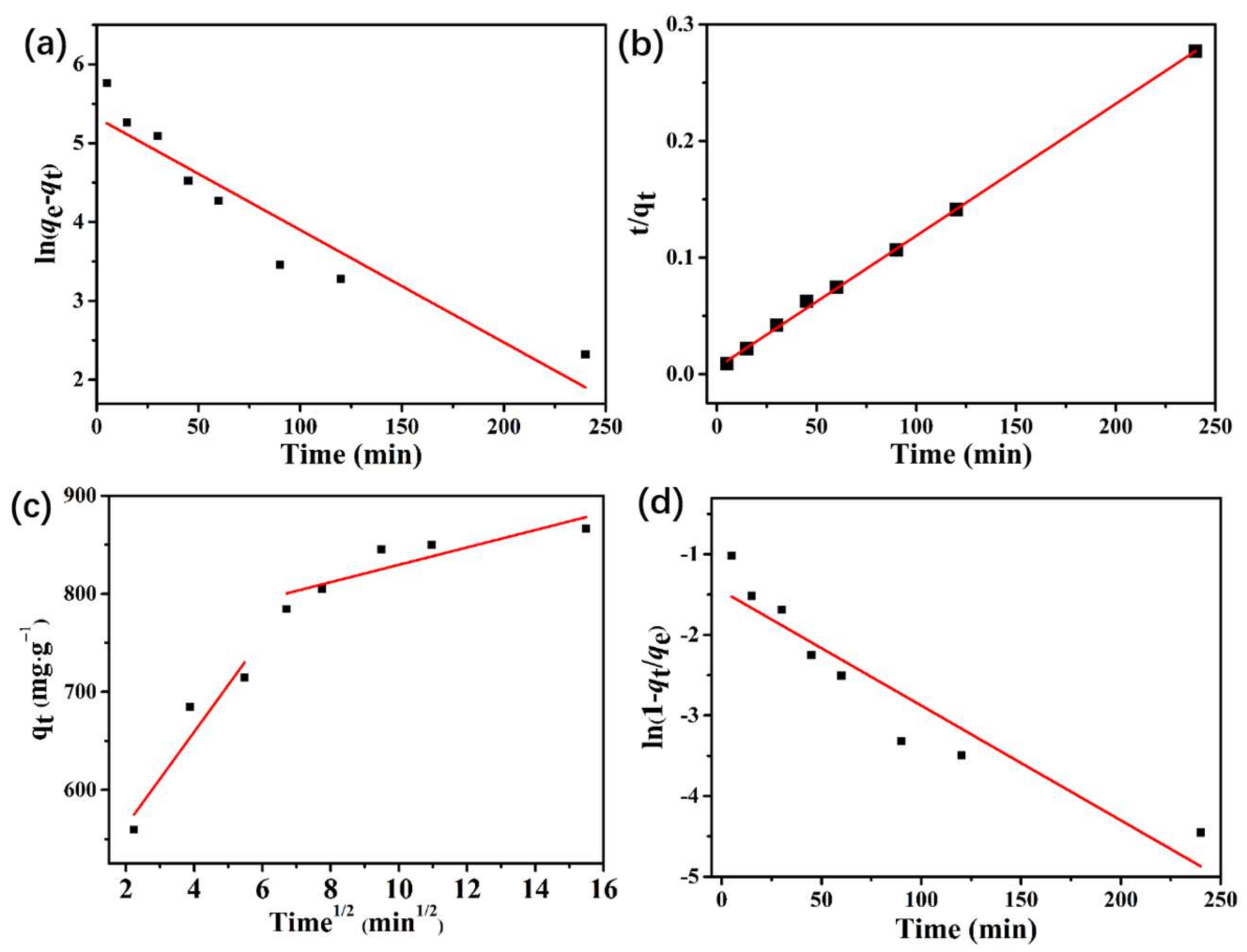
| qe,exp (mg·g−1) | Pseudo-First Order | Pseudo-Second Order | ||||
| qe,cal (mg·g−1) | k1 × 10−3 (min−1) | R2 | qe,cal (mg·g−1) | k2×10−3 (g·mg−1·min−1) | R2 | |
| 876.7694 | 205.0779 | 14.24 | 0.8741 | 877.1929 | 2.3 | 0.9997 |
| Intraparticle Diffusion | Liquid-Film Diffusion | |||||
| kd (mg·g−1·min−1) | I | R2 | kf (min−1) | A | R2 | |
| 8.8577 | 740.97517 | 0.7251 | 0.01424 | −1.45284 | 0.8741 | |
| Isotherm Model | Parameters | Temperature (K) | ||
|---|---|---|---|---|
| 298 | 308 | 318 | ||
| Langmuir | b | 8.106 × 10−3 | 7.744 × 10−3 | 6.654 × 10−3 |
| qm | 1223.135 | 1163.643 | 892.857 | |
| R2 | 0.973 | 0.986 | 0.983 | |
| Freundlich | KF | 33.693 | 30.905 | 28.817 |
| 1/n | 0.527 | 0.594 | 0.603 | |
| R2 | 0.992 | 0.994 | 0.985 | |
| Temperature (K) | lnKc | ∆Gθ (KJ·mol−1) | ∆Hθ (KJ·mol−1) | ∆Sθ (KJ·mol−1) |
|---|---|---|---|---|
| 298 | 2.3619 | −5.85 | −12.61 | 0.0228 |
| 308 | 2.1347 | −5.46 | ||
| 318 | 2.0434 | −5.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-J.; Wang, L.-J.; Xu, G.-J.; Wang, X.; Zhao, R.-S. Highly Stable Zr(IV)-Based Porphyrinic Metal−Organic Frameworks as an Adsorbent for the Effective Removal of Gatifloxacin from Aqueous Solution. Molecules 2018, 23, 937. https://doi.org/10.3390/molecules23040937
Chen J-J, Wang L-J, Xu G-J, Wang X, Zhao R-S. Highly Stable Zr(IV)-Based Porphyrinic Metal−Organic Frameworks as an Adsorbent for the Effective Removal of Gatifloxacin from Aqueous Solution. Molecules. 2018; 23(4):937. https://doi.org/10.3390/molecules23040937
Chicago/Turabian StyleChen, Jing-Jing, Li-Juan Wang, Gui-Ju Xu, Xia Wang, and Ru-Song Zhao. 2018. "Highly Stable Zr(IV)-Based Porphyrinic Metal−Organic Frameworks as an Adsorbent for the Effective Removal of Gatifloxacin from Aqueous Solution" Molecules 23, no. 4: 937. https://doi.org/10.3390/molecules23040937




