Pectin from Citrus Canning Wastewater as Potential Fat Replacer in Ice Cream
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition of Pectin
2.2. Rheological Properties of Pectin
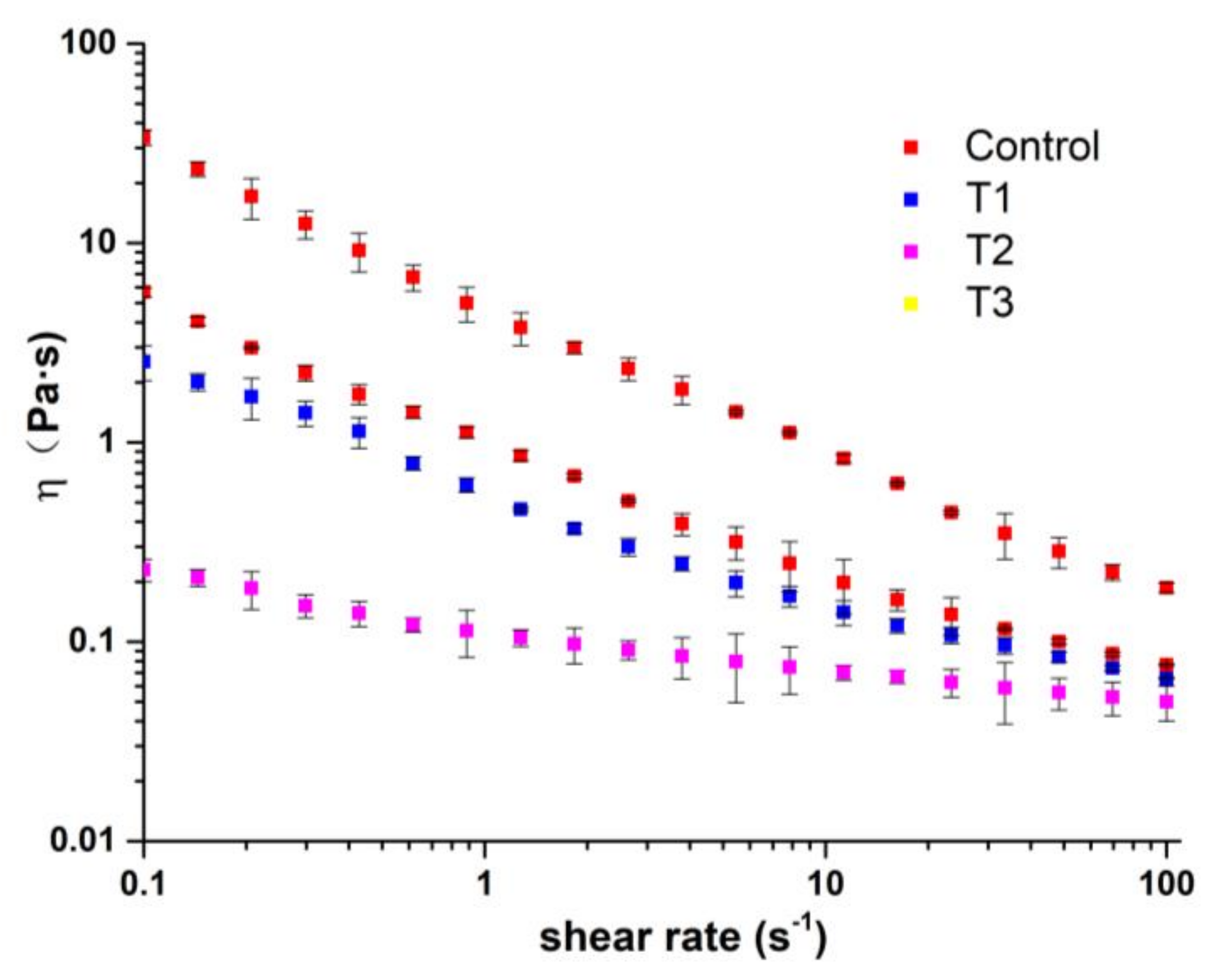
2.3. Rheological Properties of the Ice Cream Mix
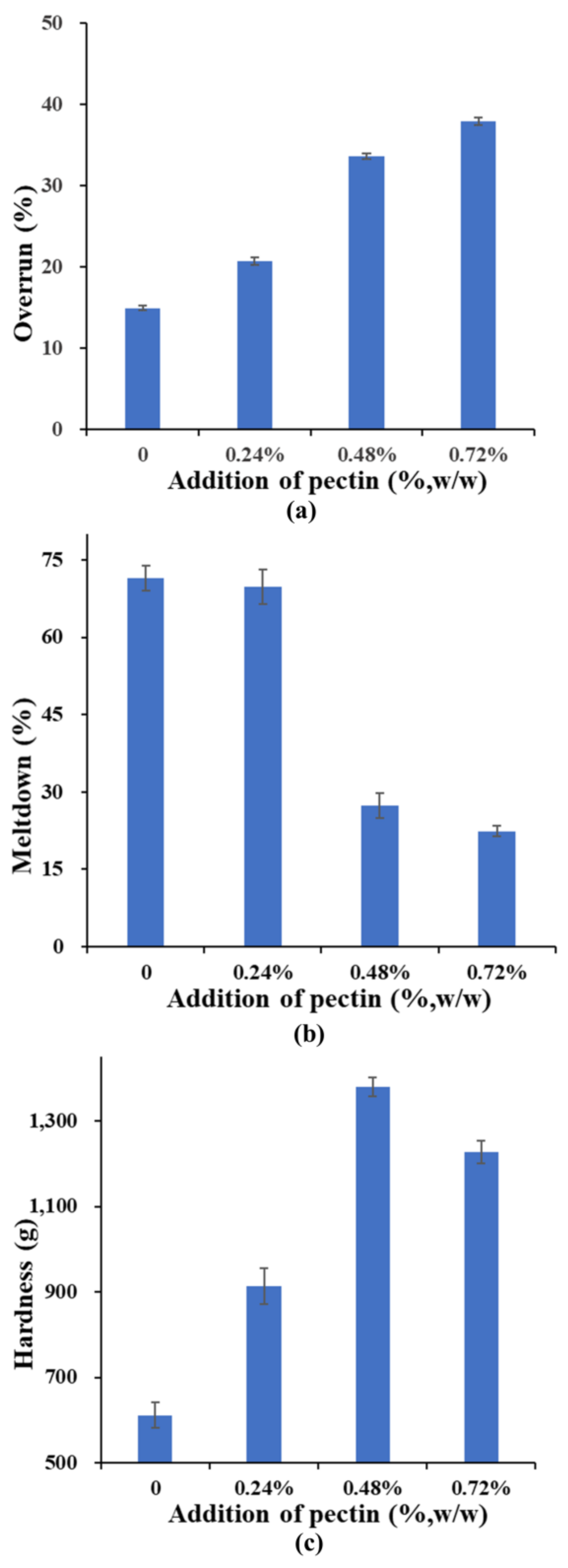
2.4. Physicochemical Properties of Ice Cream
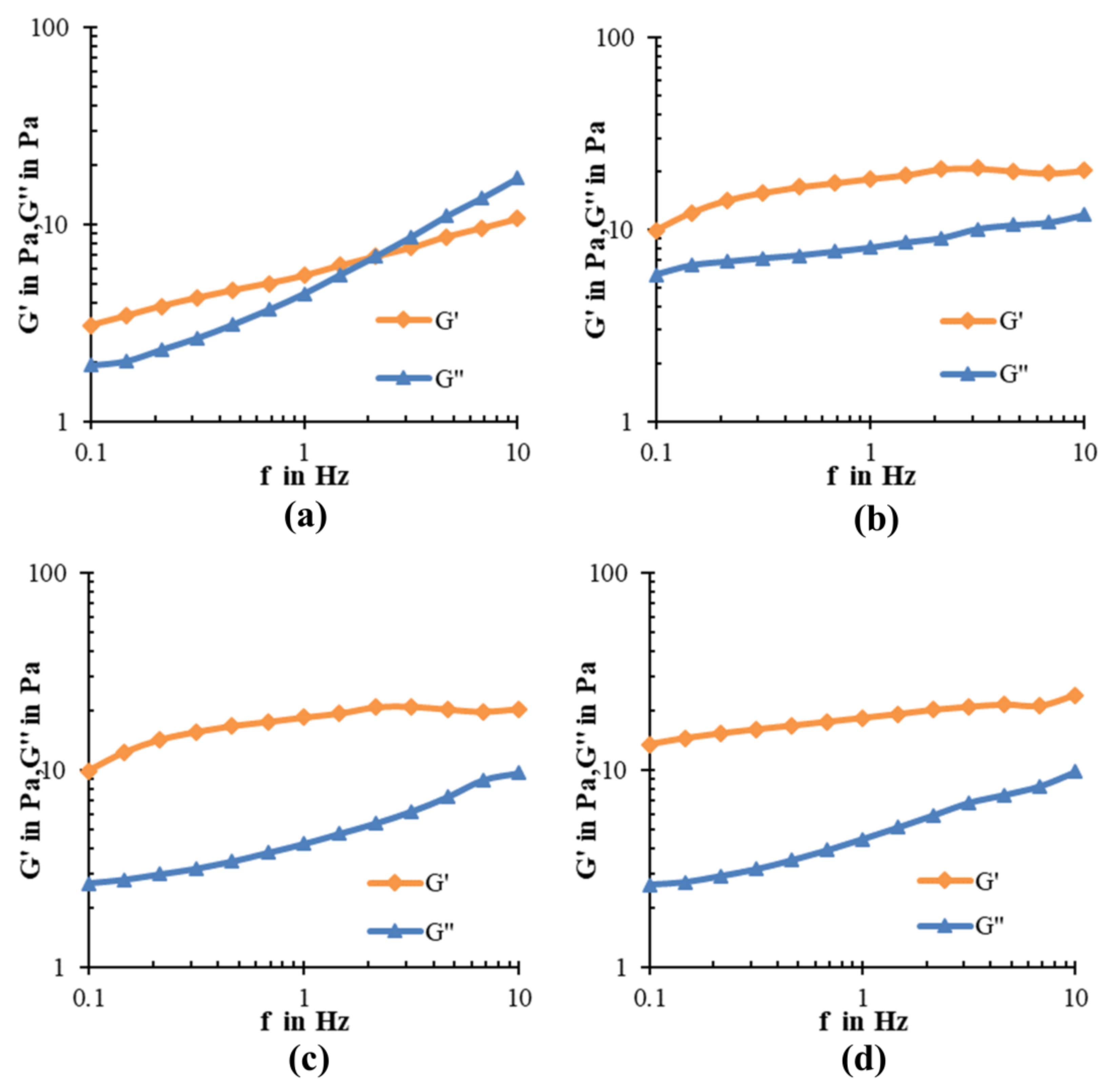
2.5. Dynamic-Viscoelasticity of Ice Cream
2.6. Sensory Properties of Ice Creams
3. Materials and Methods
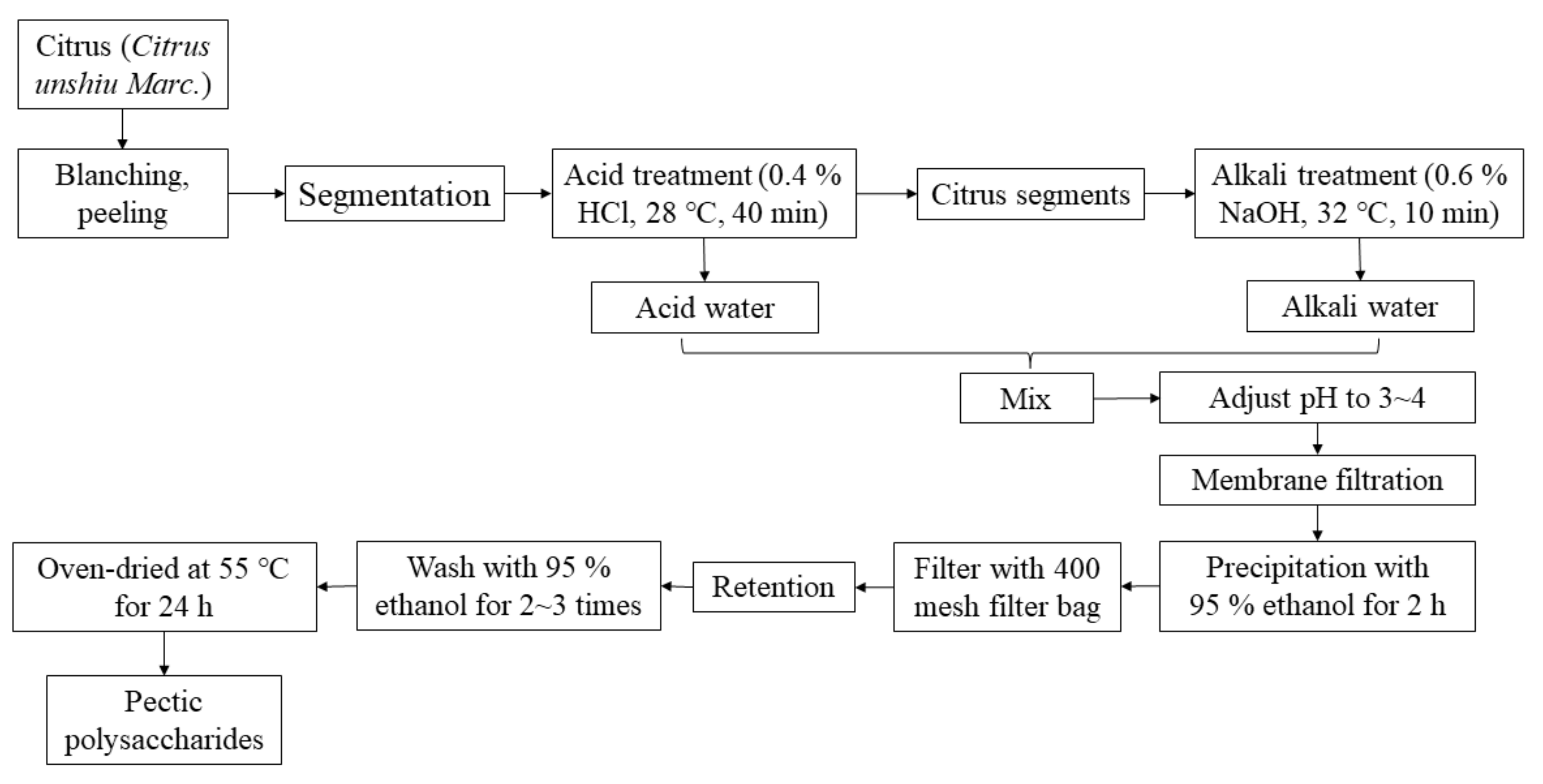
3.1. Extraction of Pectin
3.2. Monosaccharide Analysis of Pectin
3.3. Rheological Analysis of Pectin
3.4. Preparation of Ice Cream
3.5. Overrun and Meltdown Test of Ice Creams
3.6. Viscosity Determination of the Ice Cream Mixtures
3.7. Hardness Determination of Ice Creams
3.8. Dynamic-Viscoelastic Determination of Ice Creams
3.9. Sensory Evaluation
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, D.; Cao, Y.; Chen, J.; Gao, H.; Ye, X.; Liu, D.; Chen, S. Feasibility study on water reclamation from the sorting/grading operation in mandarin orange canning production. J. Clean. Prod. 2015, 113, 224–230. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, H.; Wu, D.; Linhardt, R.J.; Zhi, Z.; Yan, L.; Chen, S.; Ye, X. Green recovery of pectic polysaccharides from citrus canning processing water. J. Clean. Prod. 2016, 144, 459–469. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Chavarría-Hernández, N. Pectin: A new perspective from the biorefi nery standpoint. Biofuels Bioprod. Biorefin. 2015, 9, 368–377. [Google Scholar]
- Lim, J.; Ko, S.; Lee, S. Use of yuja (Citrus junos) pectin as a fat replacer in baked foods. Food Sci. Biotechnol. 2014, 23, 1837–1841. [Google Scholar] [CrossRef]
- Min, B.K.; Inyoung, B.; Hyeongyu, L.; Sangho, Y.; Suyong, L. Utilization of pectin-enriched materials from apple pomace as a fat replacer in a model food system. Bioresour. Technol. 2010, 101, 5414–5418. [Google Scholar] [CrossRef] [PubMed]
- Zaidel, A.; Hamidon, N.H.; Zahir, N.M. Extraction and characterization of pectin from sweet potato (ipomoea batatas) peels using alkaline extraction method. Acta Hortic. 2017, 1152, 211–218. [Google Scholar] [CrossRef]
- Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Pereira, P.H.F.; Moates, G.K.; Wellner, N.; Mazzetto, S.E.; Waldron, K.W.; Azeredo, H.M.C. Optimization of pectin extraction from banana peels with citric acid by using response surface methodology. Food Chem. 2016, 198, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hua, X.; Yang, R.; Chen, Y.; Yang, H. Characterization of natural low-methoxyl pectin from sunflower head extracted by sodium citrate and purified by ultrafiltration. Food Chem. 2015, 180, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Prentice, J.H. Dairy Rheology: A Concise Guide; VCH Publishers Inc.: New York, NY, USA, 1992. [Google Scholar]
- Mcghee, C.E.; Jones, J.O.; Park, Y.W. Evaluation of textural and sensory characteristics of three types of low-fat goat milk ice cream. Small Rumin. Res. 2015, 123, 293–300. [Google Scholar] [CrossRef]
- Akalın, A.S.; Karagözlü, C.; Ünal, G. Rheological properties of reduced-fat and low-fat ice cream containing whey protein isolate and inulin. Eur. Food Res. Technol. 2008, 227, 889–895. [Google Scholar] [CrossRef]
- Lim, J.; Inglett, G.E.; Lee, S. Response to consumer demand for reduced-fat foods; multi-functional fat replacers. Jpn. J. Food Eng. 2015, 11, 147–152. [Google Scholar]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocoll. 2014, 35, 383–391. [Google Scholar] [CrossRef]
- Velasco, S.E.; Areizaga, J.; Irastorza, A.; Dueñas, M.T.; Santamaria, A.; Muñoz, M.E. Chemical and rheological properties of the beta-glucan produced by Pediococcus parvulus 2.6. J. Agric. Food Chem. 2009, 57, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Bera, A.; Ojha, K.; Mandal, A. Effects of alkali, salts, and surfactant on rheological behavior of partially hydrolyzed polyacrylamide solutions. J. Chem. Eng. Data 2010, 55, 4315–4322. [Google Scholar] [CrossRef]
- Xu, J.; Inglett, G.E.; Chen, D.; Liu, S.X. Viscoelastic properties of oat β-glucan-rich aqueous dispersions. Food Chem. 2013, 138, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.E.; Simas-Tosin, F.F.; Iacomini, M.; Gorin, P.A.; Cordeiro, L.M. Rheological behavior of high methoxyl pectin from the pulp of tamarillo fruit (Solanum betaceum). Carbohydr. Polym. 2016, 139, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C. The Science of Ice Cream, 1st ed.; The Royal Society of Chemistry: Cambridge, UK, 2004. [Google Scholar]
- Varela, P.; Pintor, A.; Fiszman, S. How hydrocolloids affect the temporal oral perception of ice cream. Food Hydrocoll. 2014, 36, 220–228. [Google Scholar] [CrossRef]
- Soukoulis, C.; Chandrinos, I.; Tzia, C. Study of the functionality of selected hydrocolloids and their blends with κ-carrageenan on storage quality of vanilla ice cream. LWT Food Sci. Technol. 2008, 41, 1816–1827. [Google Scholar] [CrossRef]
- Oyaberkay, K.; Mehmet, G.; Kurban, Y.; Sevim, K.; Talip, K. The functional, rheological and sensory characteristics of ice creams with various fat replacers. Int. J. Dairy Technol. 2010, 62, 93–99. [Google Scholar]
- Javidi, F.; Razavi, S.M.A.; Behrouzian, F.; Alghooneh, A. The influence of basil seed gum, guar gum and their blend on the rheological, physical and sensory properties of low fat ice cream. Food Hydrocoll. 2016, 52, 625–633. [Google Scholar] [CrossRef]
- Muse, M.R.; Hartel, R.W. Ice cream structural elements that affect melting rate and hardness. J. Dairy Sci. 2004, 87, 1–10. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Silva, H.L.; Celeguini, R.M.; Santos, R.; Pastore, G.M.; Junior, C.A.; Freitas, M.Q.; Nogueira, L.C.; Silva, M.C.; Cruz, A.G. Effect of galactooligosaccharide addition on the physical, optical, and sensory acceptance of vanilla ice cream. J. Dairy Sci. 2015, 98, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Sofjan, R.P.; Hartel, R.W. Effects of overrun on structural and physical characteristics of ice cream. Int. Dairy J. 2004, 14, 255–262. [Google Scholar] [CrossRef]
- Wildmoser, H.; Scheiwiller, J.; Windhab, E.J. Impact of disperse microstructure on rheology and quality aspects of ice cream. LWT Food Sci. Technol. 2004, 37, 881–891. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Rheological properties and structural characterization of salep improved by ethanol treatment. Carbohydr. Polym. 2015, 133, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Geresh, S.; Adin, I.; Yarmolinsky, E.; Karpasas, M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohydr. Polym. 2002, 50, 183–189. [Google Scholar] [CrossRef]
- Xu, J.L.; Zhang, J.C.; Liu, Y.; Sun, H.J.; Wang, J.H. Rheological properties of a polysaccharide from floral mushrooms cultivated in Huangshan Mountain. Carbohydr. Polym. 2016, 139, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Cengiz, A.; Kahyaoglu, T. The effect of gum tragacanth on the rheological properties of salep based ice cream mix. Carbohydr. Polym. 2016, 143, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Bahramparvar, M.; Tehrani, M.M.; Razavi, S.M.A. Effects of a novel stabilizer blend and presence of κ-carrageenan on some properties of vanilla ice cream during storage. Food Biosci. 2013, 3, 10–18. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, H.K.; Kumar, N.; Kaur, M. The effect of inulin as a fat replacer on the quality of low-fat ice cream. Int. J. Dairy Technol. 2015, 68, 374–380. [Google Scholar] [CrossRef]

| Concentration of Pectin | K (Pa·sn) | n | R2 |
|---|---|---|---|
| 0.5% | 0.11 ± 0.02 | 0.43 ± 0.01 | 0.99 ± 0.01 |
| 1.0% | 0.40 ± 0.01 | 0.42 ± 0.01 | 0.99 ± 0.00 |
| 1.5% | 1.46 ± 0.01 | 0.36 ± 0.01 | 0.98 ± 0.00 |
| Ice Cream Samples | K (Pa·sn) | n | R2 |
|---|---|---|---|
| Control | 0.12 ± 0.01 | 0.79 ± 0.01 | 0.97 ± 0.01 |
| T1 | 0.62 ± 0.01 | 0.45 ± 0.00 | 0.98 ± 0.00 |
| T2 | 1.05 ± 0.02 | 0.37 ± 0.01 | 0.99 ± 0.00 |
| T3 | 5.06 ± 0.02 | 0.25 ± 0.01 | 0.99 ± 0.00 |
| Ice Cream | Appearance | Flavor | Taste | Smoothness | Mouth Coating |
|---|---|---|---|---|---|
| Control | 8.10 ± 1.02 a | 6.13 ± 1.23 a,b | 7.89 ± 1.57 a | 5.56 ± 2.05 b | 8.33 ± 0.97 a |
| T1 | 7.22 ± 1.35 a | 6.56 ± 1.62 a | 7.78 ± 1.22 a | 7.56 ± 1.50 a | 8.00 ± 1.24 a |
| T2 | 7.56 ± 1.67 a | 5.44 ± 1.49 b | 5.44 ± 2.00 b | 8.01 ± 1.23 a | 6.11 ± 1.84 b |
| T3 | 7.24 ± 1.53 a | 6.33 ± 1.81 a,b | 7.11 ± 1.88 a | 7.46 ± 1.79 a | 6.77 ± 2.05 b |
| Compositions | Treatments | |||
|---|---|---|---|---|
| Control | T1 | T2 | T3 | |
| Skim milk powder | 20.00 | 20.00 | 20.00 | 20.00 |
| Cream | 15.00 | 12.75 | 10.50 | 8.25 |
| Sucrose | 10.00 | 10.00 | 10.00 | 10.00 |
| Egg | 1.60 | 1.60 | 1.60 | 1.60 |
| Sterilized milk | 5.00 | 5.00 | 5.00 | 5.00 |
| Water | 48.40 | 50.41 | 52.42 | 54.43 |
| Pectin | 0 | 0.24 | 0.48 | 0.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Chen, J.; Li, J.; Wei, C.; Ye, X.; Shi, J.; Chen, S. Pectin from Citrus Canning Wastewater as Potential Fat Replacer in Ice Cream. Molecules 2018, 23, 925. https://doi.org/10.3390/molecules23040925
Zhang H, Chen J, Li J, Wei C, Ye X, Shi J, Chen S. Pectin from Citrus Canning Wastewater as Potential Fat Replacer in Ice Cream. Molecules. 2018; 23(4):925. https://doi.org/10.3390/molecules23040925
Chicago/Turabian StyleZhang, Hua, Jianle Chen, Junhui Li, Chaoyang Wei, Xingqian Ye, John Shi, and Shiguo Chen. 2018. "Pectin from Citrus Canning Wastewater as Potential Fat Replacer in Ice Cream" Molecules 23, no. 4: 925. https://doi.org/10.3390/molecules23040925




