Application of High-Performance Liquid Chromatography Coupled with Linear Ion Trap Quadrupole Orbitrap Mass Spectrometry for Qualitative and Quantitative Assessment of Shejin-Liyan Granule Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Sample Preparation
2.3. Chromatographic and Mass Conditions
2.4. Method Validation for Quantitative Analysis
3. Results and Discussions
3.1. Development of the Extraction Method
3.2. Profiles of Ingredients in the SJLYKL Extract
3.3. Quantitative Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Uemura, J.; Nagpal, R.; Zerbinati, N.; Singh, B.; Marcellino, M.; Mohania, D.; Mohania, D.; Marotta, F.; He, F.; Ayala, A.; et al. Effect of VBC-1814/7J, a poly-phytocompound, on a non-infectious model of pharyngitis. Exp. Ther. Med. 2017, 13, 3075–3080. [Google Scholar] [CrossRef] [PubMed]
- Spinks, A.; Glasziou, P.P.; Del Mar, C.B. Antibiotics for sore throat. Cochrane Database Syst. Rev. 2013, 11, CD000023. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, T.; Zeng, L.; Li, S. Chinese medicinal herbs for sore throat. Cochrane Database Syst. Rev. 2012, 3, CD004877. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Grigoryan, L.; Galeone, C.; Esposito, S.; Huovinen, P.; Little, P.; Verheij, T. Guideline for the management of acute sore throat. Clin. Mocrobiol. Infect. 2012, 18, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.Z.M.; Huang, W.; Cheung, A.W.H.; Bi, C.W.C.; Duan, R.; Guo, A.J.Y.; Dong, T.T.X.; Tsim, K.W.K. Quality evaluation of Rhizoma Belamcandae (Belamcanda chinensis (L.) DC.) by using high-performance liquid chromatography coupled with diode array detector and mass spectrometry. J. Chormatogr. A 2009, 1216, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Huo, X.; Liu, H.; Chai, L.; Ding, L.; Qiu, F. Identification of absorbed constituents and in vivo metabolites in rats after oral administration of Physalis alkekengi var. franchetii by ultra-high-pressure liquid chromatography quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2017, 32, e4121. [Google Scholar]
- Liu, J.; Cai, Y.-Z.; Wong, R.N.S.; Lee, C.K.-F.; Tang, S.C.W.; Sze, S.C.W.; Tong, Y.; Zhang, Y. Comparative Analysis of Caffeoylquinic Acids and Lignans in Roots and Seeds among Various Burdock (Arctium lappa) Genotypes with High Antioxidant Activity. J. Agric. Food Chem. 2012, 60, 4067–4075. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Dong, L.-N.; Peng, X.-J.; Li, Y.; Shi, J.; Zhou, F.-Y.; Liu, Z.Q. Pharmacokinetic characterization of oxymatrine and matrine in rats after oral administration of radix Sophorae tonkinensis extract and oxymatrine by sensitive and robust UPLC–MS/MS method. J. Pharm. Biomed. 2013, 83, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.S.; Choi, H.Y. Inhibitory effect of Physalis alkekengi L. var. franchetii extract and its chloroform fraction on LPS or LPS/IFN-γ-stimulated inflammatory response in peritoneal macrophages. J. Ethnopharmacol. 2011, 135, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.N.S.; Sener, B.; Ayanoglu, F.; Ozgüven, M.; Choudhary, M.I.; Ur-Rahman, A. Two isoflavones and bioactivity spectrum of the crude extracts of Iris germanica rhizomes. Phytother. Res. 2003, 17, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Hong, S.S.; Han, X.H.; Hwang, J.S.; Lee, D.; Ro, J.S.; Hwang, B.Y. Lignans from Arctium lappa and their inhibition of LPS-induced nitric oxide production. Chem. Pharm. Bull. 2007, 55, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Q.K.; Tingting, Z.; Xiaoli, W.; Mengxue, M.; Baochang, C. Qualitative and quantitative analysis of major constituents of raw and processed Arctii Fructus by UHPLC-UV-Q-TOF-MS/MS. Acta Pharm. Sin. 2017, 52, 603–608. [Google Scholar]
- Zhang, Y.-Y.; Wang, Q.; Qi, L.-W.; Qin, X.-Y.; Qin, M.-J. Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC–DAD–ESI-MSn. J. Pharm. Biomed. 2011, 56, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, S.; Pi, Z.; Song, F.; Lin, N.; Liu, S.; Liu, Z. Chemical profiling of Wu-tou decoction by UPLC–Q-TOF-MS. Talanta 2014, 118, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Shou, L.; Chen, C.; Shi, S.; Zhou, M. Application of ultra-high-performance liquid chromatography coupled with LTQ-Orbitrap mass spectrometry for identification, confirmation and quantitation of illegal adulterated weight-loss drugs in plant dietary supplements. J. Chormatogr. B 2017, 1064, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.S.; Rozet, E.; Larondelle, Y.; Hubert, P.; Rogez, H.; Quetin-Leclercq, J. Development and validation of an UHPLC-LTQ-Orbitrap MS method for non-anthocyanin flavonoids quantification in Euterpe oleracea juice. Anal. Bioanal. Chem. 2013, 405, 9235–9249. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Picó, Y.; Barceló, D. Application of ultra-high pressure liquid chromatography linear ion-trap orbitrap to qualitative and quantitative assessment of pesticide residues. J. Chormatogr. A 2014, 1328, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, D.M.A. Belamcandae chinensis rhizome—A review of phytochemistry and bioactivity. Fitoterapia 2015, 107, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, K.; Xu, J.; Yang, D.; Zhang, C.; Wang, Z.; Li, M. Belamcanda chinensis (L.) DC-An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2016, 186, 1–13. [Google Scholar] [PubMed]
- He, L.; Chen, Y.; Liang, Z.; Li, Y.; Zhou, M.; Yuan, Z.; Luo, L.; Jin, Z.; Yang, Y.; Chen, J. A rapid and comprehensive quality assessing method of Yin-Qiao-Jie-Du tablets using UHPLC-QTOF-MS in combination with multivariate statistical analysis. J. Pharm. Biomed. 2016, 124, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xu, Q.; Chen, C.; Song, C.; Xu, Y.; Xiang, Y.; Feng, Y.; Ouyang, H.; Zhang, Y.; Jiang, H. The rapid discovery and identification of physalins in the calyx of Physalis alkekengi L.var.franchetii (Mast.) Makino using ultra-high performance liquid chromatography–quadrupole time of flight tandem mass spectrometry together with a novel three-step data mining strategy. J. Chormatogr. A 2014, 1361, 139–152. [Google Scholar]
- Dong, H.; Yan, G.-L.; Han, Y.; Sun, H.; Zhang, A.-H.; Li, X.-N.; Wang, X.-J. UPLC-Q-TOF/MS-based metabolomic studies on the toxicity mechanisms of traditional Chinese medicine Chuanwu and the detoxification mechanisms of Gancao, Baishao, and Ganjiang. Chin. J. Nat. Med. 2015, 13, 687–698. [Google Scholar] [CrossRef]
- Wang, Q.; Qiao, X.; Qian, Y.; Liu, C.-F.; Yang, Y.-F.; Ji, S.; Li, J.; Guo, D.-A.; Ye, M. Metabolites identification of glycyrin and glycyrol, bioactive coumarins from licorice. J. Chormatogr. B 2015, 983–984, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.-H.; Lee, C.-K.; Ha, N.-J.; Yim, D. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.P.Y.M.; Lim, S.S.; Lee, S.H.; Ryu, N.; Shin, K.H.; Ohuchi, K. Inhibition by tectorigenin and tectoridin of prostaglandin E2 production and cyclooxygenase-2 induction in rat peritoneal macrophages. Biochim. Biophys. Acta 1999, 1438, 399–407. [Google Scholar] [CrossRef]
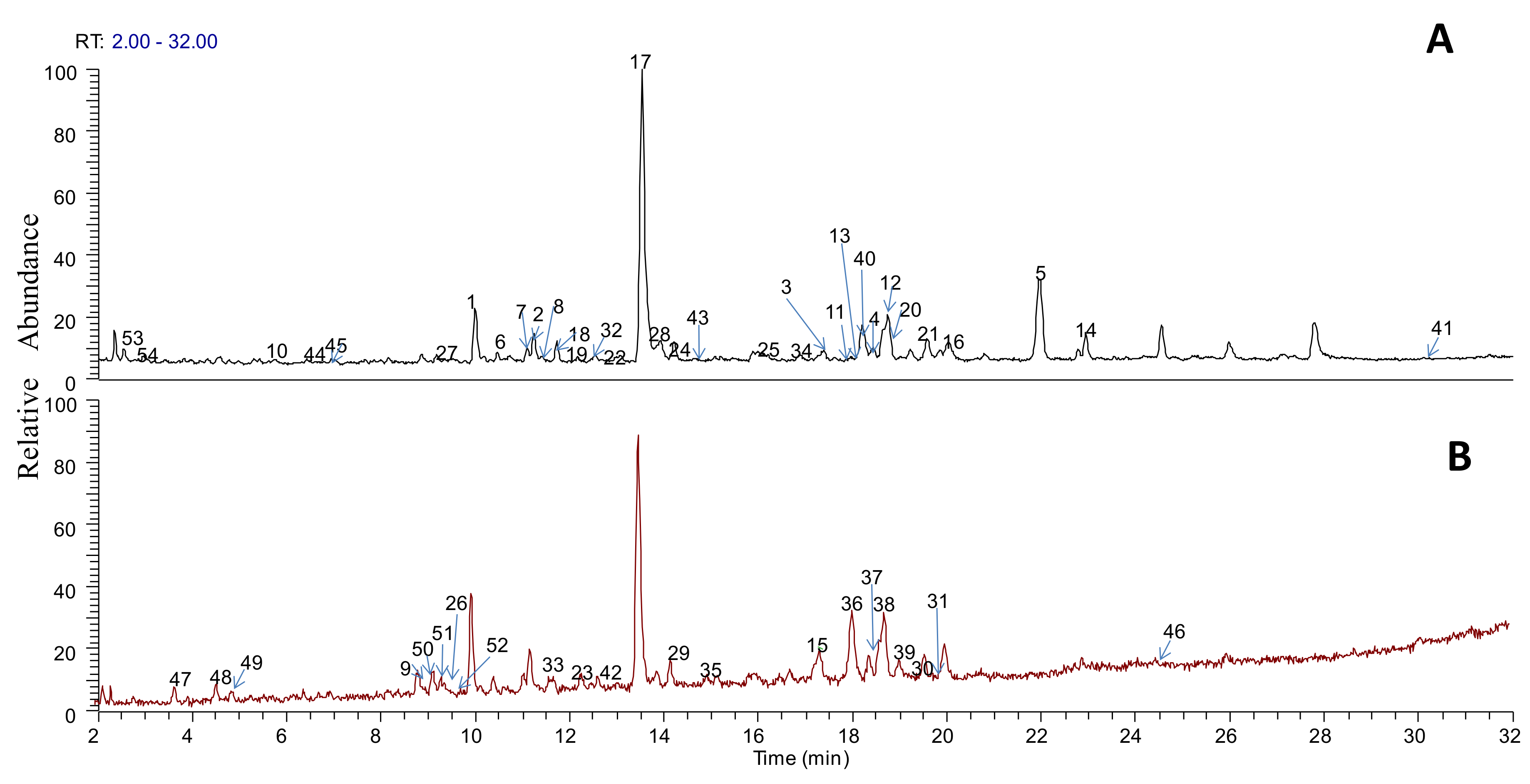
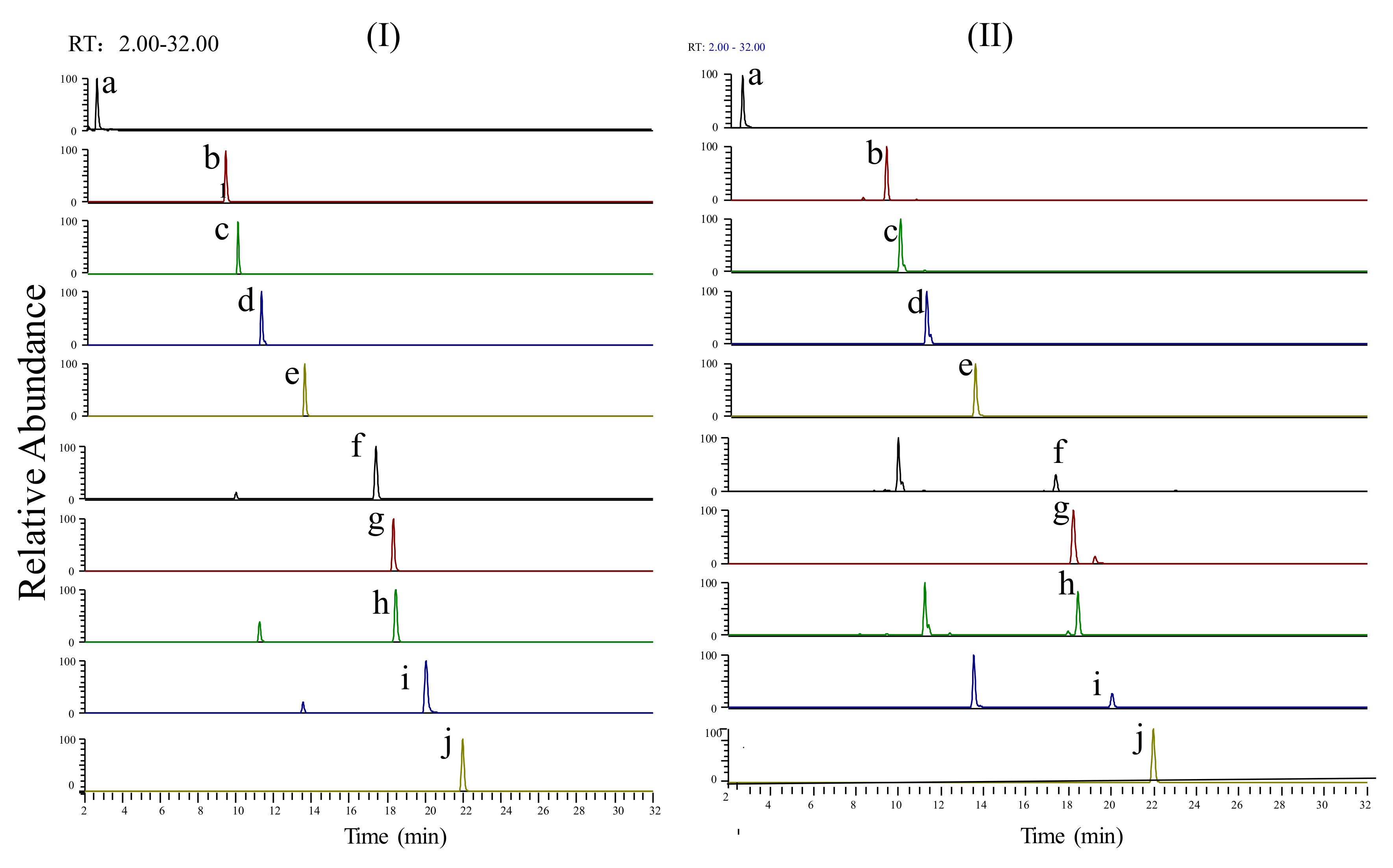

| No. | Retention Time (min) | Experimental Mass (m/z) | Theoretical Mass (m/z) | Ion Type | Identification Compound | MS/MS Fragments |
|---|---|---|---|---|---|---|
| 1 | 9.99 | 463.1237 | 463.124 | [M + H]+ | tectoridin | 301.0706, 286.0472 |
| 2 | 11.23 | 523.1447 | 523.1342 | [M + H]+ | iridin | 361.0919 |
| 3 | 17.38 | 301.0706 | 301.0711 | [M + H]+ | tectorigenin | 286.0475 |
| 4 | 18.43 | 361.0921 | 361.0923 | [M + H]+ | irigenin | 346.0685 |
| 5 | 21.97 | 387.109 | 387.109 | [M + H]+ | irisflorentin | 357.0606 |
| 6 | 10.6 | 493.1342 | 493.134 | [M + H]+ | iristectorin B | 331.0815, 316.0579 |
| 7 | 11.1 | 493.1342 | 493.134 | [M + H]+ | iristectorin A | 331.0815, 316.0579 |
| 8 | 11.46 | 523.1447 | 523.1452 | [M + H]+ | isoiridin | 523.1447, 361.0919 |
| 9 | 12.78 | 535.1446 | 535.1452 | [M + H]+ | 3,5-dimethoxyirisolone-4-O-d-glucoside | 373.0919, 358.0685 |
| 10 | 6.16 | 625.1765 | 625.1767 | [M + H]+ | tectorigenin-7-O-glucosyl-4-O-glucoside | 463.1236, 301.0707, 286.0473 |
| 11 | 17.98 | 331.0815 | 331.0818 | [M + H]+ | iristectorigenin A | 316.0582, 301.0347 |
| 12 | 18.5 | 331.0813 | 331.0818 | [M + H]+ | iristectorigenin B | 316.0582, 301.0348 |
| 13 | 18.12 | 373.0918 | 373.0923 | [M + H]+ | noririsflorentin | 358.0687 |
| 14 | 22.37 | 359.0761 | 359.0764 | [M + H]+ | dichotomitin | 326.0424 |
| 15 | 17.28 | 357.1342 | 357.1338 | [M − H]− | matairesinol | 342.0380, 327.0149 |
| 16 | 20.02 | 373.1643 | 373.1651 | [M + H]+ | arctigenin | 355.1539 |
| 17 | 13.53 | 552.2443 | 552.2445 | [M + NH4]+ | arctiin | 373.1647, 355.1542 |
| 18 | 11.72 | 538.2286 | 538.2288 | [M + NH4]+ | matairesinoside | 359.1491 |
| 19 | 11.99 | 773.2786 | 773.2785 | [M + Na]+ | lappaol H | 755.2684, 725.2574 |
| 20 | 18.8 | 559.1938 | 559.1944 | [M + Na]+ | lappaol A | 397.1259 |
| 21 | 19.6 | 732.302 | 732.302 | [M + NH4]+ | lappaol F | 531.2014 |
| 22 | 12.8 | 577.2048 | 577.205 | [M + Na]+ | lappaol C/isolappaol C/lappaol E/arctignan A | 559.1945, 562.1801 |
| 23 | 13.94 | 577.2048 | 577.205 | [M + Na]+ | lappaol C/isolappaol C/lappaol E/arctignan A | 559.1945, 562.1804 |
| 24 | 14.2 | 577.2046 | 577.205 | [M + Na]+ | lappaol C/isolappaol C/lappaol E/arctignan A | 559.1947, 562.1801 |
| 25 | 16.1 | 577.2042 | 577.205 | [M + Na]+ | lappaol C/isolappaol C/lappaol E/arctignan A | 559.1944, 562.1802 |
| 26 | 9.18 | 417.1187 | 417.1186 | [M − H]− | liquiritin | 255.0653 |
| 27 | 9.34 | 449.1083 | 449.1084 | [M + H]+ | galuteolin | 287.0555 |
| 28 | 12.34 | 417.1187 | 417.1186 | [M − H]− | isoliquiritin | 255.0653 |
| 29 | 14.24 | 255.0662 | 255.0657 | [M − H]− | liquiritigenin | 153.0917 |
| 30 | 19.08 | 255.0663 | 255.0657 | [M − H]− | isoliquiritigenin | 153.0918 |
| 31 | 19.8 | 267.0653 | 267.0657 | [M − H]− | formononetin | 252.0431 |
| 32 | 8.86 | 549.1608 | 549.1608 | [M − H]− | liquiritin apioside | 417.1192, 255.0665 |
| 33 | 11.84 | 549.1608 | 549.1608 | [M − H]− | isoliquiritin apioside | 417.1192, 255.0666 |
| 34 | 12.97 | 543.1833 | 543.1866 | [M − H]− | physalin D’ | 525.1763, 515.1919 |
| 35 | 15.22 | 543.1833 | 543.1866 | [M − H]− | physalin D | 525.1763, 515.1919 |
| 36 | 17.92 | 525.1759 | 525.1761 | [M − H]− | physalin F | 507.1663, 497.1819 |
| 37 | 18.18 | 525.176 | 525.1761 | [M − H]− | physalin A | 507.1663, 497.1819 |
| 38 | 18.53 | 527.1915 | 527.1917 | [M − H]− | physalin O | 509.1815 |
| 39 | 19.01 | 527.1916 | 527.1917 | [M − H]− | physalin L | 509.1815 |
| 40 | 18.22 | 823.4116 | 823.4116 | [M + H]+ | glycyrrhizic acid | 647.3791, 471.3467, 453.3363 |
| 41 | 30.26 | 471.3464 | 471.3474 | [M + H]+ | glycyrrhetinic acid | 425.3416 |
| 42 | 16.89 | 839.407 | 839.4065 | [M + H]+ | licorice-saponin G2 | 663.3747, 469.3312, 451.3210 |
| 43 | 14.53 | 985.4645 | 985.4644 | [M + H]+ | licorice saponin A3 | 823.4116, 647.3806, 615.3897, 453.3366 |
| 44 | 6.44 | 423.0923 | 423.0924 | [M + H]+ | mangiferin | 405.0819, 333.0607, 303.0501 |
| 45 | 6.77 | 423.0923 | 423.0924 | [M + H]+ | isomangiferin | 405.0819, 333.0607, 303.0501 |
| 46 | 24.63 | 365.1028 | 365.1025 | [M − H]− | glycyrol | 307.0244, 295.0244 |
| 47 | 3.7 | 353.0876 | 353.0873 | [M − H]− | chlorogenic acid | 191.0565, 179.0352 |
| 48 | 4.58 | 353.0876 | 353.0873 | [M − H]− | neochlorogenic acid | 191.0565, 179.0352 |
| 49 | 4.97 | 353.0876 | 353.0873 | [M − H]− | cryptochlorogenic acid | 191.0565, 179.0352 |
| 50 | 8.94 | 515.1188 | 515.119 | [M − H]− | isochlorogenic acid B | 353.0878 |
| 51 | 9.14 | 515.1193 | 515.119 | [M − H]− | isochlorogenic acid A | 353.087 |
| 52 | 9.42 | 515.1191 | 515.119 | [M − H]− | isochlorogenic acid C | 353.0876 |
| 53 | 2.49 | 249.1962 | 249.1967 | [M + H]+ | matrine | 148.1122 |
| 54 | 3.14 | 265.1913 | 265.1916 | [M + H]+ | oxymatrine | 247.1810, 205.1339 |
| Compound | Regression Equation | r2 | Liner Range (μg/mL) | LOD (μg/mL) |
|---|---|---|---|---|
| matrine | y = 46117 + 5066555x | 0.9914 | 0.020–2.5 | 0.002 |
| galuteolin | y = 1550 + 1112359x | 0.9985 | 0.023–3.0 | 0.002 |
| tectoridin | y = 30165 + 1743884x | 0.9988 | 0.027–3.5 | 0.002 |
| iridin | y = 4672 + 1112803x | 0.9921 | 0.016–2.0 | 0.002 |
| arctiin | y = 7991 + 2241923x | 0.9988 | 0.195–25 | 0.002 |
| tectorigenin | y = 34605 + 11608484x | 0.9987 | 0.023–3.0 | 0.002 |
| glycyrrhizic acid | y = 67891 + 2468972x | 0.9954 | 0.023–3.0 | 0.002 |
| irigenin | y = 1793 + 447209x | 0.9972 | 0.025–3.2 | 0.002 |
| arctigenin | y = 7894 + 1314941x | 0.9994 | 0.02–2.5 | 0.002 |
| irisflorentin | y = 54529 + 50506549x | 0.9989 | 0.016–2.0 | 0.0005 |
| Compound | Concentration (μg/mL) | Intra-Day | Inter-Day | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Accuracy (Bias %) | Mean | ± | SD | Accuracy (Bias %) | ||
| matrine | 0.040 | 0.038 | ± | 0.0014 | −5.00 | 0.0398 | ± | 0.002 | −0.50 |
| 0.640 | 0.651 | ± | 0.022 | 1.70 | 0.669 | ± | 0.015 | 4.50 | |
| 1.875 | 1.782 | ± | 0.087 | −5.00 | 1.822 | ± | 0.082 | −2.80 | |
| galuteolin | 0.046 | 0.0438 | ± | 0.002 | −4.80 | 0.044 | ± | 0.002 | −4.30 |
| 0.736 | 0.769 | ± | 0.028 | 4.50 | 0.755 | ± | 0.006 | 2.60 | |
| 2.250 | 2.21 | ± | 0.061 | −1.80 | 2.34 | ± | 0.059 | 4.00 | |
| tectoridin | 0.054 | 0.052 | ± | 0.001 | −3.70 | 0.0516 | ± | 0.001 | −4.40 |
| 0.864 | 0.811 | ± | 0.009 | −6.10 | 0.827 | ± | 0.010 | −4.30 | |
| 2.625 | 2.63 | ± | 0.102 | 0.20 | 2.56 | ± | 0.079 | −2.50 | |
| iridin | 0.032 | 0.033 | ± | 0.001 | 1.90 | 0.0307 | ± | 0.0004 | −4.10 |
| 0.512 | 0.531 | ± | 0.005 | 3.70 | 0.533 | ± | 0.002 | 4.10 | |
| 1.500 | 1.55 | ± | 0.069 | 3.30 | 1.543 | ± | 0.035 | 2.90 | |
| arctiin | 0.400 | 0.42 | ± | 0.014 | 5.00 | 0.38 | ± | 0.013 | −5.00 |
| 6.400 | 6.303 | ± | 0.046 | −1.50 | 6.21 | ± | 0.054 | −3.00 | |
| 18.750 | 17.9 | ± | 0.456 | −4.50 | 17.89 | ± | 0.432 | −4.60 | |
| tectorigenin | 0.046 | 0.0496 | ± | 0.0007 | 7.80 | 0.0464 | ± | 0.0004 | 0.90 |
| 0.094 | 0.091 | ± | 0.002 | −2.90 | 0.09 | ± | 0.003 | −4.00 | |
| 2.250 | 2.15 | ± | 0.056 | −4.40 | 2.36 | ± | 0.081 | 4.90 | |
| glycyrrhizic acid | 0.046 | 0.045 | ± | 0.002 | −2.20 | 0.0438 | ± | 0.002 | −4.80 |
| 0.736 | 0.769 | ± | 0.009 | 4.50 | 0.772 | ± | 0.009 | 4.90 | |
| 2.250 | 2.32 | ± | 0.060 | 3.10 | 2.33 | ± | 0.065 | 3.60 | |
| irigenin | 0.050 | 0.052 | ± | 0.0008 | 4.00 | 0.0522 | ± | 0.0004 | 4.40 |
| 0.800 | 0.797 | ± | 0.002 | −0.40 | 0.809 | ± | 0.001 | 1.10 | |
| 2.400 | 2.32 | ± | 0.016 | −3.30 | 2.35 | ± | 0.018 | −2.10 | |
| arctigenin | 0.040 | 0.038 | ± | 0.001 | −5.00 | 0.0391 | ± | 0.001 | −2.20 |
| 0.640 | 0.653 | ± | 0.005 | 2.00 | 0.652 | ± | 0.006 | 1.90 | |
| 1.875 | 1.86 | ± | 0.053 | −0.80 | 1.85 | ± | 0.071 | −1.30 | |
| irisflorentin | 0.032 | 0.0336 | ± | 0.0003 | 5.00 | 0.031 | ± | 0.0004 | −3.10 |
| 0.500 | 0.512 | ± | 0.001 | 2.40 | 0.513 | ± | 0.001 | 2.60 | |
| 1.500 | 1.465 | ± | 0.013 | −2.30 | 1.457 | ± | 0.015 | −2.90 | |
| Compound. | Recovery | Reproducibility (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Spiked Amount (μg) | Dectected Amount (μg) | Accuracy (Bias %) | Mean | ± | SD | RSD | |||
| matrine | 1.0 | 1.027 | ± | 0.04 | 4.80 | 0.19 | ± | 0.003 | 1.67 |
| galuteolin | 7.3 | 7.28 | ± | 0.25 | −0.10 | 1.39 | ± | 0.03 | 2.27 |
| tectoridin | 125.8 | 119.6 | ± | 5.85 | −4.90 | 24.33 | ± | 0.53 | 2.18 |
| iridin | 27.3 | 26.9 | ± | 1.23 | −1.50 | 5.68 | ± | 0.25 | 4.40 |
| arctiin | 863.5 | 862.1 | ± | 31.56 | −0.20 | 169.60 | ± | 2.50 | 1.47 |
| tectorigenin | 56.7 | 55.6 | ± | 1.52 | −1.90 | 11.00 | ± | 0.54 | 4.91 |
| glycyrrhizic acid | 38.8 | 37.1 | ± | 0.64 | −4.50 | 7.49 | ± | 0.37 | 4.94 |
| irigenin | 19.0 | 18.2 | ± | 0.19 | −4.30 | 3.92 | ± | 0.15 | 3.83 |
| arctigenin | 20.3 | 19.5 | ± | 0.75 | −3.90 | 4.25 | ± | 0.19 | 4.47 |
| irisflorentin | 7.2 | 6.9 | ± | 0.26 | −3.80 | 1.37 | ± | 0.07 | 4.91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Wu, W.; Huang, M.; Long, F.; Liu, X.; Zhu, Y. Application of High-Performance Liquid Chromatography Coupled with Linear Ion Trap Quadrupole Orbitrap Mass Spectrometry for Qualitative and Quantitative Assessment of Shejin-Liyan Granule Supplements. Molecules 2018, 23, 884. https://doi.org/10.3390/molecules23040884
Gu J, Wu W, Huang M, Long F, Liu X, Zhu Y. Application of High-Performance Liquid Chromatography Coupled with Linear Ion Trap Quadrupole Orbitrap Mass Spectrometry for Qualitative and Quantitative Assessment of Shejin-Liyan Granule Supplements. Molecules. 2018; 23(4):884. https://doi.org/10.3390/molecules23040884
Chicago/Turabian StyleGu, Jifeng, Weijun Wu, Mengwei Huang, Fen Long, Xinhua Liu, and Yizhun Zhu. 2018. "Application of High-Performance Liquid Chromatography Coupled with Linear Ion Trap Quadrupole Orbitrap Mass Spectrometry for Qualitative and Quantitative Assessment of Shejin-Liyan Granule Supplements" Molecules 23, no. 4: 884. https://doi.org/10.3390/molecules23040884




