Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C
Abstract
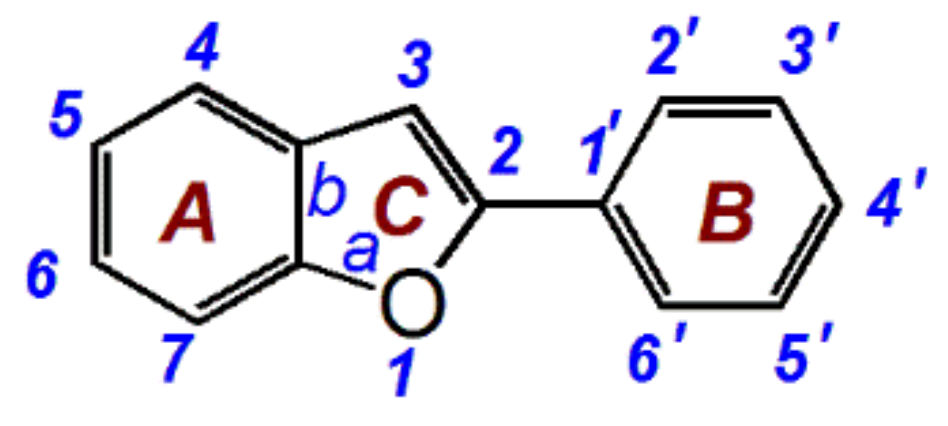
1. Introduction
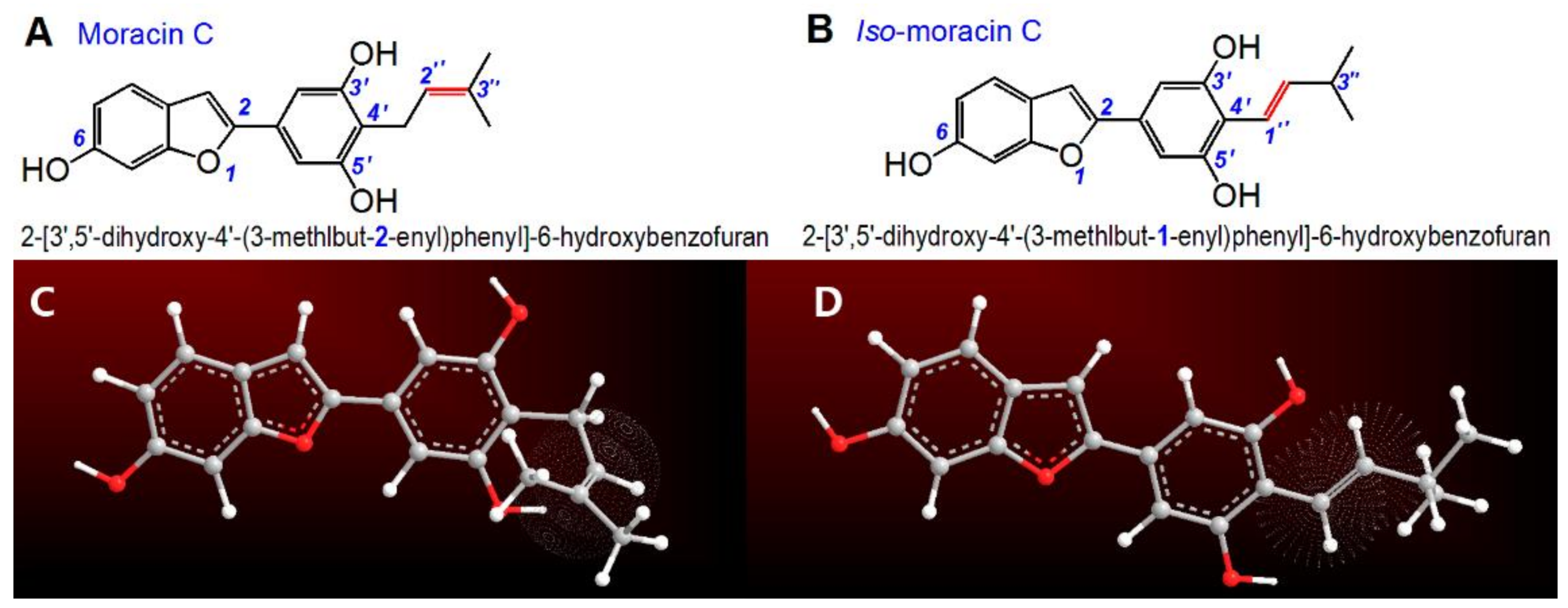
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Superoxide Anion (•O2−) Inhibiting Assay (Spectrophotometry)
3.3. CUPRAC Assay (Spectrophotometry)
3.4. DPPH•-Scavenging and ABTS•+-Scavenging Assays (Spectrophotometry)
3.5. Determining DPPH• Reaction Products with Moracin C or Iso-Moracin C (UPLC-ESI-Q-TOF-MS/MS Analysis)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis (3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt |
| DPPH• | 1,1-diphenyl-2-picryl-hydrazl |
| ET | electron-transfer |
| FBS | fetal bovine serum |
| FRAP | ferric reducing antioxidant power |
| HAT | hydrogen atom transfer |
| ROS | reactive oxygen species |
| RAF | radical-adduct-formation |
| SD | standard deviation |
| TPTZ | 2,4,6-tris(2-pyridyl-s-triazine) |
| Trolox | (±)-6-hydroxyl-2,5,7,8-tetramethlychromane-2-carboxylic acid |
References
- Meisinger, M.A.P.; Kuehl, F.A., Jr.; Rickes, E.L.; Brink, N.G.; Folkers, K.; Forbes, M.; Zilliken, F.; Gyorgy, P. The Structure of a New Product from Yeast: 2-(6-Hydroxy-2-methoxy-3,4-methylenedioxyphenyl)-benzofuran. J. Am. Chem. Soc 1959, 81, 4979–4982. [Google Scholar] [CrossRef]
- Qin, H.L.; Yu, D.O. 1H-NMR Spectroscopic Databook of Natural Products, 1st ed.; Chemical Industry Press: Beijing, China, 2011; pp. 2224–2235. [Google Scholar]
- Pel, P.; Chae, H.S.; Nhoek, P.; Kim, Y.M.; Chin, Y.W. Chemical Constituents with Proprotein Convertase Subtilisin/Kexin Type 9 mRNA Expression Inhibitory Activity from Dried Immature Morus alba Fruits. J. Agric. Food Chem. 2017, 65, 5316–5321. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Harmalkar, D.S.; Xu, X.; Jang, K.; Lee, K. Bioactive benzofuran derivatives: Moracins A-Z in medicinal chemistry. Eur. J. Med. Chem. 2015, 90, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Hakim, E.H.; Fahriyati, A.; Kau, M.S.; Achmad, S.A.; Makmur, L.; Ghisalberti, E.L.; Nomura, T. Artoindonesianins A and B, two new prenylated flavones from the root of Artocarpus champeden. J. Nat. Prod. 1999, 62, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Hoang, D.M.; Njamen, D.; Mbafor, J.T.; Fomum, Z.T.; Thuong, P.T.; Ahn, J.S.; Oh, W.K. Inhibitory effect of 2-arylbenzofurans from Erythrina addisoniae on protein tyrosine phosphatase-1B. Bioorg. Med. Chem. Lett. 2007, 17, 3868–3871. [Google Scholar] [CrossRef] [PubMed]
- Kapche, D.; Lekane, N.M.; Kulabas, S.S.; Ipek, H.; Tok, T.T.; Ngadjui, B.T.; Demirtas, I.; Tumer, T.B. Aryl benzofuran derivatives from the stem bark of Calpocalyx dinklagei attenuate inflammation. Phytochemistry 2017, 141, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Mei, H.; Tan, P.; Lu, W.; Zhu, J.; Wang, W.; Huang, J.; Li, J. Total synthesis of the 2-arylbenzo[b]furan-containing natural products from Artocarpus. Tetrahedron Lett. 2015, 56, 4383–4387. [Google Scholar] [CrossRef]
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M.A.; Keri, R.S. A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol. Rep 2017, 69, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wu, D.; Dong, N.; Ouyang, P.; Pu, J.; Hu, Q.; Wang, J.; Lu, W.; Huang, J.; Moracin, C. A Phenolic Compound Isolated from Artocarpus heterophyllus, Suppresses Lipopolysaccharide-Activated Inflammatory Responses in Murine Raw264.7 Macrophages. Int. J. Mol. Sci 2016, 17, 1199. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Mai, Y.; Wen, B.; Wang, X. Concordance between antioxidant activities in vitro and chemical components of Radix astragali (Huangqi). Nat. Prod. Res. 2012, 26, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Lin, J.; Wang, T.; Huang, J.; Lin, Y.; Chen, D. Protective Effects of Dihydromyricetin against •OH-Induced Mesenchymal Stem Cells Damage and Mechanistic Chemistry. Molecules 2016, 21, 604. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mai, W.Q.; Chen, D.F. Chemical study on protective effect against hydroxyl-induced DNA damage and antioxidant mechanism of myricitrin. J. Chin. Chem. Soc. 2014, 61, 383–391. [Google Scholar] [CrossRef]
- Li, X.; Han, W.; Mai, W.Q.; Wang, L. Antioxidant activity and mechanism of Tetrahydroamentoflavone in vitro. Nat. Prod. Commun. 2013, 8, 787–789. [Google Scholar]
- Lin, J.; Li, X.; Chen, L.; Lu, W.; Chen, X.; Han, L.; Chen, D. Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of [6]-gingerol: A Chemical Study. Bull. Korean Chem. Soc. 2014, 35, 1633–1638. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Liu, J.; Liu, Y.; Zhang, J.; Lin, J.; Zhao, Z.; Chen, D. Effect and mechanism of wedelolactone as antioxidant-coumestan on •OH-treated mesenchymal stem cells. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Li, Y.; Zhang, J.; Chen, J.; Lu, W.; Zhao, X.; Lai, Y.; Chen, D.; Wei, G. Protective Effect of Sinapine against Hydroxyl Radical-Induced Damage to Mesenchymal Stem Cells and Possible Mechanisms. Chem. Pharm. Bull. (Tokyo) 2016, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Huang, L. Correlation between Antioxidant Activities and Phenolic Contents of Radix Angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Li, X.; Xu, Z.; Liu, X.; Du, S.H.; Li, H.; Zhou, J.H.; Zeng, H.P.; Hua, Z.C. Hexadecanoic acid from Buzhong Yiqi decoction induced proliferation of bone marrow mesenchymal stem cells. J. Med. Food. 2010, 13, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Hu, Q.P.; Jiang, S.X.; Li, F.; Lin, J.; Han, L.; Hong, Y.L.; Lu, W.B.; Gao, Y.X.; Chen, D.F. Flos Chrysanthemi Indici protects against hydroxyl-induced damages to DNA and MSCs via antioxidant mechanism. J. Saudi Chem. Soc. 2015, 19, 454–460. [Google Scholar] [CrossRef]
- Zelová, H.; Hanáková, Z.; Čermáková, Z.; Šmejkal, K.; Dalĺ, A.S.; Babula, P.; Cvačka§, J.; Hošek, J. Evaluation of Anti-Inflammatory Activity of Prenylated Substances Isolated from Morus alba and Morus nigra. J. Nat. Prod. 2014, 77, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sohn, M.J.; Kim, W.G. Chalcomoracin and moracin C, new inhibitors of Staphylococcus aureus enoyl-acyl carrier protein reductase from Morus alba. Biol. Pharm. Bull. 2012, 35, 791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lang, L.; Dong, N.N.; Wu, D.Y.; Yao, X.; Lu, W.Q.; Zhang, C.; Ouyang, P.; Zhu, J.; Tang, Y.; Wang, W.; et al. 2-Arylbenzo[b]furan derivatives as potent human lipoxygenase inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Jiang, Q.; Wang, T.T.; Liu, J.J.; Chen, D.F. Comparison of the Antioxidant Effects of Quercitrin and Isoquercitrin: Understanding the Role of the 6 ''-OH Group. Molecules 2016, 21, 1246. [Google Scholar] [CrossRef] [PubMed]
- Tersey, S.A.; Maier, B.; Nishiki, Y.; Maganti, A.V.; Nadler, J.L.; Mirmira, R.G. 12-lipoxygenase promotes obesity-induced oxidative stress in pancreatic islets. Mol. Cell Biol. 2014, 34, 3735–3745. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [PubMed]
- Li, X.C.; Wei, G.; Wang, X.Z.; Liu, D.H.; Deng, R.D.; Li, H.; Zhou, J.H.; Li, Y.W.; Zeng, H.P.; Chen, D.F. Targeting of the Shh pathway by atractylenolides promotes chondrogenic differentiation of mesenchymal stem cells. Biol. Pharm. Bull. 2012, 35, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, R.A.; Estevez, L.; Bugarin, M.G. Computational studies on conformation, electron density distributions, and antioxidant properties of anthocyanidins. Methods Mol. Biol. 2015, 1208, 257–276. [Google Scholar] [PubMed]
- Sedlak, E.; Musatov, A. Inner mechanism of protection of mitochondrial electron-transfer proteins against oxidative damage. Focus on hydrogen peroxide decomposition. Biochimie 2017, 142, 152–157. [Google Scholar] [PubMed]
- Cekic, S.D.; Baskan, K.S.; Tutem, E.; Apak, R. Modified cupric reducing antioxidant capacity (CUPRAC) assay for measuring the antioxidant capacities of thiol-containing proteins in admixture with polyphenols. Talanta 2009, 79, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Solomons, T.W.G; Fryhle, B.C. Organic Chemistry, 8th ed.; Chemical Industry Press: Beijing, China, 2004; pp. 1015–1016. [Google Scholar]
- Chen, Y.; Xiao, H.; Zheng, J.; Liang, G. Structure-thermodynamics-antioxidant activity relationships of selected natural phenolic acids and derivatives: An experimental and theoretical evaluation. PLoS ONE 2015, 10, e0121276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; Gao, Y.; Li, F.; Liang, A.; Xu, Z.; Bai, Y.; Mai, W.; Han, L.; Chen, D. Maclurin protects against hydroxyl radical-induced damages to mesenchymal stem cells: Antioxidant evaluation and mechanistic insight. Chem. Biol. Interact. 2014, 219, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Rugeles, L.; Alvarez-Idaboy, J.R. A proton-electron sequential transfer mechanism: Theoretical evidence about its biological relevance. Phys. Chem. Chem. Phys. 2015, 17, 28525–28528. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Uno, B. Importance of Proton-Coupled Electron Transfer from Natural Phenolic Compounds in Superoxide Scavenging. Chem. Pharm. Bull. (Tokyo) 2015, 63, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Saumeth, J.; Rincon, D.A.; Doerr, M.; Daza, M.C. Concerted double proton-transfer electron-transfer between catechol and superoxide radical anion. Phys. Chem. Chem. Phys. 2017, 19, 26179–26190. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G.; Malaj, N.; Russo, N.; Toscano, M. Density functional study of the antioxidant activity of some recently synthesized resveratrol analogues. Food Chem. 2013, 141, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.; Pinto, S.; Weisbecker, C.; Attygalle, A.B. Competitive Deprotonation and Superoxide (•O2−) Radical-Anion Adduct Formation Reactions of Carboxamides under Negative-Ion Atmospheric-Pressure Helium-Plasma Ionization (HePI) Conditions. J. Am. Soc. Mass Spectrom. 2016, 27, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.R.; Li, W.G.; Chen, L.F.; Xiao, B.K.; Yang, J.Y.; Yang, L.; Zhang, C.G.; Huang, R.Q.; Dong, J.X. ABTS•+ scavenging potency of selected flavonols from Hypericum perforatum L. by HPLC-ESI/MS QQQ: Reaction observation, adduct characterization and scavenging activity determination. Food Res. Int. 2014, 58, 47–58. [Google Scholar] [CrossRef]
- Li, X. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Guclu, K.; Ozyurek, M.; Bektas Oglu, B.; Bener, M. Cupric ion reducing antioxidant capacity assay for food antioxidants: Vitamins, polyphenolics, and flavonoids in food extracts. Methods Mol. Biol. 2008, 477, 163–193. [Google Scholar] [PubMed]
- Jiang, Q.; Li, X.; Tian, Y.; Lin, Q.; Xie, H.; Lu, W.; Chi, Y.; Chen, D. Lyophilized aqueous extracts of Mori Fructus and Mori Ramulus protect Mesenchymal stem cells from •OH-treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Zeng, H. Synthesis, antioxidation activity of (E)-9-p-Tolyl-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and (E)-9-(p-Anisyl)-3-[2-(8-hydroxy-quinol-2-yl)vinyl]-carbazole and their induction proliferation of mesenchymal stem cells. Acta Chim. Sin. 2009, 67, 974–982. [Google Scholar]
- Wang, T.T.; Zeng, G.C.; Li, X.C.; Zeng, H.P. In vitro studies on the antioxidant and protective effect of 2-substituted-8-hydroxyquinoline derivatives against H2O2-induced oxidative stress in BMSCs. Chem. Biol. Drug Des. 2010, 75, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Li, X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) Radical Scavenging: A New and Simple Antioxidant Assay In Vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xie, H.; Zhan, R.; Chen, D. Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C. Molecules 2018, 23, 754. https://doi.org/10.3390/molecules23040754
Li X, Xie H, Zhan R, Chen D. Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C. Molecules. 2018; 23(4):754. https://doi.org/10.3390/molecules23040754
Chicago/Turabian StyleLi, Xican, Hong Xie, Ruicai Zhan, and Dongfeng Chen. 2018. "Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C" Molecules 23, no. 4: 754. https://doi.org/10.3390/molecules23040754
APA StyleLi, X., Xie, H., Zhan, R., & Chen, D. (2018). Effect of Double Bond Position on 2-Phenyl-benzofuran Antioxidants: A Comparative Study of Moracin C and Iso-Moracin C. Molecules, 23(4), 754. https://doi.org/10.3390/molecules23040754




