Reparative Effects of Astaxanthin-Hyaluronan Nanoaggregates against Retrorsine-CCl4-Induced Liver Fibrosis and Necrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
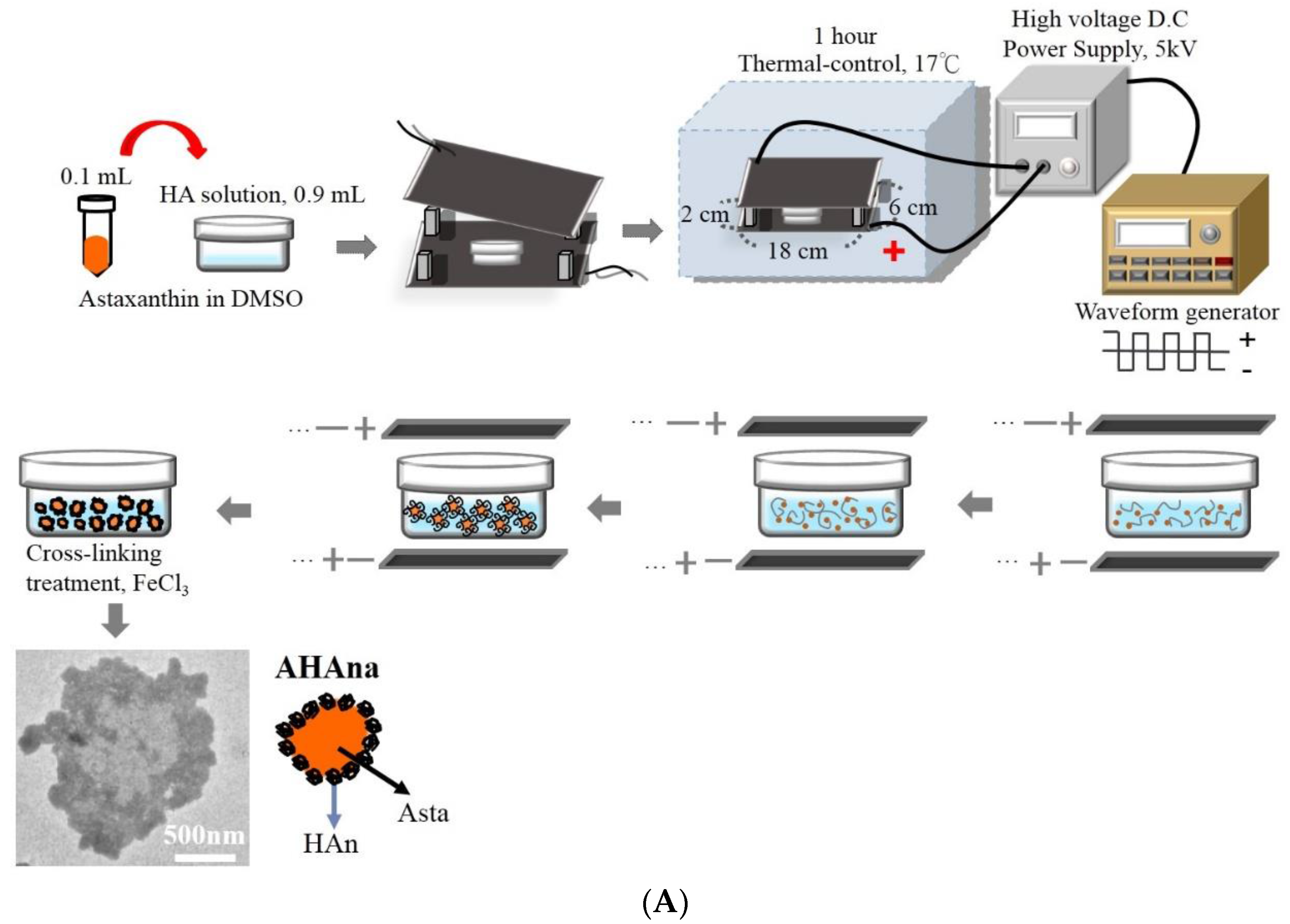
2.2. Production and Characterization of Asta-HA Nano-Aggregates (AHAna)
2.3. Incorporation Efficiency
2.4. In Vitro Release Study
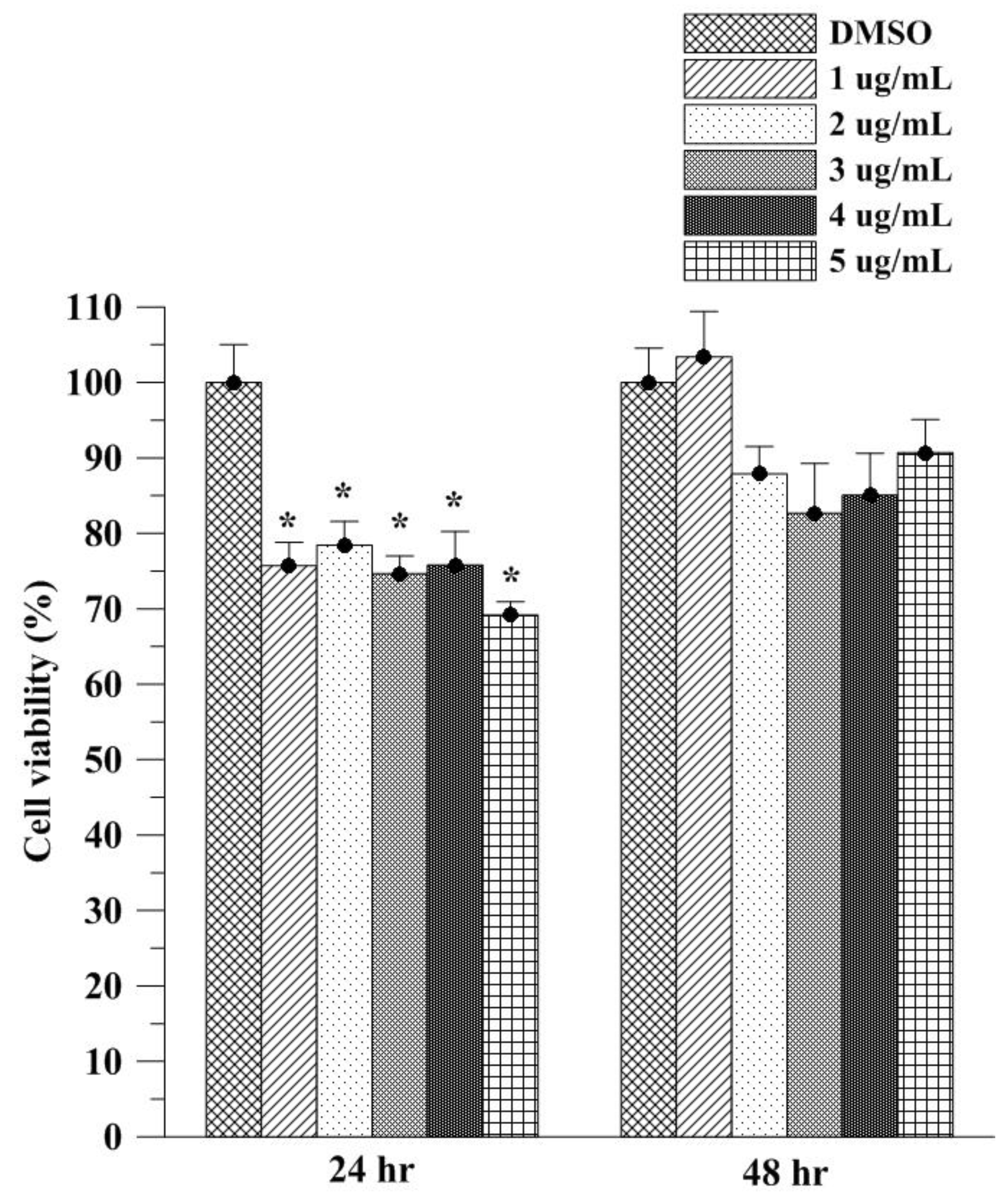
2.5. In Vitro Cell Viability Study of Asta on L929 Fibroblasts
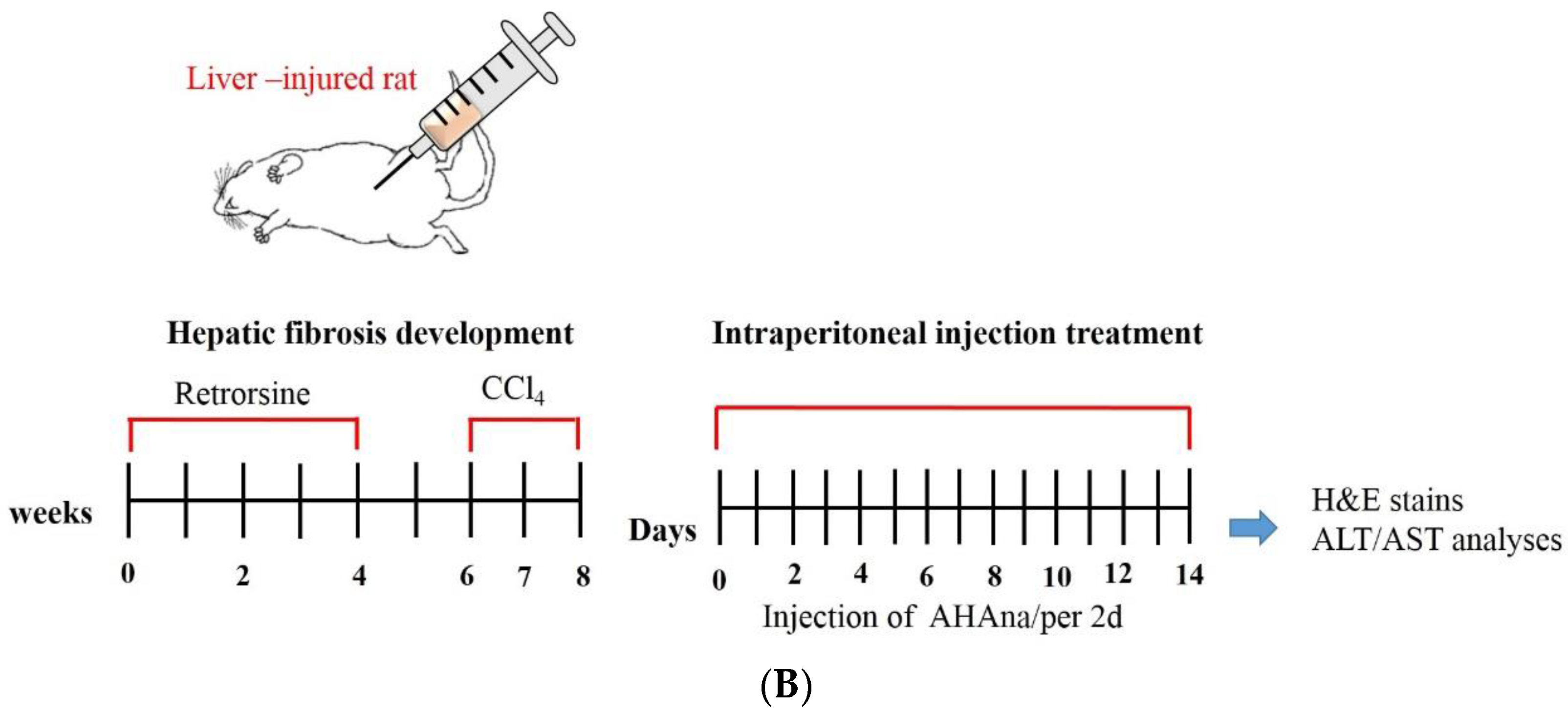
2.6. Animals and Experimental Design
2.7. Histopathological Analysis
2.8. Alanine Aminotransferase and Aspartate Aminotransferase Assays
2.9. Statistical Analysis
3. Results and Discussion
3.1. Observation of Asta-HA Nano-Aggregates (AHAna)
3.2. Characteristics of AHAna
3.3. Assessment of Cell Viability
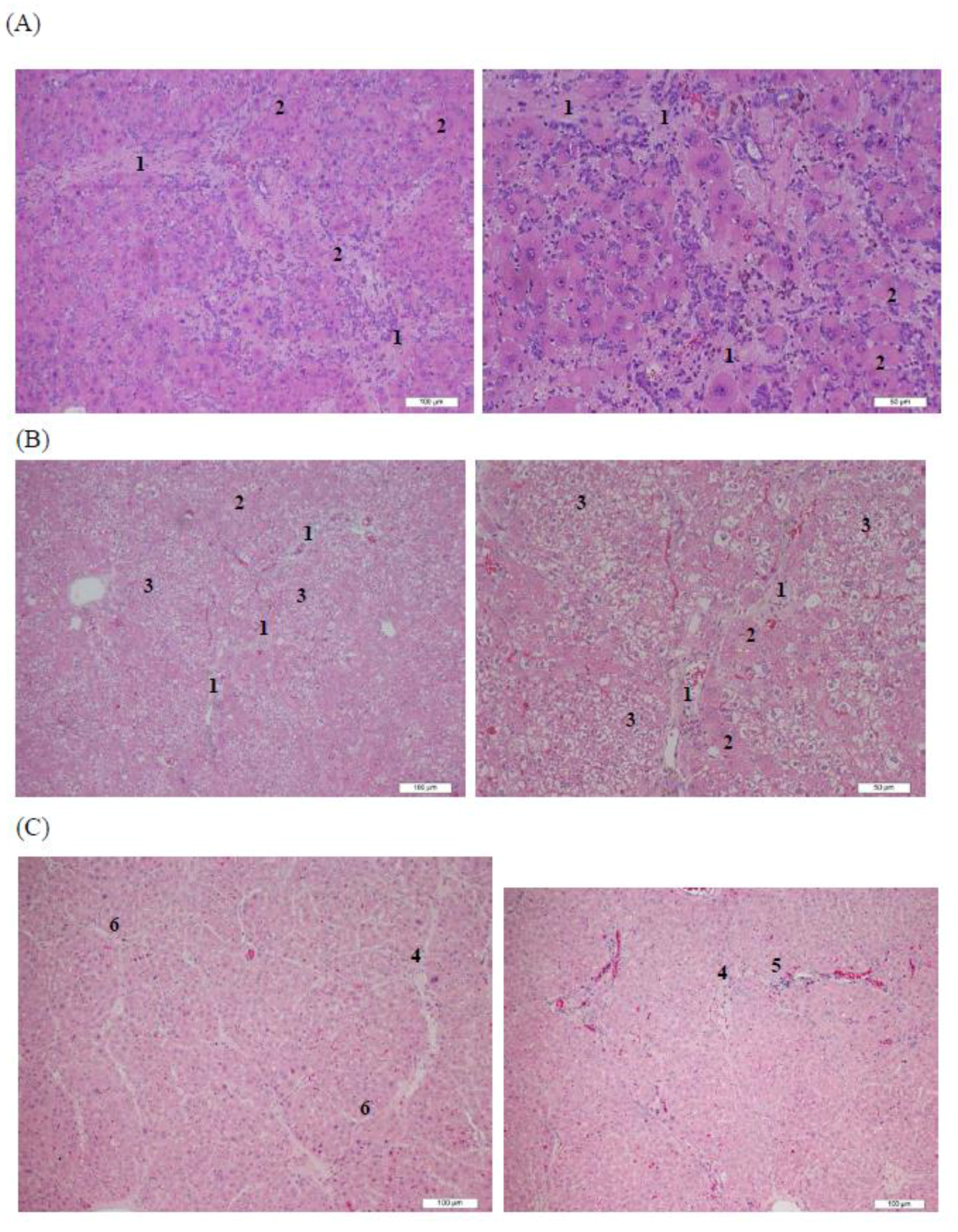
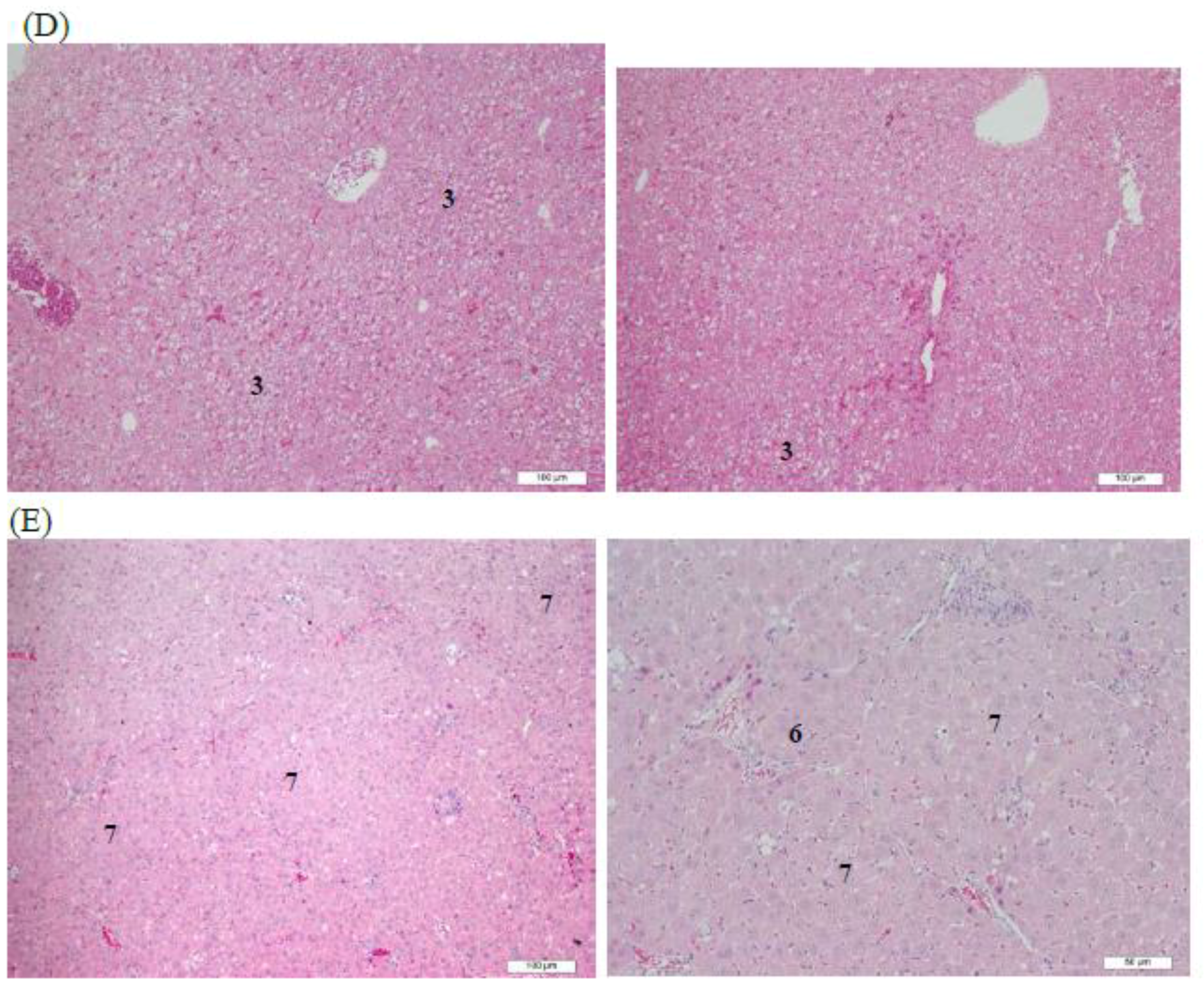
3.4. Histopathological Analysis of Rat Livers Treated with AHAna
3.5. ALT an AST Analysis of Rat Sera
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Farruggia, C.; Ku, C.S.; Pham, T.X.; Yang, Y.; Bae, M.; Wegner, C.J.; Farrell, N.J.; Harness, E.; Park, Y.K.; et al. Astaxanthin inhibits inflammation and fibrosis in the liver and adipose tissue of mouse models of diet-induced obesity and nonalcoholic steatohepatitis. J. Nutr. Biochem. 2017, 43, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Dreon, M.S.; Schinella, G.; Heras, H.; Pollero, R.J. Antioxidant defense system in the apple snail eggs, the role of ovorubin. Arch. Biochem. Biophys. 2004, 422, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Chang, C.C.; Lin, S.T.; Chyau, C.C.; Peng, R.Y. Improved hepatoprotective effect of liposome-encapsulated astaxanthin in lipopolysaccharide-induced acute hepatotoxicity. Int. J. Mol. Sci. 2016, 17, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ashtari, S.; Pourhoseingholi, M.A.; Zali, M.R. Non-alcohol fatty liver disease in Asia: Prevention and planning. World J. Hepatol. 2015, 7, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gong, G.; Yang, W.; Li, Y.; Jiang, M.; Li, L. Antifibrotic activity of galangin, a novel function evaluated in animal liver fibrosis model. Environ. Toxicol. Pharmacol. 2013, 36, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, Y.; Tajima, F.; Nakamura, Y.; Shibasaki, E.; Wakejima, M.; Shimomura, T.; Murai, R.; Murawaki, Y.; Hashiguchi, K.; Kanbe, T.; et al. Analyses to clarify rich fractions in hepatic progenitor cells from human umbilical cord blood and cell fusion. Biochem. Biophys. Res. Commun. 2004, 324, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Best, D.H.; Coleman, W.B. Liver regeneration by small hepatocyte-like progenitor cells after necrotic injury by carbon tetrachloride in retrorsine-exposed rats. Exp. Mol. Pathol. 2010, 89, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.Z.; Wang, B.; Liang, Y.K.; Bao, Y.Y.; Gu, Y. Hepatoprotective and antioxidant effects of licorice extract against CCL4-induced oxidative damage in rats. Int. J. Mol. Sci. 2011, 12, 6529–6543. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Kotani, K. Astaxanthin as a potential protector of liver function: A review. J. Clin. Med. Res. 2016, 8, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, K.Y.; Wu, Y.J.; Liu, Z.N.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Anticancer effects of sinulariolide-conjugated hyaluronan nanoparticles on lung adenocarcinoma cells. Molecules 2016, 21, 297. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Kuo, S.M.; Wu, Y.J.; Su, J.H. Improvement and enhancement of antibladder carcinoma cell effects of heteronemin by the nanosized hyaluronan aggregation. Int. J. Nanomed. 2016, 11, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Teong, B.; Chen, I.F.; Chang, S.J.; Gao, J.; Kuo, S.M. Enhanced apoptotic effects of dihydroartemisinin-aggregated gelatin and hyaluronan nanoparticles on human lung cancer cells. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Teong, B.; Lin, C.Y.; Chang, S.J.; Niu, G.C.; Yao, C.H.; Chen, I.F.; Kuo, S.M. Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J. Mater. Sci. Mater. Med. 2015, 26, 5357. [Google Scholar] [CrossRef] [PubMed]
- Skrede, G.; Storebakken, T. Instrumental colour analysis of farmed and wild Atlantic salmon when raw, baked and smoked. Aquaculture 1986, 53, 279–286. [Google Scholar] [CrossRef]
- Baccouche, B.; Mbarek, S.; Dellaa, A.; Hammoum, I.; Messina, C.M.; Santulli, A.; Chaouacha-Chekir, R.B. Protective effect of astaxanthin on primary retinal cells of the gerbil Psammomys obesus cultured in diabetic milieu. J. Food Biochem. 2017, 41, e12274. [Google Scholar] [CrossRef]
- Srinivas, S.; Sironmani, T.A.; Shanmugam, G. Dimethyl sulfoxide inhibits the expression of early growth-response genes and arrests fibroblasts at quiescence. Exp. Cell Res. 1991, 196, 279–286. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, J.; Guo, J.; Bai, L.; Marshall, C.; Cai, Z.; Wang, L.; Xiao, M. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS ONE 2014, 9, e107447. [Google Scholar] [CrossRef] [PubMed]
- Franceschelli, S.; Pesce, M.; Ferrone, A.; De Lutiis, M.A.; Patruno, A.; Grilli, A.; Felaco, M.; Speranza, L. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2− production. PLoS ONE 2014, 9, e88359. [Google Scholar] [CrossRef] [PubMed]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Nagashimada, M.; Zhuge, F.; Zhan, L.; Nagata, N.; Tsutsui, A.; Nakanuma, Y.; Kaneko, S.; Ota, T. Astaxanthin prevents and reverses diet-induced insulin resistance and steatohepatitis in mice: A comparison with vitamin E. Sci. Rep. 2015, 5, 17192. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Cadenas, E. Oxidative stress: Damage to intact cells and organsm. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 311, 617–663. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Mendez-Sanchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Kim, B.; Park, Y.K.; Koo, S.I.; Lee, J.Y. Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells. Biochim. Biophys. Acta 2015, 1850, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bae, M.; Kim, B.; Park, Y.K.; Koo, S.I.; Lee, J.Y. Astaxanthin prevents and reverses the activation of mouse primary hepatic stellate cells. J. Nutr. Biochem. 2016, 29, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Gasso, M.; Rubio, M.; Varela, G.; Cabre, M.; Caballeria, J.; Alonso, E.; Deulofem, R.; Camps, J.; Gimenez, A.; Pajares, M.; et al. Effects of s-adenosylmethionine on lipid peroxidation and liver fibrogenesis in carbon tetrachloride-induced cirrhosis. J. Hepatol. 1996, 25, 200–205. [Google Scholar] [CrossRef]
- Cho, M.K.; Lee, G.H.; Park, E.Y.; Kim, S.G. Hyaluronic acid inhibits adhesion of hepatic stellate cells in spite of its stimulation of DNA synthesis. Tissue Cell 2004, 36, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Wartewig, S.; Bottcher, R.; Poppl, A.; Hoentsch, J.; Ozegowski, J.H.; Neubert, R.H. The effects of hyaluronan and its fragments on lipid models exposed to UV irradiation. Int. J. Pharm. 2003, 254, 223–234. [Google Scholar] [CrossRef]
- Trabucchi, E.; Pallotta, S.; Morini, M.; Corsi, F.; Franceschini, R.; Casiraghi, A.; Pravettoni, A.; Foschi, D.; Minghetti, P. Low molecular weight hyaluronic acid prevents oxygen free radical damage to granulation tissue during wound healing. Int. J. Tissue React. 2002, 24, 65–71. [Google Scholar] [PubMed]
Sample Availability: Astaxanthin samples are available from Bioptik Technology Inc. (Miaoli, Taiwan). |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.J.; Wu, Y.C.; Chen, I.-F.; Wu, Y.-L.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Reparative Effects of Astaxanthin-Hyaluronan Nanoaggregates against Retrorsine-CCl4-Induced Liver Fibrosis and Necrosis. Molecules 2018, 23, 726. https://doi.org/10.3390/molecules23040726
Wu YJ, Wu YC, Chen I-F, Wu Y-L, Chuang CW, Huang HH, Kuo SM. Reparative Effects of Astaxanthin-Hyaluronan Nanoaggregates against Retrorsine-CCl4-Induced Liver Fibrosis and Necrosis. Molecules. 2018; 23(4):726. https://doi.org/10.3390/molecules23040726
Chicago/Turabian StyleWu, Yi Jhen, Yu Chiuan Wu, I-Fen Chen, Yi-Lung Wu, Chin Wen Chuang, Han Hsiang Huang, and Shyh Ming Kuo. 2018. "Reparative Effects of Astaxanthin-Hyaluronan Nanoaggregates against Retrorsine-CCl4-Induced Liver Fibrosis and Necrosis" Molecules 23, no. 4: 726. https://doi.org/10.3390/molecules23040726
APA StyleWu, Y. J., Wu, Y. C., Chen, I.-F., Wu, Y.-L., Chuang, C. W., Huang, H. H., & Kuo, S. M. (2018). Reparative Effects of Astaxanthin-Hyaluronan Nanoaggregates against Retrorsine-CCl4-Induced Liver Fibrosis and Necrosis. Molecules, 23(4), 726. https://doi.org/10.3390/molecules23040726



