Cis–Trans Configuration of Coumaric Acid Acylation Affects the Spectral and Colorimetric Properties of Anthocyanins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Spectrophotometric Properties of Acylated Anthocyanin Derivatives
2.2. Colorimetric Properties of Acylated Anthocyanin Derivatives
2.3. Color Stability over Time
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Anthocyanin Preparation for Pigment Isolation
3.2.2. Anthocyanin Isolation
3.2.3. Isolated Anthocyanin Identity—HPLC-MS
3.2.4. Sample Preparation
3.2.5. Visible Spectrophotometry of Samples
3.2.6. Colorimetry of Samples
3.2.7. Calculation of Sample Half-Lives
3.2.8. Statistical Evaluation of Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Potera, C. The Artificial Food Dye Blues. Environ. Health Perspect. 2010, 118, 428. [Google Scholar] [CrossRef] [PubMed]
- Dornblaser, L.; Jago, D. Colors and Flavors: The move to more natural. Mintel 2013, 1, 1–24. [Google Scholar]
- Sigurdson, G.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Ø.M.; Jordheim, M. Basic Anthocyanin Chemistry and Dietary Sources. In Anthocyanins in Health and Disease; Wallace, T.C., Giusti, M.M., Eds.; CRC Press (Taylor & Francis Group): Boca Raton, FL, USA, 2014; pp. 13–90. [Google Scholar]
- Ananga, A.; Georgiev, V.; Ochieng, J.; Phills, B.; Tsolova, V. Production of Anthocyanins in Grape Cell Cultures: A Potential Source of Raw Material for Pharmaceutical, Food, and Cosmetic Industries. In The Mediterranean Genetic Code-Grapevine and Olive; Puljuha, D., Sladonja, B., Eds.; InTech: Rijeka, Croatia, 2013; pp. 247–288. [Google Scholar]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Rakic, V.; Skrt, M.; Miljkovic, M.; Kostic, D.; Sokolovic, D.; Poklar-Ulrih, N. Effects of pH on the stability of cyanidin and cyanidin 3-O-β-glucopyranoside in aqueous solution. Hem. Ind. 2015, 69, 511–522. [Google Scholar] [CrossRef]
- Zhao, C.L.; Chen, Z.J.; Bai, X.S.; Ding, C.; Long, T.J.; Wei, F.G.; Miao, K.R. Structure-activity relationships of anthocyanidin glycosylation. Mol. Divers. 2014, 18, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Cabrita, L.; Andersen, O.M. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chem. 1998, 63, 435–440. [Google Scholar] [CrossRef]
- Mazza, G.; Brouillard, R. Recent developments in the stabilization of anthocyanins in food products. Food Chem. 1987, 25, 207–225. [Google Scholar] [CrossRef]
- Torskangerpoll, K.; Andersen, Ø.M. Colour stability of anthocyanins in aqueous solutions at various pH values. Food Chem. 2005, 89, 427–440. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Malcıoğlu, O.B.; Calzolari, A.; Gebauer, R.; Varsano, D.; Baroni, S. Dielectric and Thermal Effects on the Optical Properties of Natural Dyes: A Case Study on Solvated Cyanin. J. Am. Chem. Soc. 2011, 133, 15425–15433. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Molar absorptivity (ε) and spectral characteristics of cyanidin-based anthocyanins from red cabbage. Food Chem. 2016, 197, 900–906. [Google Scholar] [CrossRef] [PubMed]
- George, F.; Figueiredo, P.; Toki, K.; Tatsuzawa, F.; Saito, N.; Brouillard, R. Influence of trans-cis isomerisation of coumaric acid substituents on colour variance and stabilisation in anthocyanins. Phytochemistry 2001, 57, 791–795. [Google Scholar] [CrossRef]
- Yoshida, K.; Kondo, T.; Kameda, K.; Goto, T. Structure of Anthocyanins Isolated from Purple Leaves of Perilla ocimoides L. var. crispa Benth and Their Isomerization by Irradiation of Light. Agric. Biol. Chem. 1990, 54, 1745–1751. [Google Scholar] [CrossRef]
- Hosokawa, K.; Fukushi, E.; Kawabata, J.; Fujii, C.; Ito, T.; Yamamura, S. Seven acylated anthocyanins in blue flowers of Hyacinthus orientalis. Phytochemistry 1997, 45, 167–171. [Google Scholar] [CrossRef]
- Ichiyanagi, T.; Kashiwada, Y.; Shida, Y.; Ikeshiro, Y.; Kaneyuki, T.; Konishi, T. Nasunin from Eggplant Consists of Cis−Trans Isomers of Delphinidin 3-[4-(p-Coumaroyl)-l-rhamnosyl(1→6)glucopyranoside]-5-glucopyranoside. J. Agric. Food Chem. 2005, 53, 9472–9477. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Inami, O.; Tamura, I.; Kikuzaki, H.; Nakatami, N. Stability of anthocyanins of Sambucus canadensis and Sambucus nigra. J. Agric. Food Chem. 1996, 44, 3090–3096. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Guo, Z.; Yang, F.; Wang, J.; Li, X.; Peng, X.; Liang, X. High-performance liquid chromatography separation of cis–trans anthocyanin isomers from wild Lycium ruthenicum Murr. employing a mixed-mode reversed-phase/strong anion-exchange stationary phase. J. Agric. Food Chem. 2015, 63, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Comparative Biochemistry of the Flavonoids; Academic Press: London, UK; New York, NY, USA, 1967. [Google Scholar]
- Brouillard, R.; Delaporte, B. Chemistry of Anthocyanin Pigments. 2. * Kinetic and Thermodynamic Study of Proton Transfer, Hydration, and Tautomeric Reactions of Malvidin 3-Glucoside. J. Am. Chem. Soc. 1977, 99, 8461–8468. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Spectral and colorimetric characteristics of metal chelates of acylated cyanidin derivatives. Food Chem. 2017, 221, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Giusti, M.M. Bathochromic and Hyperchromic Effects of Aluminum Salt Complexation by Anthocyanins from Edible Sources for Blue Color Development. J. Agric. Food Chem. 2014, 62, 6955–6965. [Google Scholar] [CrossRef] [PubMed]
- Asenstorfer, R.E.; Iland, P.G.; Tate, M.E.; Jones, G.P. Charge equilibria and pKa of malvidin-3-glucoside by electrophoresis. Anal. Biochem. 2003, 318, 291–299. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Galano, A.; Alvarez-Idaboy, J.R. Deprotonation routes of anthocyanidins in aqueous solution, pKa values, and speciation under physiological conditions. RSC Adv. 2016, 6, 53421–53429. [Google Scholar] [CrossRef]
- Rodríguez-Saona, L.E.; Wrolstad, R.E. Extraction, Isolation, and Purifification of Anthocyanins. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. F1.1.1–F1.1.11. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Separation and Characterization of Anthocyanins by HPLC. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. F1.3.1–F1.3.13. [Google Scholar]
- Azuma, K.; Ohyama, A.; Ippoushi, K.; Ichiyanagi, T.; Takeuchi, A.; Saito, T.; Fukuoka, H. Structures and antioxidant activity of anthocyanins in many accessions of eggplant and its related species. J. Agric. Food Chem. 2008, 56, 10154–10159. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Anthocyanins, colour and antioxidant properties of eggplant (Solanum melongena L.) and violet pepper (Capsicum annuum L.) peel extracts. Z. Naturforsch. Sect. C J. Biosci. 2006, 61, 527–535. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R. Characterization and measurement of anthocyanins by UV- Visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2001; Volume 1, pp. F1.2.1–F1.2.13. [Google Scholar]
- Farr, J.E.; Giusti, M.M. ColorbySpectra-Academic License. Available online: https://buckeyevault.com/products/colorbyspectra (accessed on 15 January 2018).
Sample Availability: Samples of the compounds are not available from the authors. |
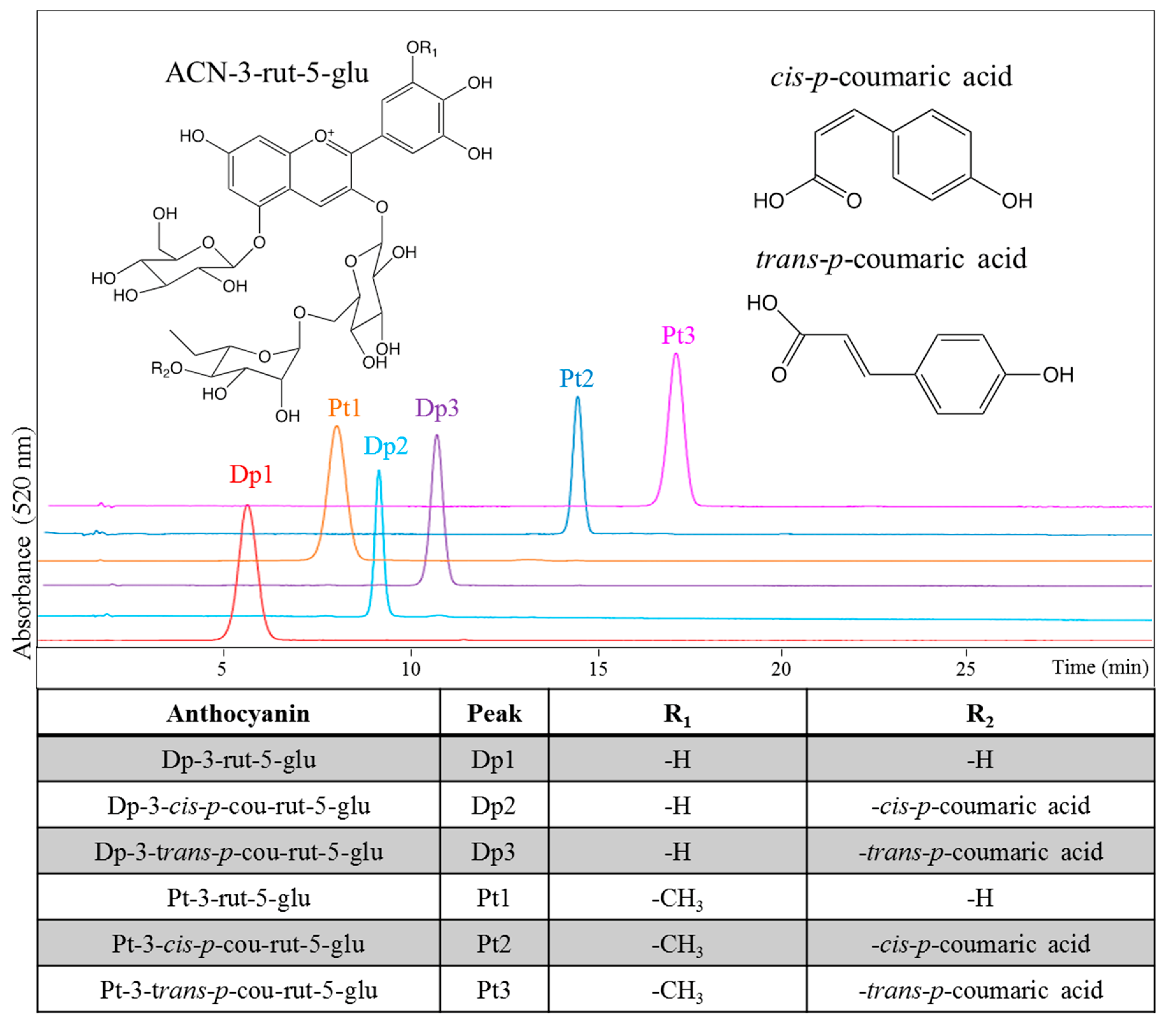
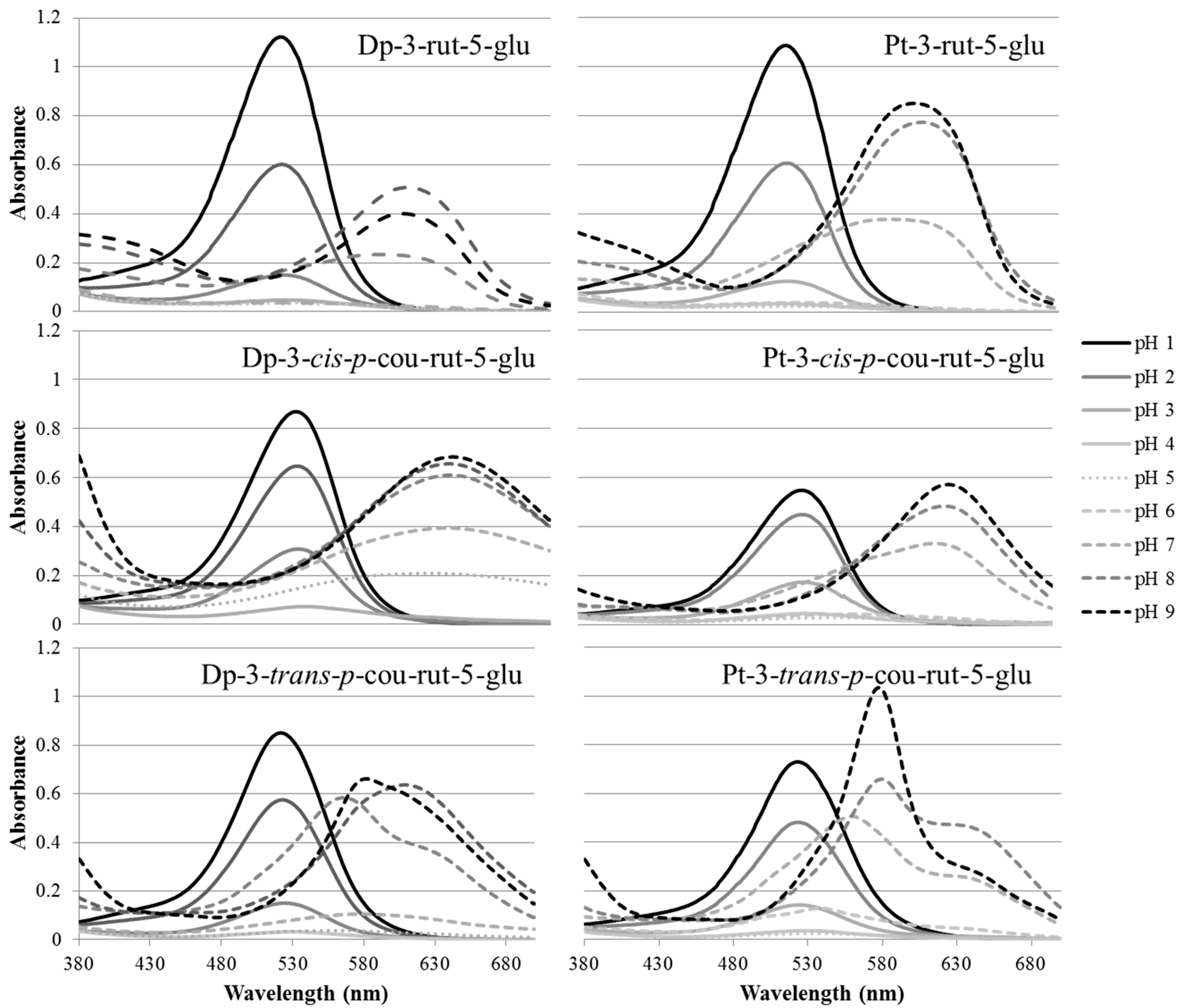
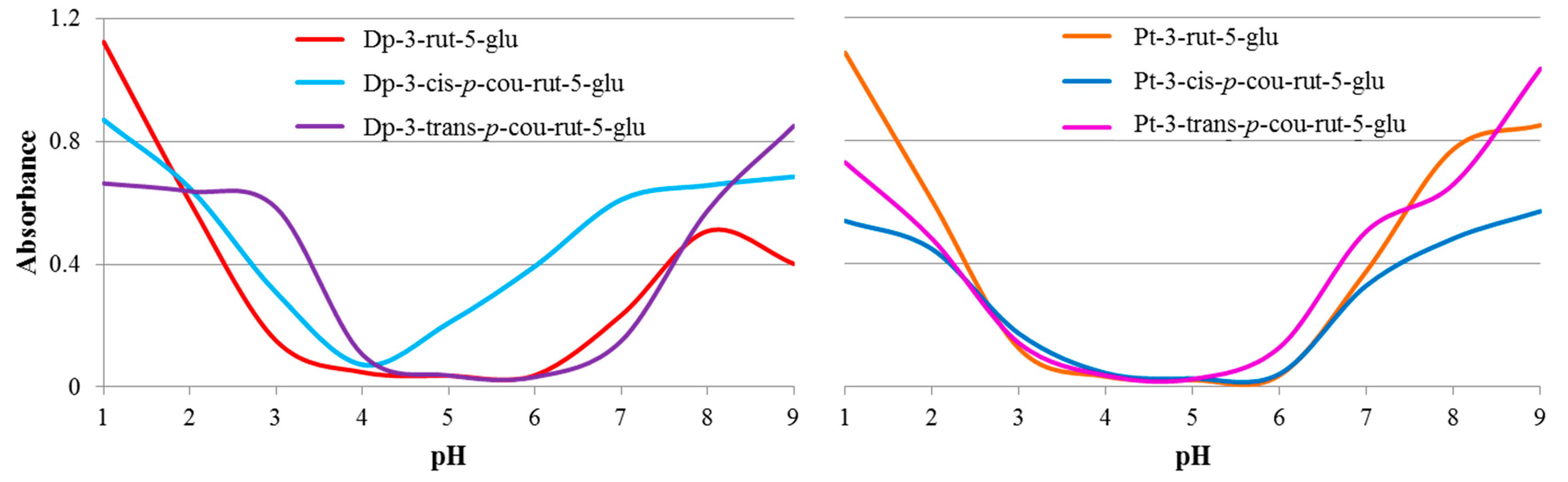

| Anthocyanin | pH 1 | pH 2 | pH 3 | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | pH 9 |
|---|---|---|---|---|---|---|---|---|---|
| λmax (nm) | |||||||||
| Dp-3-rut-5-glu | 517 c (0) | 518 c (0) | 518 c (2) | 521 c (0) | 522 c (4) | 450 c (1) | 583 b (0) | 603 c (1) | 600 b (1) |
| Dp-3-cis-p-cou-rut-5-glu | 528 a (1) | 528 a (1) | 529 a (1) | 534 a (2) | 617 a (1) | 630 a (1) | 632 a (0) | 631 a (1) | 632 a (1) |
| Dp-3-trans-pcou-rut-5-glu | 521 b (1) | 523 b (0) | 523 b (1) | 531 b (1) | 563 b (2) | 576 b (1) | 566 c (0) | 608 b (2) | 584 c (4) |
| Pt-3-rut-5-glu | 518 c (0) | 519 c (0) | 521 c (2) | 519 b (6) | 522 b (9) | 524 c (2) | 589 b (1) | 610 b (1) | 604 b (1) |
| Pt-3-cis-p-cou-rut-5-glu | 531 a (0) | 531 a (0) | 532 a (1) | 534 a (0) | 553 a (6) | 564 a (1) | 621 a (1) | 627 a (0) | 630 a (1) |
| Pt-3-trans-p-cou-rut-5-glu | 523 b (0) | 524 b (1) | 525 b (2) | 535 a (4) | 535b (2) | 538 b (0) | 559 c (0) | 579 c (0) | 578 c (0) |
| % absorbance retention | |||||||||
| Dp-3-rut-5-glu | 53.7 c (2.4) | 13.4 c (0.1) | 4.3 b (0.1) | 3.3 c (0.4) | 3.4 c (0.0) | 20.8 b (0.3) | 45.2 b (1.6) | 35.7 b (4.2) | |
| Dp-3-cis-p-cou-rut-5-glu | 77.0 a (1.1) | 36.3 a (1.2) | 9.0 a (0.3) | 25.6 a (0.8) | 45.8 a (2.5) | 70.6 a (2.2) | 70.6 a (2.3) | 71.1 a (1.3) | |
| Dp-3-trans-pcou-rut-5-glu | 68.2 b (0.7) | 18.6 b (0.2) | 4.1 b (0.1) | 4.9 b (0.2) | 14.3 b (0.2) | 70.5 a (2.4) | 73.5 a (0.2) | 68.4 a (3.0) | |
| Pt-3-rut-5-glu | 55.9 c (2.1) | 11.5 c (0.2) | 3.1 c (0.2) | 2.1 c (0.1) | 3.5 c (0.1) | 34.8 c (4.1) | 71.3 b (4.7) | 78.3 c (3.1) | |
| Pt-3-cis-p-cou-rut-5-glu | 82.0 a (1.8) | 31.8 a (0.4) | 8.3 a (0.3) | 5.1 a (0.1) | 7.9 b (1.0) | 60.4 b (3.3) | 88.3 a (0.6) | 104.4 b (0.7) | |
| Pt-3-trans-p-cou-rut-5-glu | 66.1 b (0.9) | 19.6 b (1.1) | 5.1 b (0.3) | 3.5 b (0.3) | 17.8 a (0.7) | 69.4 a (0.8) | 90.3 a (3.4) | 141.8 a (4.8) | |
| Anthocyanin | pH 1 | pH 2 | pH 3 | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | pH 9 |
|---|---|---|---|---|---|---|---|---|---|
| Lightness (L*) | |||||||||
| Dp-3-rut-5-glu | 74.1 (0.1) | 82.2 (0.5) | 93.7 (0.1) | 97.2 (0.1) | 97.5 (0.2) | 97.2 (0.0) | 84.4 (0.3) | 77.3 (0.4) | 80.5 (1.5) |
| Dp-3-cis-pcou-rut-5-glu | 77.7 (0.1) | 81.4 (0.5) | 89.8 (0.6) | 95.9 (0.2) | 89.0 (0.6) | 82.8 (0.7) | 78.7 (0.5) | 79.3 (0.8) | 79.4 (0.2) |
| Dp-3-trans-pcou-rut-5-glu | 78.0 (0.3) | 83.0 (0.4) | 94.3 (0.1) | 98.3 (0.1) | 97.4 (0.2) | 93.1 (0.1) | 75.3 (1.2) | 78.1 (0.2) | 79.3 (1.0) |
| Pt-3-rut-5-glu | 74.6 (0.2) | 82.3 (0.3) | 94.9 (0.0) | 98.2 (0.1) | 98.4 (0.1) | 97.3 (0.1) | 77.2 (2.3) | 72.1 (1.1) | 70.3 (1.2) |
| Pt-3-cis-pcou-rut-5-glu | 81.1 (0.2) | 83.7 (0.2) | 92.0 (0.6) | 97.3 (0.1) | 98.1 (0.1) | 96.9 (0.3) | 83.5 (0.5) | 83.3 (0.4) | 83.7 (0.3) |
| Pt-3-trans-pcou-rut-5-glu | 76.1 (0.3) | 82.5 (0.1) | 93.5 (0.5) | 97.9 (0.1) | 98.3 (0.1) | 92.3 (0.3) | 73.4 (0.3) | 73.5 (0.6) | 70.4 (0.3) |
| Chroma (C*ab) | |||||||||
| Dp-3-rut-5-glu | 58.3 (0.1) | 41.3 (1.0) | 12.2 (0.1) | 3.1 (0.2) | 2.0 (0.3) | 2.3 (0.1) | 9.0 (0.4) | 22.3 (0.5) | 16.2 (2.0) |
| Dp-3-cis-pcou-rut-5-glu | 50.2 (0.2) | 42.4 (1.1) | 21.8 (1.3) | 4.0 (0.3) | 10.1 (0.5) | 17.0 (0.8) | 23.8 (0.6) | 24.2 (0.8) | 22.8 (0.6) |
| Dp-3-trans-pcou-rut-5-glu | 48.4 (0.6) | 38.2 (0.7) | 11.7 (0.1) | 2.0 (0.1) | 1.9 (0.2) | 6.4 (0.1) | 28.2 (1.5) | 28.4 (0.2) | 35.1 (1.6) |
| Pt-3-rut-5-glu | 58.6 (0.5) | 42.5 (0.7) | 10.6 (0.0) | 2.4 (0.1) | 1.5 (0.1) | 1.9 (0.1) | 22.2 (2.3) | 37.5 (1.2) | 39.0 (1.2) |
| Pt-3-cis-p-cou-rut-5-glu | 44.5 (0.4) | 38.4 (0.4) | 16.0 (0.2) | 3.3 (0.2) | 1.2 (0.1) | 1.3 (0.3) | 19.2 (0.8) | 27.9 (0.3) | 29.9 (0.4) |
| Pt-3-trans-pcou-rut-5-glu | 52.0 (0.7) | 38.8 (0.4) | 12.4 (0.9) | 2.5 (0.1) | 1.0 (0) | 9.5 (0.3) | 33.0 (0.3) | 34.2 (0.6) | 36.9 (0.3) |
| Hue angle (hab) | |||||||||
| Dp-3-rut-5-glu | 0.3 c (0.1) | 356.4 a (0.2) | 359.0 a (0.3) | 26.3 c (2.2) | 55.5 c (2.6) | 75.7 c (2.7) | 272.3 b (1.1) | 218.7 c (1.3) | 198.4 c (5.3) |
| Dp-3-cis-pcou-rut-5-glu | 343.9 b (0.2) | 344.0 c (0.2) | 341.2 b (0.4) | 327.6 b (2.8) | 255.5 b (0.4) | 247.5 b (0.3) | 231.3 c (0.5) | 223.2 b (0.3) | 218.6 b (0.5) |
| Dp-3-trans-pcou-rut-5-glu | 344.5 a (0.1) | 343.4 b (0.2) | 341.7 b (0.1) | 342.0 a (1.1) | 293.9 a (3.1) | 280.8 a (0.5) | 283.6 a (1.1) | 237.0 a (0.2) | 236.8 a (1.7) |
| Pt-3-rut-5-glu | 357.3 a (0.1) | 354.2 a (0.1) | 358.2 a 90.5) | 34.5 c (2.0) | 58.2 c (2.5) | 33.7 c (1.5) | 274.5 b (1.2) | 231.5 b (0.7) | 224.6 b (0.6) |
| Pt-3-cis-p-cou-rut-5-glu | 341.2 c (0.1) | 340.7 c (0.2) | 336.6 c (3.2) | 337.8 b (1.4) | 325.0 b (3.6) | 299.1 b (8.3) | 246.3 c (0.2) | 221.5 c (0.1) | 215.0 c (0.9) |
| Pt-3-trans-pcou-rut-5-glu | 342.8 b (0.1) | 342.2 b (0.1) | 342.2 b (0.4) | 352.6 a (1.0) | 356.3 a (3.9) | 322.3 c (1.1) | 296.8 a (0.3) | 250.1 a (0.4) | 265.0 a (0.2) |
| Anthocyanin | pH 1 | pH 2 | pH 3 | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | pH 9 |
|---|---|---|---|---|---|---|---|---|---|
| t1/2 (h) | |||||||||
| Dp-3-rut-5-glu | 675.0 b (9.3) | 378.7 a (16.8) | 102.7 a (6.2) | 34.2 b (5.5) | * NM | * NM | 1.7 c (0.0) | 1.1 c (0.0) | 0.7 c (0.0) |
| Dp-3-cis-p-cou-rut-5-glu | 1077.2 a (21.6) | 424.0 a (128.9) | 52.9 b (0.6) | 16.0c (0.2) | 13.08 b (0.2) | 12.8 b (0.0) | 12.6 a (0.1) | 8.6 b (0.1) | 7.5 b (0.4) |
| Dp-3-trans-p-cou-rut-5-glu | 666.3 b (47.6) | 457.5 a (26.2) | 89.2 a (7.2) | 53.9 a (1.6) | 67.5 a (8.4) | 18.3 a (7.7) | 7.7 b (0.1) | 13.2 a (0.7) | 13.7 a (0.6) |
| Pt-3-rut-5-glu | 183.9 b (26.0) | 65.3 b (5.3) | 28.5 a (1.9) | 18.0(1.5) | 19.5(2.5) | 6.5 a (0.2) | 1.7 b (0.0) | 2.5 b (0.0) | 1.1 c (0.0) |
| Pt-3-cis-p-cou-rut-5-glu | 546.8 b (20.3) | 137.4 a (19.8) | 17.8 b (1.7) | * NM | * NM | 1.9 b (0.1) | 1.8b (0.0) | 3.3 b (0.1) | 4.0 b (0.7) |
| Pt-3-trans-p-cou-rut-5-glu | 2309.6 a (389.1) | 109.7 a (11.8) | 25.3 a (2.6) | * NM | * NM | 7.1 a (0.8) | 4.2 a (0.1) | 7.3 a (0.6) | 5.5 a (0.6) |
| k | |||||||||
| Dp-3-rut-5-glu | 0.0010 (0.0000) | 0.0018 (0.0001) | 0.0068 (0.0004) | 0.0206 (0.0032) | * NM | * NM | 0.4004 (0.0034) | 0.6110 (0.0078) | 1.0377 (0.0583) |
| Dp-3-cis-p-cou-rut-5-glu | 0.0006 (0.0000) | 0.0017 (0.0005) | 0.0131 (0.0001) | 0.0433 (0.0006) | 0.0532 (0.0001) | 0.0540 (0.0001) | 0.0552 (0.0005) | 0.0808 (0.0005) | 0.0929 (0.0049) |
| Dp-3-trans-p-cou-rut-5-glu | 0.0010 (0.0001) | 0.0015 (0.0001) | 0.0078 (0.0007) | 0.0129 (0.0004) | 0.0104 (0.0012) | 0.0379 (0.0015) | 0.0900 (0.0009) | 0.0527 (0.0028) | 0.0507 (0.0020) |
| Pt-3-rut-5-glu | 0.0038 (0.0001) | 0.0107 (0.0001) | 0.0244 (0.0016) | 0.0386 (0.0032) | 0.0359 (0.0049) | 0.1073 (0.0029) | 0.4019 (0.0064) | 0.2780 (0.0033) | 0.6163 (0.0071) |
| Pt-3-cis-p-cou-rut-5-glu | 0.0013 (0.0000) | 0.0051 (0.0007) | 0.0391 (0.0040) | * NM | * NM | 0.3745 (0.0124) | 0.3832 (0.0091) | 0.2127 (0.0054) | 0.1751 (0.0329) |
| Pt-3-trans-p-cou-rut-5-glu | 0.0003 (0.0001) | 0.0064 (0.0001) | 0.0277 (0.0030) | * NM | * NM | 0.0988 (0.0118) | 0.1664 (0.0029) | 0.0956 (0.0070) | 0.1273 (0.0122) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigurdson, G.T.; Tang, P.; Giusti, M.M. Cis–Trans Configuration of Coumaric Acid Acylation Affects the Spectral and Colorimetric Properties of Anthocyanins. Molecules 2018, 23, 598. https://doi.org/10.3390/molecules23030598
Sigurdson GT, Tang P, Giusti MM. Cis–Trans Configuration of Coumaric Acid Acylation Affects the Spectral and Colorimetric Properties of Anthocyanins. Molecules. 2018; 23(3):598. https://doi.org/10.3390/molecules23030598
Chicago/Turabian StyleSigurdson, Gregory T., Peipei Tang, and M. Mónica Giusti. 2018. "Cis–Trans Configuration of Coumaric Acid Acylation Affects the Spectral and Colorimetric Properties of Anthocyanins" Molecules 23, no. 3: 598. https://doi.org/10.3390/molecules23030598
APA StyleSigurdson, G. T., Tang, P., & Giusti, M. M. (2018). Cis–Trans Configuration of Coumaric Acid Acylation Affects the Spectral and Colorimetric Properties of Anthocyanins. Molecules, 23(3), 598. https://doi.org/10.3390/molecules23030598





